Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev Col Gastroenterol vol.24 no.3 Bogotá July/Sept. 2009
Etiology of duodenal ulcers in a Colombian population
William Otero Regino, MD. (1), Martín Gómez Zuleta, MD. (2), Xiomara Ruiz Lobo, MD.(3)
1. Professor of Medicine, Gastroenterology Unit, Universidad Nacional de Colombia, Gastroenterologist, Clínica Fundadores, Clínica Carlos Lleras Restrepo, Fundación Hospital San Carlos, Bogotá, Colombia.
2. Professor of Medicine, Gastroenterology Unit, Universidad Nacional de Colombia, Gastroenterologist, Hospital El Tunal, Clínica Carlos Lleras Restrepo, Fundación Hospital San Carlos, Bogotá, Colombia.
3. Gastroenterologist, Hospital El Tunal, SaludCoop, Clìnica Carlos Lleras, Fundación Hospital San Carlos, Bogotá, Colombia
Received: 22-04-09 Accepted: 18-08-09
Summary
Introduction: In the last decade the number of duodenal ulcers secondary to Helicobacter pylori has decreased in the United States. During the same period there has been an increase in the number reported cases of duodenal ulcers associated with NSAID as well as an increase in the number of reported idiopathic ulcers (H. pylori and NSAID/Aspirin negative). Since the prevalence rates of duodenal ulcers secondary to both H. pylori and NSAID/Aspirin are to date unknown in Colombia, we decided to undertake this study.
Materials and Methods: This study looks at adult patients who were diagnosed with duodenal ulcers after undergoing esophagogastroduodenoscopies (EGD) in the gastroenterology unit in the Hospital el Tunal and Clinica Fundadores in Bogotá, Colombia.
Results: Between January 2007 and November 2008, 104 patients with duodenal ulcer were recruited to this study. Their mean age was 56 +/- 13 years. 61% were men. H. pylori infections were identified in 76 patients (73%), while 12 patients (11.5%) were at risk from NSAID/Aspirin. 31 patients (29.8%) had both risks factors while no risk factors were identified in 12 patients (11.5%).
Conclusions: 14.4% of the duodenal ulcers in our study could not be explained by either H pylori or NSAID/Aspirin. Additional studies are necessary to determine other causes. H. pylori was by far the leading cause of duodenal ulcers, while NSAID/Aspirin was a distant second. Given these results, we recommend treatment to eradicate H. pylori even in patients whose diagnostic tests have not indicated its presence.
Key words
Helicobacter pylori, NSAID/Aspirin, duodenal ulcer.
INTRODUCCION
Since the discovery of Helicobacter pylori (H. pylori) in 1982 (1), diverse works have solidly demonstrated that it is the main etiological agent of chronic gastritis (CG) as well as gastric and duodenal peptic ulcers (PUs) (2, 3). It has also been found that the removal of this infection is necessary to cure CG and avoid the reappearance of peptic ulcers (4-8), and it is well documented that the removal of H. pylori is more effective than anti-acid secretors to cure duodenal ulcers (DUs) (8). The main causes of DUs are H. pylori and non-steroidal anti-inflammatory drugs (NSAIDs), including aspirin (ASA) in antithrombotic doses (9-11); although there are also less common causes such as hypersecretion of acids other than that from Zollinger-Ellison syndrome (ZE), Crohns disease, Behçet disease, malignant ulcers, ischemia and cytomegalovirus caused infections (9-13). NSAIDs and H. pylori caused infections are considered independent and synergistic risk factors for complicated and non complicated PUs (14). In many previous studies H. pylori has been found in more than 90% of the patients with DUs and 70% of the patients with gastric ulcers (15, 16). In other publications the prevalence of the infection is 100% in patients with DUs (17). However, in the last decade it has been documented that in some parts of the world the etiology of PUs has changed. The prevalence of H. pylori infections, and consequently H. pylori associated ulcers, has declined while the prevalence of NSAID and ASA use, and consequently associated ulcers, has increased (18). In the United States of America (USA) it has been found that from 20% to 40% of PUs are idiopathic, meaning that they are not associated with H. pylori or to NSAIDs (18,19). However, after excluding NSAID related ulcers, H. pylori was found in 61% of the patients with PUs (19). In an analysis of six clinical trials that included 2900 patients Ciociola found the infection in 73% of the patients with DUs (20). This contrasts with Japan where idiopathic PUs represent only 1.3% and ulcers associated with H. pylori still occur very frequently (21). Treatment guidelines such as those of The American College of Gastroenterologys (22) and the recent Maastricht III Consensus (23) recommend that all patients with duodenal ulcers and H. pylori infection must undergo treatment with antibiotics and proton pump inhibitors (PPIs) to eradicate the bacteria. Although nearly two decades ago a study using Gram stains and bacterial cultures found H. pylori in 80% of the adults studied (24), and in 77% of the patients with DUs studied, the current prevalence of H. pylori in the population and among people with ulcers remains unknown. The same can be said about the prevalence of NSAID and ASA use in Colombia, and their association with ulcers. Considering these facts along with the therapeutic impact of eradicating H. pylori in patients with duodenal ulcers, we decided undertake the present work. We used upper digestive endoscopy (UDE).to determine the prevalence of H. pylori and NSAIDs/ASA in patients with association with peptic ulcers, as well as determining the percentage of ulcers which are not associated with either diagnosis.
MATERIALS AND METHODS
The study included immunocompetent adult patients over 18 years of age who were diagnosed with DU between January 2007 and November 2008 at the gastroenterology units of El Tunal Hospital and Fundadores Clinic. The research protocol and the informed consent procedure were approved by the respective ethics committees of the participating institutions. Even though the Colombian Ministry of Healths Resolution 08430 of 1993 classifies this type of study as non-risk research which does not require written informed consent, written consent was obtained in case the patient was required for an additional interview. The characteristics of each patient were recorded on a form produced specifically for this research project.
EXCLUSION CRITERIA
The following classes of patients were excluded: patients with gastric tumors (adenocarcinoma, lymphoma, sarcoma, carcinoids, etc.), patients with histories of previous gastric surgery, patients with HVI/AIDS, patients undergoing chemotherapy, patients with Crohns disease, patients with Behçet disease, patients with weight loss over 10% of normal weight, patients with cirrhosis, patients with advanced chronic renal failure (on dialysis), patients with organ transplants, patients with uncontrolled diabetes, patients with malignant disease or coagulopathy, patients who had previously undergone H. pylori eradication therapy (or who were undergoing it at the time of the study), patients who were alcoholics, patients who had suffered recent acute myocardial infarct, cerebrovascular disease, congestive heart failure grade 4, severe respiratory failure, patients with suspected perforation of hollow viscera, pregnant women, and patients with any other contraindication for undergoing upper digestive endoscopy. Patients who did not agree to participate in the study, and those who did not sign the informed consent form, were also excluded.
UDEs were performed as usual: with patients in left lateral decubitus position after six or more hours fasting, without sedation, using Olympus Exera CFQ145L or Fujynon 2200 endoscopes. An ulcer was defined as a lesion with loss of mucosal integrity that was 5 mm or more in diameter and which had an apparent depth of 1 mm or more. The lesions diameters were measured using standard gastric biopsy forceps which open 6mm wide. Larger ulcers did not require use of this measure. Depth greater than or equal to 1mm was estimated based on the experience of the gastroenterologists who performed the endoscopies. Four biopsies of the stomach were taken for histology with hematoxylin and eosin: two from the antrum, one each from the greater and lesser curvatures two to three cm from the pylorus; and two from the stomach body, one each from the greater and lesser curvatures taken eight cm from the cardia (24). If the hematoxylin and eosin tests did not identify H. pylori, Giemsa stains were used. A biopsy of the antrum was also taken for rapid urease test using a solution of urea and phenol red produced in house according to published recommendations (25). H. pylori infections were considered to be present when detected either by rapid urease and/or histology. Data was entered into Excel 2003 and analyzed with Stata 9.0 software. Descriptive statistics were used for the study variables while frequencies and distribution percentages were analyzed with categorical variables. Numeric variables were analyzed with measures of central tendency and dispersion. Statistical tests were evaluated at a significance level of 5% (p <0.05). Continuous variables were reported as frequencies and averages. The differences between averages were determined using the Students t-test.
RESULTS
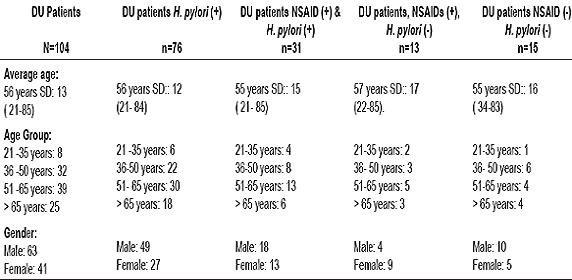
Between January 2007 and October 2008 104 patients prospectively recruited. The average age was 56 + / - 13 years. 61% were men. H. pylori infection was identified in 76 patients (73%). Consumption of NSAIDs and/or ASA as well as H. pylori infection was documented in 31 patients (29.8%). The cause of DU was exclusively NSAIDs (H. pylori negative) in 12 patients (11.5%). 15 patients (14.4%) were diagnosed as H. pylori negative and had not consumed NSAIDs. Among the different groups there were no statistically significant differences concerning average age or education. Patients characteristics are shown in table 1.
Table 1. Duodenal ulcer patients characteristics.
DISCUSSION
The prevalence of H. pylori in patients with duodenal ulcers in this study (73%) is lower than that reported in other countries in the 1990s (> / = 90%) (14.15). However, we have set aside the issue of whether or not our data indicate any change from previous prevalence in Colombia for several reasons. We were unable to find studies on subject except for a work published by the National University group (24). In that study overall prevalence was 80% in people over 30 years of age, and 77% in patients with DUs. Although this date is similar to that found in this study, there is a possibility that the rate of infection was higher than reported in the National University study. That study used Gram staining and bacterial cultures to diagnose H. pylori. Both methods tend to under diagnose the presence of the bacteria compared to diagnoses with invasive methods such as the rapid urease test or histology with complementary staining techniques like Giemsa and silver staining used when the microorganism is not found with hematoxylin and eosin (HE) (22). (Gram staining has a lower yield than invasive methods, and bacterial cultures have lower sensitivity than invasive methods.) Considering the drawbacks of the techniques used in the work of the National University, our findings may or may not indicate that DUs are less often associated with H. pylori than in the past. In a study by Bravo and colleagues on the prevalence of H. pylori in gastric biopsies (27) the prevalence of the bacteria was found to be 69.1%. This number is also likely to underestimate the true prevalence of the infection in the general population. The patient sample in that study was not screened for previous therapy for H. pylori eradication or for use of antisecretory or antimicrobials drugs. All of these factors can influence the detection of the infection (28), even when sensitive testing methods such as the urea breath test are used (28.29). In addition, complementary staining, such as Giemsa, was not used. Neither was specific staining used when hematoxylin and eosin were negative. (26). Finding H. pylori in 73% of the DUs in the present study shows that in our environment the prevalence of the infection remains high, although have more accurate and reliable data about the prevalence in the general population would be required for the development of well-designed prevalence studies with suitably calculated sample sizes and samples controlled for the aforementioned variables that can influence the detection of the infection. The prevalence of H. pylori found in this study is similar to that found in an analysis of six clinical trials in the USA (73%) a decade ago (20). However, that study included patients who might have taken taken antimicrobials which could have temporarily suppressed the growth of H. pylori. Consequently, the result may have been erroneously high. In contrast, another study during the same period in the same country found a prevalence rate of 61% (18). In this latter study the population was stratified. H. pylori prevalence was found 52% in the white population and 78% in the non-white population. The latter number is similar to that found by us, possibly reflecting socio-economic similarities between the two population groups ten years earlier. We believe that the relatively high frequency of the infection, although its accuracy is debatable, could justify empirical eradication treatment in some cases when detection tests are not available.
The finding that 14% of the duodenal ulcers studies were H. pylori negative and NSAIDs/ASA negative is lower than found in U.S., where this percentage has fluctuated between 27% and 40% (18, 20). However, it is 10 times higher than the percentage found in Japan (21) and more than 15 times higher than the one found in Spain where H. pylori infections prevalence is over 95% (17). In Italy, Meucci et al. (30) found that among 409 patients with peptic ulcers, 4.6% had neither H. pylori infections nor had consumed NSAIDs/ASA.
Our definition of positive infection combines test results. The tests applied were both highly sensitive and highly specific. They included the rapid urease test with a sensitivity of 88-95% and specificity of 95-100% (28); and histology with 99% sensitivity when three biopsies are taken (31). Although this combination should theoretically be more efficient for identifying infection than separate use of these tests, and therefore our probability of obtaining false negative results should be low, we do not rule out the possibility of some errors. One potential source of errors is in the questioning of patients. If a patient had forgotten to mention a history of using antisecretory drugs, antibiotics or NSAIDs / ASAs, our date would have been affected. The consumption of NSAIDs is considered to be the most common cause of peptic ulcers in H. pylori negative patients, with a prevalence of about 50% (32, 33). This contrasts to the 12% we found. One possible explanation for this notable difference could be that the use of NSAIDs may be greater than that reported by patients. Recognition of such drugs may be low in this environment, as has been found in other countries where the use of ASA, for example, has been demonstrated by urine and serum analyses (34). Although it is unlikely that these circumstances have significantly influenced the results, the results of this study suggest that in our environment there could be other causes of DUs which have hitherto been masked by the main causes, H. pylori and NSAIDs/ASA. From a practical standpoint it is necessary to start research into this possibility in patients with DUs. We need to look into the use of drugs other than NSAIDs/ASA which might injure the gastroduodenal mucosa. These include bisphosphonates, colchicine, and clopidogrel among others (29). In cases where consumption of all of the possible drugs which might cause DUs are ruled out, and if the patient does not improve with the antisecretory treatment, we believe it is justified to investigate causes of DUs traditionally considered rare. They include ZE (with serum gastrin), biopsies of the angles of the ulcer and adjacent mucosa to exclude malignancy, lymphoma, Crohns disease and Helicobacter heilmannii (29). It has been reported that the latter microorganism can produce DUs which can be treated with quadruple therapy (35). In our environment the prevalence of ZE in patients with peptic ulcers is unknown, although colloquially it is considered be a very rare entity, as in the USA. There it is thought less than 1% of DUs are caused by ZE (36).
In conclusion, H. pylori infection is the main cause of DUs in our population. However DUs which are H. pylori negative and NSAIDs/ASA negative represent a relatively large percentage of DUs (greater than NSAIDs alone). Therefore we believe that further studies are needed to determine the underlying etiologies.
REFERENCES
1. Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983; 1: 1273-5.
2. Kuipers EJ, Thies JC, Festen HPM. The prevalence of Helicobacter pylori infection in peptic ulcer disease. Aliment Pharmacol Ther 1995; 9: 59-70.
3. Liu Yi Ponsioen CIJ, Xiao S, Tytgat GNJ, Ten FJW. Geographic pathology of Helicobacter pylori gastritis. Helicobacter 2005; 10: 107-113.
4. Tatsuta M, Lishi H, Yokata Y. Effects of helicobacter pylori infection on healing and recurrence of gastric ulcers. Am J Gastroenterol 1995; 90: 406-10.
5. Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ, Jr, Saeed ZA, et al. Effect of treatment of Helicobacter infection on the long term recurrence of gastric or duodenal ulcer: A randomized, controlled study. Ann Intern Med 1992; 116: 705-8.
6. Tytgat GNJ. Treatments that impact favorably upon the eradication of Helicobacter pylori and ulcer recurrence. Aliment Pharmacol Ther 1994; 8: 359-68.
7. Logan RPH, Bardham, Celestin LR. Eradication of Helicobacter pylori and prevention of recurrence of duodenal ulcer: A randomized double blind, multicentric trial of omeprazole with or without clarithromycin. Alimen Pharmacol Ther 1995; 9: 417- 24.
8. Gisbert JP, Pajares JM. Systematic review and metanalysis: is 1 week proton pump inhibitor-based triple therapy sufficient to heal peptic ulcer? Aliment Pharmacol Ther 2005; 21: 795-804.
9. Dore MP, Graham DY. Pathogenesis of duodenal ulcer disease: the rest of story. Baillieres Best Pract Res Clin Gastroenterol 2000; 14: 97-107.
10. Chan FK. NSAID-induced peptic ulcers and Helicobacter pylori infection: implications for patient management. Drug Saf 2005; 28: 287-300.
11. Weisman SM, Graham DY. Evaluation of the benefits and risks of low-dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events. Arch Intern Med 2002; 162: 2197-2202.
12. Yeomans ND, Lanas AI, Talley NJ, Thomson AB, Daneshjoo R, Ercksson B, et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther 2005; 22: 795-801.
13. Papatheodoridis GP, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and Nonsteroidal anti-inflammatory drugs on peptic ulcer disease: A sytematic review. Clin Gastroenterol Hepatol 2006; 4: 130-42.
14. Marshall BJ, McGechie DB, Rogers PA, Glancy RJ. Pyloric Campylobacter infection and gastroduodenal disease . Ms J Aust 1985; 142: 439-44.
15. Graham DY, Klein P, Opekun AR, Malaty HM, Boutton TW. Effect of age on the frequency of active Campylobacter pylori infection diagnosed by the C13 urea breath test in normal subjects and patients with peptic ulcer disease. J Infect Dis 1988; 157: 777-80.
16. Cow DKL, Sung JY. Is the prevalence of idiopathic ulcers really on the increase? Nature Clin Pract Gastroenterol Hepatol 2007; 4: 176-77.
17. Gisbert JP, Blanco M, Mateos JM, Fernández-Salazar L, Fernández-Bermejo M, Cantero J, et al. H. pylori-negative duodenal ulcer prevalence and causes in 774 patients. Dig Dis Sci 1999; 44: 2295-302.
18. Jyotheeswaran, S, Shah, AN, Jin, HO, Potter GD, Ona FV, Chey Wy, et al. Prevalence of Helicobacter pylori in peptic ulcer patients in greater Rochester, NY: is empirical triple therapy justified? Am J Gastroenterol 1998; 93: 574-8.
19. Quan C, Tallley NJ. Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs. Am J Gastroenterol 2002; 97: 2950-61.
20. Ciociola AA, McSorley DJ, Turner K, Sykers D, Palmer J. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol 1999; 94: 1834-40.
21. Nishikawa K, Sugiyama T, Kato M, Ishizuka J, Komatzu Y, Kagaya H, et al. Non- Helicobacter pylori and non-NSAID peptic ulcer disease in the Japanese population. Eur J Gastroenterol Hepatol 2000; 12: 635-40.
22. Chey WD, Wong BCY. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102: 1808-1825.
23. Malfertheiner P, Megarud F, O`Morain C, Bazzoli F, El-Omar E, Graham DY, et al. Current concepts in the management of Helicobacter pylori infection: The Masstricht III consensus report. Gut 2007; 56: 772-81.
24. Sierra F, Gutiérrez O, Gómez MC, Camargo H, Serrano B, Otero W. Campylobacter pylori en úlcera duodenal, gastritis crónica y dispepsia no ulcerosa. Acta Med Col 1990; 15: 74-83.
25. El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: role of the Sydney system. Human pathol 1999; 30: 72-7.
26. Genta RM, Graham DY. Diagnosis and treatment of Helicobacter pylori infection. En: Gastritis, Graham DY, Genta RM, Dixon MF, (eds) Lippincott Wiliams & Wilkins Phil 1999. p. 189-201.
27. Bravo LE, Cortés A, Carrascal E, Jaramillo R, García LS, Bravo PA, et al. Helicobacter pylori: Patología y prevalencia en biopsias gástricas en Colombia. Clomb Med 2003; 34: 124-31.
28. Megraud F, Lerhours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clinical Microbiol Rev 2007; 20: 280-322.
29. McKoll KEL. How I manage H. pylori-negative, ASAID/Aspirin negative peptic ulcers. Am J Gastroenterol 2009; 104: 190-93.
30. Meucci G, Di Battista R, Abbiati C, Benassi R, Bierti L, Batoli A, et al. Prevalence and risk factors of Helicobacter pylori-negative peptic ulcer. J Clin Gastroenterol 2000; 31: 42-7.
31. Guarner J, Herrera-Goepfert R, Mohar A, Smith C, Scofield A, Halperin D, et al. Diagnostic of gastric biopsy specimens when screening for preneoplasic lesions. Human Pathol 2003; 34: 28-31.
32. Di Battista R, Bierti L, Benassi R, Bortoli A, Ferrara A, Redaelli D, et al. Prevalence and risk factors in Helicobacter pylori-negative peptic ulcer in an area of northern Italy. Gastroenterology 1997; 112: A 102.
33. McColl KEL, El-Nujumi AM, Chittajallu RS, Dahill SW, Dorrian CA, El-Omar. A study of the pathogenesis of Helicobacter pylori negative chronic duodenal ulceration. GUT 1993; 34: 762-8.
34. Hirschowitz BI. Intractable peptic ulceration due to aspirin abuse in patients who have not had gastric surgery. Gastroenterology 1997; 112: A962.
35. Goddard AF, Logan RPH, Atherton JC. Healing of duodenal ulcer after eradication of Helicobacter heilmanii. Lancet 1997; 249: 1815.
36. Isenberg JI, Walsh JH, Grossman MI. Zollinger-Ellison syndrome. Gastroenterology 1973; 65: 140-65.
1. Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983; 1: 1273-5. [ Links ]
2. Kuipers EJ, Thies JC, Festen HPM. The prevalence of Helicobacter pylori infection in peptic ulcer disease. Aliment Pharmacol Ther 1995; 9: 59-70. [ Links ]
3. Liu Yi Ponsioen CIJ, Xiao S, Tytgat GNJ, Ten FJW. Geographic pathology of Helicobacter pylori gastritis. Helicobacter 2005; 10: 107-113. [ Links ]
4. Tatsuta M, Lishi H, Yokata Y. Effects of helicobacter pylori infection on healing and recurrence of gastric ulcers. Am J Gastroenterol 1995; 90: 406-10. [ Links ]
5. Graham DY, Lew GM, Klein PD, Evans DG, Evans DJ, Jr, Saeed ZA, et al. Effect of treatment of Helicobacter infection on the long term recurrence of gastric or duodenal ulcer: A randomized, controlled study. Ann Intern Med 1992; 116: 705-8. [ Links ]
6. Tytgat GNJ. Treatments that impact favorably upon the eradication of Helicobacter pylori and ulcer recurrence. Aliment Pharmacol Ther 1994; 8: 359-68. [ Links ]
7. Logan RPH, Bardham, Celestin LR. Eradication of Helicobacter pylori and prevention of recurrence of duodenal ulcer: A randomized double blind, multicentric trial of omeprazole with or without clarithromycin. Alimen Pharmacol Ther 1995; 9: 417- 24. [ Links ]
8. Gisbert JP, Pajares JM. Systematic review and metanalysis: is 1 week proton pump inhibitor-based triple therapy sufficient to heal peptic ulcer? Aliment Pharmacol Ther 2005; 21: 795-804. [ Links ]
9. Dore MP, Graham DY. Pathogenesis of duodenal ulcer disease: the rest of story. Baillieres Best Pract Res Clin Gastroenterol 2000; 14: 97-107. [ Links ]
10. Chan FK. NSAID-induced peptic ulcers and Helicobacter pylori infection: implications for patient management. Drug Saf 2005; 28: 287-300. [ Links ]
11. Weisman SM, Graham DY. Evaluation of the benefits and risks of low-dose aspirin in the secondary prevention of cardiovascular and cerebrovascular events. Arch Intern Med 2002; 162: 2197-2202. [ Links ]
12. Yeomans ND, Lanas AI, Talley NJ, Thomson AB, Daneshjoo R, Ercksson B, et al. Prevalence and incidence of gastroduodenal ulcers during treatment with vascular protective doses of aspirin. Aliment Pharmacol Ther 2005; 22: 795-801. [ Links ]
13. Papatheodoridis GP, Sougioultzis S, Archimandritis AJ. Effects of Helicobacter pylori and Nonsteroidal anti-inflammatory drugs on peptic ulcer disease: A sytematic review. Clin Gastroenterol Hepatol 2006; 4: 130-42. [ Links ]
14. Marshall BJ, McGechie DB, Rogers PA, Glancy RJ. Pyloric Campylobacter infection and gastroduodenal disease . Ms J Aust 1985; 142: 439-44. [ Links ]
15. Graham DY, Klein P, Opekun AR, Malaty HM, Boutton TW. Effect of age on the frequency of active Campylobacter pylori infection diagnosed by the C13 urea breath test in normal subjects and patients with peptic ulcer disease. J Infect Dis 1988; 157: 777-80. [ Links ]
16. Cow DKL, Sung JY. Is the prevalence of idiopathic ulcers really on the increase? Nature Clin Pract Gastroenterol Hepatol 2007; 4: 176-77. [ Links ]
17. Gisbert JP, Blanco M, Mateos JM, Fernández-Salazar L, Fernández-Bermejo M, Cantero J, et al. H. pylori-negative duodenal ulcer prevalence and causes in 774 patients. Dig Dis Sci 1999; 44: 2295-302. [ Links ]
18. Jyotheeswaran, S, Shah, AN, Jin, HO, Potter GD, Ona FV, Chey Wy, et al. Prevalence of Helicobacter pylori in peptic ulcer patients in greater Rochester, NY: is empirical triple therapy justified? Am J Gastroenterol 1998; 93: 574-8. [ Links ]
19. Quan C, Tallley NJ. Management of peptic ulcer disease not related to Helicobacter pylori or NSAIDs. Am J Gastroenterol 2002; 97: 2950-61. [ Links ]
20. Ciociola AA, McSorley DJ, Turner K, Sykers D, Palmer J. Helicobacter pylori infection rates in duodenal ulcer patients in the United States may be lower than previously estimated. Am J Gastroenterol 1999; 94: 1834-40. [ Links ]
21. Nishikawa K, Sugiyama T, Kato M, Ishizuka J, Komatzu Y, Kagaya H, et al. Non- Helicobacter pylori and non-NSAID peptic ulcer disease in the Japanese population. Eur J Gastroenterol Hepatol 2000; 12: 635-40. [ Links ]
22. Chey WD, Wong BCY. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007; 102: 1808-1825. [ Links ]
23. Malfertheiner P, Megarud F, O`Morain C, Bazzoli F, El-Omar E, Graham DY, et al. Current concepts in the management of Helicobacter pylori infection: The Masstricht III consensus report. Gut 2007; 56: 772-81. [ Links ]
24. Sierra F, Gutiérrez O, Gómez MC, Camargo H, Serrano B, Otero W. Campylobacter pylori en úlcera duodenal, gastritis crónica y dispepsia no ulcerosa. Acta Med Col 1990; 15: 74-83. [ Links ]
25. El-Zimaity HM, Graham DY. Evaluation of gastric mucosal biopsy site and number for identification of Helicobacter pylori or intestinal metaplasia: role of the Sydney system. Human pathol 1999; 30: 72-7. [ Links ]
26. Genta RM, Graham DY. Diagnosis and treatment of Helicobacter pylori infection. En: Gastritis, Graham DY, Genta RM, Dixon MF, (eds) Lippincott Wiliams & Wilkins Phil 1999. p. 189-201. [ Links ]
27. Bravo LE, Cortés A, Carrascal E, Jaramillo R, García LS, Bravo PA, et al. Helicobacter pylori: Patología y prevalencia en biopsias gástricas en Colombia. Clomb Med 2003; 34: 124-31. [ Links ]
28. Megraud F, Lerhours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clinical Microbiol Rev 2007; 20: 280-322. [ Links ]
29. McKoll KEL. How I manage H. pylori-negative, ASAID/Aspirin negative peptic ulcers. Am J Gastroenterol 2009; 104: 190-93. [ Links ]
30. Meucci G, Di Battista R, Abbiati C, Benassi R, Bierti L, Batoli A, et al. Prevalence and risk factors of Helicobacter pylori-negative peptic ulcer. J Clin Gastroenterol 2000; 31: 42-7. [ Links ]
31. Guarner J, Herrera-Goepfert R, Mohar A, Smith C, Scofield A, Halperin D, et al. Diagnostic of gastric biopsy specimens when screening for preneoplasic lesions. Human Pathol 2003; 34: 28-31. [ Links ]
32. Di Battista R, Bierti L, Benassi R, Bortoli A, Ferrara A, Redaelli D, et al. Prevalence and risk factors in Helicobacter pylori-negative peptic ulcer in an area of northern Italy. Gastroenterology 1997; 112: A 102. [ Links ]
33. McColl KEL, El-Nujumi AM, Chittajallu RS, Dahill SW, Dorrian CA, El-Omar. A study of the pathogenesis of Helicobacter pylori negative chronic duodenal ulceration. GUT 1993; 34: 762-8. [ Links ]
34. Hirschowitz BI. Intractable peptic ulceration due to aspirin abuse in patients who have not had gastric surgery. Gastroenterology 1997; 112: A962. [ Links ]
35. Goddard AF, Logan RPH, Atherton JC. Healing of duodenal ulcer after eradication of Helicobacter heilmanii. Lancet 1997; 249: 1815. [ Links ]
36. Isenberg JI, Walsh JH, Grossman MI. Zollinger-Ellison syndrome. Gastroenterology 1973; 65: 140-65. [ Links ]











 text in
text in