Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957versión On-line ISSN 2500-7440
Rev Col Gastroenterol v.24 n.3 Bogotá jul./sep. 2009
Prevalence of different types of colitis among the elderly
William Otero Regino MD.(1), Angélica González MD.(2), Martín Gómez Zuleta MD.(3)
(1) Professor of Medicine, Gastroenterology Unit, Universidad Nacional de Colombia, Gastroenterologist at the Clínica Fundadores, Clínica Carlos Lleras Restrepo, Hospital Fundación San Carlos. Bogotá, Colombia.
(2) Internal Medicine Clínica Fundadores. Bogotá, Colombia.
(3) Professor of Medicine, Gastroenterology Unit, Universidad Nacional de Colombia, Gastroenterologist, Hospital El Tunal. Bogotá, Colombia.
Received: 16-06-09 Accepted: 18-08-09
Summary
Colitis is an inflammatory process of the colon with diverse etiologies, although clinical conditions and endoscopic examinations look similar. The attending physician should determine if the condition is acute (less than four weeks duration), or chronic (more than four weeks duration). The most frequent cause of acute bloody diarrhea in adults is infection, although in adults over 65 years of age causes include ischemic colitis and, upon rare occasions, inflammatory intestinal disease. Information is lacking on colitis among the population of Colombia. The objectives of this study are to determine the prevalence, causes and locations within the colon of colitis in elderly patients (over the age of 65).
Materials and methods: An observational study of elderly patients who were through diagnosed with colitis colonoscopy. Diagnoses were confirmed histologically and clinically. Study was conducted between March 2002 and December 2006.
Results: 2244 colonoscopy results were checked. 321 elderly patients were identified among these cases. 49 of them (15%) had colitis diagnosed through endoscopy, histology and clinical profiles. Main causes were ischemia 30%, infections 20% (40% Clostridium difficile - C. difficile), ulcerative colitis 18%, radiotherapy after effects 18% and others 14% (NSAIDs, neoplasia, etc.). In 23% of the cases the colitis was on the right side.
Conclusions: The prevalence of colitis diagnosed in elderly patients through colonoscopy was 15%. The main causes were ischemia, infections (40% C. difficile), and ulcerative and post radiotherapy colitis. A complete colonoscopy is indicated in all elderly patients with acute colitis.
Key words
Colitis, elderly, ischemic colitis, ulcerative colitis, colonoscopy
INTRODUCTION
Colitis is a colonic inflammatory process which includes a wide spectrum of pathologies whose physio-pathologic mechanisms differ, but often have similar clinical, endoscopic, and histological manifestations (1). The characteristic symptom is an inflammatory type diarrhea characterized by the presence of blood and mucus in feces, although frequently there is only rectal bleeding (1, 2). With a patient who has bloody diarrhea the diagnosis is colitis until the contrary has been proven (2). Among the etiologic possibilities are parasites, bacteria, tuberculosis (TBC), virus, medications, ischemia, radiation therapy, and idiopathic intestinal inflammatory disease (1, 3). The profile is considered acute if symptoms have existed for less than four weeks and chronic if symptoms have existed for more than four weeks (2). The most frequent cause of recently started bloody diarrhea in adult patients is colitis with an infectious origin (2, 3). In elderly patients the main causes are infectious agents, ischemic colitis and less frequently intestinal inflammatory disease (1). Other possible etiologies are medications, especially non-steroid anti-inflammatory medicines, tumors, and stercoraceous ulcers (1, 3). Much rarer causes include allergic colitis and amyloidosis. Taking into account that the colon has a limited number of responses towards different noxae, it is not surprising that endoscopy, imaging, and pathologic findings overlap for different types of colitis. Therefore, colonoscopy alone has very limited value for differentiating different types of colitis (4). Additionally the patient's clinical profile, pathology, and the course of the disease must be taken into account to establish a definite diagnosis (1-4). The same is true for histopathological alterations when considered in isolation (1, 3, 5).
In the adult population, the epidemiology of colitis has been well characterized. However, in the elderly population the epidemiology is not yet clear. Studies in the international community report that infectious colitis and ischemic colitis are the main causes (1). Nonetheless, to date, there are no known population studies about the prevalence of colitis among the elderly in Colombia, nor do studies exist identifying precipitating or aggravating risk factors. Taking these factors into account we decided to carry out the present study in a general clinic in Bogotá, Colombia. This study has the following objectives: to define the prevalence of colitis in elderly patients who undergo a colonoscopy, to identify the frequencies of different causes of colitis among the elderly, to compare the prevalence of different causes of colitis between elderly men and women, to determine the most frequently affected location in the colon, and to establish the presence and type of risk factors for colitis in elderly adults.
MATERIALS AND METHODS
This study is a cross sectional observational study of elderly patients identified through the colonoscopy data base of the Gastroenterology Unit at the Clinica Fundadores in Bogotá, Colombia from March 2002 to December 2006. Patients had been diagnosed with colitis through colonoscopies. Diagnoses had been confirmed histologically, and clinical profiles were checked. The research protocol was approved by the Ethics Committee of the institution where the study was carried out. Because this research was no risk, signed patient authorizations were not requested (as per Resolution 8430, 1993 of the Colombian Ministry of Health).
Inclusion criteria
The study's inclusion criteria included the following: patients older than 65 years of age, immunocompetent with endoscopic diagnose of colitis, diagnosis confirmed by histology and check of clinical profile. Although endoscopic manifestations of inflammation of the colon mucosa for colitis are not specific, we considered that there was colitis when the mucosa presented erythma or edema (loss of vascular pattern), erosions, alterations in the light reflex, friability with bleeding with or without ulcers, and exuded or fibrin membranes. In addition an important factor for any of the above was appearance in a diffuse homogeneous way, in patches, in which unaffected areas were within affected areas in any location and independently from the extension of the affected areas.
To diagnose the most frequent types of colitis, the following endoscopic and/or colonoscopic and histological characteristics were taken into consideration (6):
Idiopathic ulcerative colitis (proctitis, proctosigmoiditis, extensive colitis or pan colitis):
Colonoscopic indications: edema, diffuse erythma, granularity, lost of vascular pattern, friability with spontaneous bleeding or at contact with colonoscope, exuded or confluences, superficial ulcerations.
Histological indications from studies of biopsies taken from unaffected and affected areas: distortion of the crypt architecture (atrophy, distorted or ramified glands), basal plasmocitosis, lymphoid hyperplasia, mucus depletion, microabcesses, inflammation of the mucosa and lamina propia, inflammation of the sub-mucosa (rare), acute and chronic infiltrated inflammation.
Crohn's Disease:
Endoscopic indications: aphthoid ulcers, lineal or serpiginous, superficial or profound, interposed within unaffected mucosa; stony appearance with intersections of lineal or transverse ulcers, and swollen thickened mucus appearing within the ulcers.
Histological indications: focal inflammation (polymorphonuclear or chronic inflammatory cells), segmented inflammation (with the possibility of trans mural or sub mucosal inflammation), abscesses in crypts, and epitheloid granuloma (3, 6, 7).
Radiation therapy after effects:
Endoscopic indications: paleness, friability, multiple telangiectasias (lineal or serpiginous). These alterations may be continuous or patchy, and may or may not be accompanied by friability with bleeding (8).
Histologic indications: superficial necrosis, neovascularization, hyalinized capillary walls, indications of abdominopelvic radiation therapy.
Pseudomembranous colitis caused by Clostridium difficile:
Colonoscopic indications: erythma, edema, loss of vascular pattern, friability with easy bleeding, high yellowish plaques from 2 to 10 mm in diameter which may coalesce, easily detachable adherents (9, 10).
Histological indications: patchy necrosis lamina propia, crypt dilatation, typical "volcano" lesion in which inflamed cells, fibrin and mucus emerge from micro ulcerations towards the epithelium (3, 6).
Risk factors: previous use of antibiotics, subjacent severe disease, previous hospitalization, diarrhea during hospitalization, tube feeding, hospitalization in ICU (10).
Ischemic Colitis:
Colonoscopic indications: pale mucosa with petechial bleeding, hemorrhagic bluish nodules which represent sub mucosa hemorrhaging (equivalent to the "thumbprint" identified by radiology), cyanotic mucosa and hemorrhaging ulcerations (severe cases), there may be occasional yellowish plaques that resemble those observed in C. difficile infections (10, 11). The lesions have segmental distribution with abrupt transitions between affected and unaffected areas. There are lineal ulcers following the longitudinal axel of the colon (12). Lesions are located primarily in vulnerable areas of the colon (splenic flexure, rectosigmoid junction, hepatic angle of transverse colon, and (rarely) rectum) (13).
Histological indications: hemorrhaging, crypt distortion, capillary micro thrombosis, extravasation of red blood cells, plasma proteins in the lamina propia, initial stages of scarce cellularity, tissue granulation with cryptic abscesses, "ghost" cells. In chronic phases there may be mucosal atrophy and tissue granulation (3).
Infectious or self-limiting Colitis:
Colonoscopic indications: erythma, edema, loss of vascular pattern, friability, mucosa compromised either continuously or in patches.
Histological indications: preservation of glandular architecture, erosion, edema, neutrophils in the lamina propia, little or no increase in mononuclear leukocytes, on rare occasion basal plasmacytosis in the CU or EC (14), decrease of mucus layer (3, 6).
After reviewing results of colonoscopies, clinical histories and pathological findings were reviewed. All patients in the study were given a form to fill out to provide basic information including age at onset, gender, etiology, location of colitis within digestive tract and risk factors.
Exclusion criteria
Colonoscopic diagnosis of colitis unsupported by biopsy results. No access to clinical history.
Statistical analysis
Data was entered into Excel 2003 and analyzed with Strata 9.0 software. Descriptive statistics were used in this study. Nominal and ordinal numbers, distribution medians and averages were used for categorical variables. Numerical variables are expressed with central tendency measures and standard deviations. Statistical evidence is analyzed at a 5% degree of significance (p<0.05). Continuous variables are reported as frequencies and averages determined through the use of the Student T test.
RESULTS
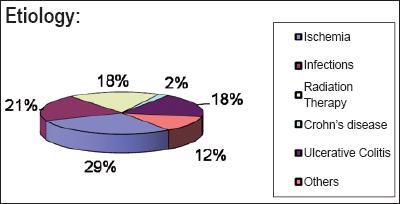
Results of 2244 colonoscopies performed during the period of the study were reviewed. 321 patients over the age of 65 were identified, 49 (15.26%) of whom had been diagnosed with colitis, and that diagnosis had been confirmed histologically. 20 (41%) of these patients were men, 29 (59%) were women. Total colonoscopies were done without sedation for all patients. There were no complications in any of the procedures. Average age of the male patients was 72 +/- 5 years. Average age of the female patients was73 +/- 7 years. The different types of colitis identified are shown in figure 1. In descending order the principal causes were the following: ischemic colitis: 15 patients (30%), probability of infectious colitis: 10 patients (20%), primary ulcerative colitis and colitis resulting from radiation therapy: 9 patients (18%) each, other causes: 5 patients (12%). Among the possible "other causes" are NSAIDs, colitis associated with neoplasia, Crohn's disease (encountered in 1 patient) and others without clear etiologies.
Figure 1. Etiology of colitis.
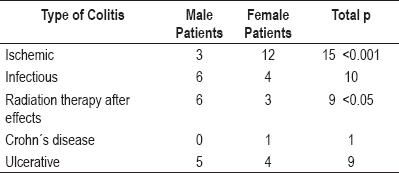
Presentation of ischemic colitis shows no differences between the two different age groups studied. Distribution of different types of colitis by gender is shown in table 1. There were two significant differences. Ischemic colitis was four times more frequent in women (11 women and three men, p<0.001) while colitis due to radiation therapy was twice as frequent among men (6 men and 3 women).
Table 1. Presentation of colitis by gender.
Location within digestive tract. The rectum was the most frequent location with 29 patients (32%). The sigmoid colon followed with 23 patients (25%). The descending colon was next with 19 patients (20%). The transverse colon followed with 10 patients (6%). The ascending colon followed with 6%. 6% also had pancolitis. It should be noted that one patient may have colitis in more than one location. Locations of colitis within the digestive tract are shown in figure 2.
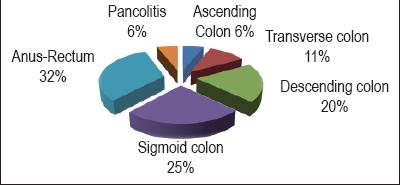
Figure 2. Locations of colitis.
Risk factors
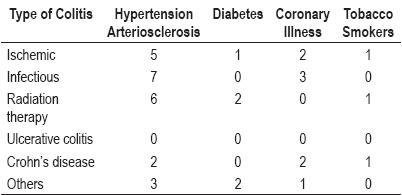
We correlated the different causes of colitis in this population with the risk factors classically considered to be associated with different types of colitis (with the exception of prior use of antibiotics in patients with Pseudomembranous colitis). There were no statistically significant differences among patients with previous coronary illness, hypertension or arteriosclerosis with ischemic colitis (table 2). We could not correlate prior smoking with different types of colitis, although this information was not clearly reported in the patients' clinical histories.
Table 2. Risk Factors.
Among the 10 patients with probable infectious colitis, four (40%) presented pseudomembranous colitis. All of these patients had experienced prolonged hospital stays and had been previously treated with wide-spectrum antibiotics.
DISCUSSION
In this study the presence of endoscopically and histologically diagnosed colitis was 15% of the patients in the targeted age group (over 65 years of age) who had undergone total colonoscopies. When a patient has bloody diarrhea the most likely origin of the bleeding is the colon. For this reason a total colonoscopy should be performed. Although the objectives of this study did not include determining the effectiveness of colonoscopy in patients with a probability of acute colitis, it is worth noting that in our protocol for handling colitis in the emergency room total colonoscopies are included, implying that the procedure is requested for patients with this clinical picture. However the diagnostic approach can vary according to the available resources and the training of the attending physician. Ideally a quick and efficient patient interview to determine if the colitis is acute, chronic or recurrent, which risk factors exist (use of antibiotics, radiation therapy, family and personal history of EII) and which types of colitis they are associated with). Then a physical examination should be conducted to identify relevant symptoms such as abdominal pain and bleeding rectum. Following the examination routine laboratory tests of blood and fecal material should be conducted to look for inflammation. If more specific relevant tests are available, such as those for C. difficile, they should also be conducted. (2). In our view the next step should be a total colonoscopy to contribute to determination of the cause. All diagnostic tools should point to a similar result. By combining colonoscopic findings with other diagnostic tools and the clinical history the present study has been able to determine the most frequent causes of colitis in approximately 80% of the patients studied. 12% of the patients had "other" causes and 10 patients probably had infectious colitis, although we can reasonably assume that four of these were the result of C. difficile and that 40% of the probably infectious colitis cases were pseudomembranous colitis. In general terms in this investigation the most frequent types of colitis were ischemic colitis 30%, probably infectious colitis 20%, ulcerative colitis and colitis following radiation therapy 18% each.
This study is in agreement with other studies that the most frequent cause of colitis in patients over the age of 65 is ischemia (15). This type of colitis was encountered four times as frequently in women patients as in male patients, an observation which is in agreement with population studies of colitis (16) and with other studies which have demonstrated that in the absence of irritable bowel syndrome women are twice as likely as men to develop ischemic colitis (9 per 100,000 women versus 5 per 100,000 men) (17).
The fact that our study did not encounter some classical risk factors, especially arteriosclerosis (18-20), in our patient does not indicate the absence of these factors. More probably they did exist but had not been diagnosed. Another possibility is that the final diagnoses of these patients' conditions might not have been ischemic colitis. Nevertheless this is unlikely because of the identification of the classical symptoms and signs of this condition, e.g. sudden slight or moderate abdominal pain followed by urgent desire to defecate, bright red or maroon blood mixed with fecal material but without hemodynamic compromise or necessity of transfusion (19). Moreover both the histological and colonoscopic indications were compatible with this diagnosis. Colonoscopy is the examination of choice for this diagnosis (18-20). It is considered to be safe (20), as long as the endoscope is not advanced past locations in which alterations are encountered which indicate necrosis or gangrene (20).
The second most frequent type of colitis was ulcerative colitis. 10 cases were found among patients between the ages of 65 and 79 years, a surprising result given that the statistical peak for this type of colitis occurs between in the 15 to 25 year age group (21). However, one study has shown a second peak between the ages of 55 and 65 years (22). Moreover, two recent studies found that 21-23% of ulcerative colitis cases occur after the age of 50 while 5% occur after the age of (23-24). Another study has found that 15% of ulcerative colitis cases occur after the age of 60 (25).
Of the nine cases of probably infectious colitis encountered, four were established with reasonable certainty to be the result of C. difficile. Because the the C. difficile toxin test (10) was not available for these diagnoses the endoscopic evidence combined with the response to treatment with metronidazol or vancomicina formed the bases for these diagnoses. Other infectious agents which can cause colitis, and to which older patients are particularly vulnerable, include Shiguella, Salmonella, Campylobacter jejuni and less frequently Eschericia coli O157:H7 (26). We were unable to make our diagnosis more precise because of the absence of the required diagnostic tools.
In 23% of the patients studied colitis was located on the right side implying that a total colonoscopy was not required. However this would have left the following segments unexamined contrary to the recommendations of experts who advise complete colonoscopies in cases where the patient is not critically ill (1,2). Prior preparation for the total colonoscopy is an essential part of a high quality procedure (27).
In patients over the age of 65, polyethylene glycol (PEG) solutions are preferable to phophate solutions because they are safer and more easily tolerated (28). Phophate solutions are not advisable for patients over the age of 65 because of the high risks of complications including electrolyte disturbances (hypocalcemia, hypernatremia, hyperphosphatemia). Another possible complication is acute phosphate nephropathy in patients with compromised renal function or who are receiving medications which influence the balance of electrolytes which are inhibitors of the enzyme which converts angiotensinase, diuretics, or blockers of angiotensinase receptors (29-31). Nevertheless, this product can be considered safe for sleected patients over the age of 65 (32,33). A disadvantage of phosphate solutions is that they produce lesions such as erosion, blisters, and even ulcers in about 3% of patients (34). These side effects can be confused with structural macroscopic and microscopic diseases intrinsic to the colon (28) such as active inflammation, erosions, edema of the lamina propia, hyperemia of the mucosa, focal hemorraging, lymphoid nodules, and ulcers (28).
In our patients colonoscopies were complete, performed without sedation, and with minimal patient discomfort. For this reason we do not consider that routine administration of sedatives is necessary, as has been suggested for screening colonoscopies (35). We also consider that sedation does not make the colonscopy technically easier (manuscript under preparation). Even when the patient makes known a preference for sedation, the procedure should be undertaken without sedation.
The greater frequency of colitis due to the effects of radiation therapy among men is due to the fact that among the patients studied there was a greater number of cases of prostate cancer than there was of gynecological cancers.
In conclusion, the prevalence of colitis found among the patients in our study, people over the age of 65 who had undergone colonoscopies, was 15%. The principal causes were ischemic colits, ulcerative colitis, and probably infectious colitis. Complete colonoscopies should be performed on patients in this age group with symptoms of colitis. Taking into account the limitations of the present study, especially the incompletenes of the information available, we believe that prospective studies need to be conducted.
Disclosure
None
The costs of this study were born entirely by the research team.
Acknowledgements
We would like to thank Doctor Fabiola Quintero, a pathologist at the Clínica Fundadores de Bogotá for her diligence and dedication in the study of the specimens collected for this study. We thank the gastroenterology assistant, Miss Liliana Oino, for her care and punctuality in deliverying the results of pathology. We thank Doctor Héctor Sandoval for reviewing the manuscript and making critical suggestions.
REFERENCES
1. Brandt LJ. Bloody Diarrhea in an elderly patient. Gastroenterology 2005; 128: 157-163.
2. Bernstein CN. The role of an endoscopy in inflammatory bowel disease. Clinical update 2008; 15: 1-4.
3. Abreu MT, Harpaz N. Diagnosis of colitis: making the initial diagnosis. Clin Gastroenterol Hepatol 2007; 5: 295-301.
4. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc 2006; 63: 558-65.
5. Warren BF, Shepherd NA. What are the controversies in histopathological findings? In Jewell D (edit), Challenges in inflammatory bowel disease, Blackwell Publishing Ltd 2006. p. 67-84.
6. Surawicz CM. Diagnosing colitis. Annual Postgraduate Course, ACG 2002: 1B-69-1B85.
7. Estenson WF, Tremaine WJ, Cohen RD. Inflammatory Bowel disease. In Yamada T (Edit). Atlas of gastroenterology 4th edit. Blackwell Publ. 2009. p. 389-408.
8. Habu Y, Tahashi Y, Kiyota K, Matsumura K, Hirota M, Inokuchi H, et al. Reevaluation of clinical features of ischemic colitis. Analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31: 881-886.
9. Kelly CP, LaMont JT. Clostridium difficile infection. Ann Rev Med 1998; 49: 375-90.
10. Sánchez. AL, Otero W, Caminos JE Infección por Clostridium difficile. Rev Col Gastroenterol 2008; 23: 142-59.
11. Dignan CR, Greenson JK. Can ischemic colitis be differentiated from C. difficile colitis in biopsy specimens. Am J Surg pathol 1997; 21: 706-10.
12. Mitsudo F, Brandt LJ, Pathology of intestinal ischemia. Surg Clin North Am 1992; 72: 43-55.
13. Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis Clev Clin J Med 2003; 70: 820-34.
14. Surawicz CM, Haggitt RC, Husseman M, McFarland LV. Mucosal biopsy diagnosis of colitis: acute self-limited colitis and idiopathic inflammatory bowel disease. Gastroenterology 1994; 107: 755-62.
15. Brandt LJ, Mitsudo S. Clinical characteristics and natural history colitis in the elderly. Am J Gastroenterol 1982; 77: 382-6.
16. Higgins PDR, Davis KJ, Laine L. Systematic review: the epidemiology of ischemic colitis. Aliment Pharmacol Ther 2004; 19: 729-38.
17. Cole JA, Cook SF, Miller DP. The risk of colonic ischemia among patients with irritable bowel syndrome. Digestive Disease Week 2002: A91 (Abstract 726).
18. Tohda G, Higashi S, Sumiyoshi KI, Sakamuto H, Kato C, Kane T. Evaluation of clinical features of ischemic colitis comparison between Young and elderly. Dig Endosc 2005; 17: 123-30.
19. Green BT, Tendler DA. Ischemic colitis: A clinical review. South Med J 2005; 98: 217-22, Hwang RF, Schwartz RV. Ischemic colitis: A brief review. Curr Surg 2001; 58: 192-4.
20. Yang XS, Lu YM, Fu CF, Wang CW. Clinical and endoscopic features of ischemic colitis. Chin J Dig Dis 2003; 4: 64-8.
21. Stenson F, Hanauer SB, Cohen RD.Inflammatory Bowel Disease. En Tadataka Y (edi). Textbook of Gastroenterology 5th ed, Blackwell Publising 2009. p. 1386-1472.
22. Calkins BM, Lilienfeld AM, Garland CF, Mendeloff AI. Trends in the incidence rates of ulcerative colitis and Crohn's disease. Dig Dis Sci 1984; 29: 913-19.
23. Loftus EV, Jr, Silverstein MD, Sandborn WJ, Harmsen WS, Tremaine WJ, Zinsmeister AR. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence and survival. Gut 2000; 46: 336-43.
24. Riegler G, Tartaglione MT, Carratu R, D´Inca R, Valpiani D, Russo MI, et al. Age-related clinical severity at diagnosis in 1705 patients with ulcerative colitis. A study by GISC (Italian Colon-Rectum Study Group). Dig Dis Sci 2000; 45: 462-5.
25. Softley A, Myren J, Clamp SE, Bouchier IA Watkinson G, de Dombal FT. Inflammatory bowel disease in the elderly patient. Scand J Gastroenterol (Suppl) 1988; 144: 27-30.
26. Trinh C, Prabhakar K. Diarrheal Disease in the Elderly. Clin Geriatr Med 2007; 23: 833-56.
27. Parente F, Marino B, Crosta C. Bowel preparation before colonoscopy in the era of mass screening for colo-rectal cancer: a practical approach. Dig Liv Dis 2009; 41: 87-95.
28. Ramin YI, Flachuck M. Bowel preparation for colonoscopy. Up To Date 2009.
29. Khurana A, McLean L, Atkinson S, Foulk CJ. The effect of oral sodium phosphate drug produces on renal function in adults undergoing bowel endoscopy. Arch Intern Med 2008; 168: 593-7.
30. Maa KK, Ng CSH, Mui LM. Severe hyperphosphate and hypercalcemia following sodium phosphate bowel preparation: a forgotten menace. Endoscopy 2003; 35: 717-9.
31. Maekowitz GS, Stokes MB, Radhakrishnan J, D'Agati VD. Acute Phosphate nephropathy following oral sodium phosphate bowel purgative: a underrecognized cause of chronic renal failure. Am Soc Nerphrol 2005; 16: 3389-96.
32. Seinela L, Pehkonen E, Laasanen T, Ahveinanen J. Bowel preparation for colonoscopy en very old patients: a randomized prospective trial comparing oral sodium phosphate and politilen glycol electrolyte lavage solution. Scand J Gastroenterol 2003; 38: 216-20.
33. Barclay RL, Depew WT, Vanner SJ. Carbohydrate-electrolyte rehydration protects against intravascular volume contraction during colonic cleansing with orally administered sodium phosphate. Gastrointest Endosc 2002; 56: 633-8.
34. Rejchrt S, Bures J, Siroky, Kopakova M, Slezak L, Langr F. A prospective, observational study of colonic mucosal abnormalities associated with orally administered sodium phosphate for colon cleansing before colonoscopy. Gastrointest Endosc 2004; 59: 651-654.
35. Evensen ET, Hoff GS, Sauar J, Vatn MH. Patient tolerance of colonosopy without sedation during screening examination for colorectal polyps. Gastrointest Endosc 2000; 52: 606-10.
1. Brandt LJ. Bloody Diarrhea in an elderly patient. Gastroenterology 2005; 128: 157-163. [ Links ]
2. Bernstein CN. The role of an endoscopy in inflammatory bowel disease. Clinical update 2008; 15: 1-4. [ Links ]
3. Abreu MT, Harpaz N. Diagnosis of colitis: making the initial diagnosis. Clin Gastroenterol Hepatol 2007; 5: 295-301. [ Links ]
4. ASGE guideline: endoscopy in the diagnosis and treatment of inflammatory bowel disease. Gastrointest Endosc 2006; 63: 558-65. [ Links ]
5. Warren BF, Shepherd NA. What are the controversies in histopathological findings? In Jewell D (edit), Challenges in inflammatory bowel disease, Blackwell Publishing Ltd 2006. p. 67-84. [ Links ]
6. Surawicz CM. Diagnosing colitis. Annual Postgraduate Course, ACG 2002: 1B-69-1B85. [ Links ]
7. Estenson WF, Tremaine WJ, Cohen RD. Inflammatory Bowel disease. In Yamada T (Edit). Atlas of gastroenterology 4th edit. Blackwell Publ. 2009. p. 389-408. [ Links ]
8. Habu Y, Tahashi Y, Kiyota K, Matsumura K, Hirota M, Inokuchi H, et al. Reevaluation of clinical features of ischemic colitis. Analysis of 68 consecutive cases diagnosed by early colonoscopy. Scand J Gastroenterol 1996; 31: 881-886. [ Links ]
9. Kelly CP, LaMont JT. Clostridium difficile infection. Ann Rev Med 1998; 49: 375-90. [ Links ]
10. Sánchez. AL, Otero W, Caminos JE Infección por Clostridium difficile. Rev Col Gastroenterol 2008; 23: 142-59. [ Links ]
11. Dignan CR, Greenson JK. Can ischemic colitis be differentiated from C. difficile colitis in biopsy specimens. Am J Surg pathol 1997; 21: 706-10. [ Links ]
12. Mitsudo F, Brandt LJ, Pathology of intestinal ischemia. Surg Clin North Am 1992; 72: 43-55. [ Links ]
13. Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis Clev Clin J Med 2003; 70: 820-34. [ Links ]
14. Surawicz CM, Haggitt RC, Husseman M, McFarland LV. Mucosal biopsy diagnosis of colitis: acute self-limited colitis and idiopathic inflammatory bowel disease. Gastroenterology 1994; 107: 755-62. [ Links ]
15. Brandt LJ, Mitsudo S. Clinical characteristics and natural history colitis in the elderly. Am J Gastroenterol 1982; 77: 382-6. [ Links ]
16. Higgins PDR, Davis KJ, Laine L. Systematic review: the epidemiology of ischemic colitis. Aliment Pharmacol Ther 2004; 19: 729-38. [ Links ]
17. Cole JA, Cook SF, Miller DP. The risk of colonic ischemia among patients with irritable bowel syndrome. Digestive Disease Week 2002: A91 (Abstract 726). [ Links ]
18. Tohda G, Higashi S, Sumiyoshi KI, Sakamuto H, Kato C, Kane T. Evaluation of clinical features of ischemic colitis comparison between Young and elderly. Dig Endosc 2005; 17: 123-30. [ Links ]
19. Green BT, Tendler DA. Ischemic colitis: A clinical review. South Med J 2005; 98: 217-22, Hwang RF, Schwartz RV. Ischemic colitis: A brief review. Curr Surg 2001; 58: 192-4. [ Links ]
20. Yang XS, Lu YM, Fu CF, Wang CW. Clinical and endoscopic features of ischemic colitis. Chin J Dig Dis 2003; 4: 64-8. [ Links ]
21. Stenson F, Hanauer SB, Cohen RD.Inflammatory Bowel Disease. En Tadataka Y (edi). Textbook of Gastroenterology 5th ed, Blackwell Publising 2009. p. 1386-1472. [ Links ]
22. Calkins BM, Lilienfeld AM, Garland CF, Mendeloff AI. Trends in the incidence rates of ulcerative colitis and Crohns disease. Dig Dis Sci 1984; 29: 913-19. [ Links ]
23. Loftus EV, Jr, Silverstein MD, Sandborn WJ, Harmsen WS, Tremaine WJ, Zinsmeister AR. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence and survival. Gut 2000; 46: 336-43. [ Links ]
24. Riegler G, Tartaglione MT, Carratu R, D´Inca R, Valpiani D, Russo MI, et al. Age-related clinical severity at diagnosis in 1705 patients with ulcerative colitis. A study by GISC (Italian Colon-Rectum Study Group). Dig Dis Sci 2000; 45: 462-5. [ Links ]
25. Softley A, Myren J, Clamp SE, Bouchier IA Watkinson G, de Dombal FT. Inflammatory bowel disease in the elderly patient. Scand J Gastroenterol (Suppl) 1988; 144: 27-30. [ Links ]
26. Trinh C, Prabhakar K. Diarrheal Disease in the Elderly. Clin Geriatr Med 2007; 23: 833-56. [ Links ]
27. Parente F, Marino B, Crosta C. Bowel preparation before colonoscopy in the era of mass screening for colo-rectal cancer: a practical approach. Dig Liv Dis 2009; 41: 87-95. [ Links ]
28. Ramin YI, Flachuck M. Bowel preparation for colonoscopy. Up To Date 2009. [ Links ]
29. Khurana A, McLean L, Atkinson S, Foulk CJ. The effect of oral sodium phosphate drug produces on renal function in adults undergoing bowel endoscopy. Arch Intern Med 2008; 168: 593-7. [ Links ]
30. Maa KK, Ng CSH, Mui LM. Severe hyperphosphate and hypercalcemia following sodium phosphate bowel preparation: a forgotten menace. Endoscopy 2003; 35: 717-9. [ Links ]
31. Maekowitz GS, Stokes MB, Radhakrishnan J, DAgati VD. Acute Phosphate nephropathy following oral sodium phosphate bowel purgative: a underrecognized cause of chronic renal failure. Am Soc Nerphrol 2005; 16: 3389-96. [ Links ]
32. Seinela L, Pehkonen E, Laasanen T, Ahveinanen J. Bowel preparation for colonoscopy en very old patients: a randomized prospective trial comparing oral sodium phosphate and politilen glycol electrolyte lavage solution. Scand J Gastroenterol 2003; 38: 216-20. [ Links ]
33. Barclay RL, Depew WT, Vanner SJ. Carbohydrate-electrolyte rehydration protects against intravascular volume contraction during colonic cleansing with orally administered sodium phosphate. Gastrointest Endosc 2002; 56: 633-8. [ Links ]
34. Rejchrt S, Bures J, Siroky, Kopakova M, Slezak L, Langr F. A prospective, observational study of colonic mucosal abnormalities associated with orally administered sodium phosphate for colon cleansing before colonoscopy. Gastrointest Endosc 2004; 59: 651-654. [ Links ]
35. Evensen ET, Hoff GS, Sauar J, Vatn MH. Patient tolerance of colonosopy without sedation during screening examination for colorectal polyps. Gastrointest Endosc 2000; 52: 606-10. [ Links ]











 texto en
texto en