Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev Col Gastroenterol vol.24 no.3 Bogotá July/Sept. 2009
Helicobacter pylori: Current treatment
An important challenge for gastroenterology
William Otero Regino, MD(1), Alba Alicia Trespalacios, MSc (2), Elder Otero (3)
(1) Professor of Medicine, Gastroenterology Unit, Universidad Nacional de Colombia, Gastroenterologist at the Clínica Fundadores, Clínica Carlos Lleras, Hospital San Carlos. Bogotá, Colombia.
(2) Professor in the Department of Microbiology, Coordinator of the Medical Microbiology Specialty Program at the Pontificia Universidad Javeriana. Bogotá, Colombia.
(3) Gastroenterologist at Clínica Fundadores, SaludCoop, Clínica Carlos Lleras, Hospital San Carlos, Bogotá, Colombia.
Received: 18-06-09 Accepted: 18-08-09
Summary
Since the discovery of Helicobacter pylori, its eradication has been one of the most important global challenges in gastroenterology. In many places the prevalence of the microorganism's primary resistance to different antibiotics is unknown. Consequently, antibiotics are used empirically in daily practice. Tests to verify eradication are normally not used, leaving the effectiveness of treatment unknown as well. Knowledge of these two factors could make it possible to identify those still infected after treatment as well as help physicians make better informed choices of appropriate rescue therapies. Lack of information on pre-treatment resistance is a problem that makes it impossible to measure the impact of pretreatment resistance on therapeutic failure. At the global level, standard triple therapy has lost the efficacy it once had, while sequential therapy is not equally effective in every part of the world, especially in those regions where high resistance to clarithromycin and methronidazolee exists. Treatment schemes with levofloxacin triple therapies have proven effective as first line therapies and/or rescue treatments. Nevertheless, each region should develop its own treatment schemes based on the results of local susceptibility tests and pharmacogenomic studies.
Key words
Helicobacter, triple therapy, levofloxacin, sequential therapy, primary resistance.
Helicobacter pylori (H. pylori), is one of humanity's principal pathogens. We have maintained a relationship with it for around 58,000 years, since before the human migrations out of East Africa disseminated our species, and the virus, to other parts of the world (1, 2). Despite this long shared prehistory, H. pylori was only recognized as a pathogen a little more than 25 years ago (3). That discovery opened a new chapter in medicine. Possibly 25 years has been too brief a time for us to have discovered all of the complex interactions which have occurred between ourselves and this pathogen since its origin. Perhaps this can help explain the tremendous difficulties medicine has had in managing H. Pylori, compared to the management of other infectious agents such as Treponema pallidum, and Pseudomonas spp.
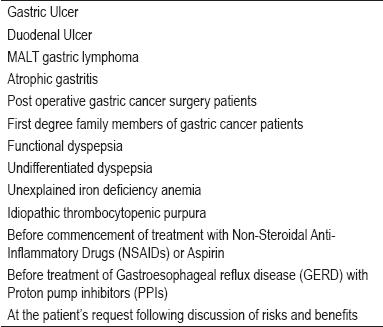
H. pylori affects about two thirds of the world's population. Its prevalence is Bly related to socio-economic conditions (4). In developing countries it affects more than 80% of all adults, whereas in developed countries it affects only between 20% and 50% of adults (5). H. Pylori is acquired in infancy, and if not eliminated with antimicrobials, persists throughout an individual's life (6-8). It produces chronic gastritis in all those infected, but only 20% of those infected develop any clinical illness (9). Ten to twenty percent of those infected are at risk of developing peptic ulcers at some point in their lives, and between one and two per cent will develop gastric cancer. One percent develop dyspepsia, while 1/100.000 will develop MALT lymphoma (9). Next to Non-Steroidal Anti-Inflammatory Drugs (NSAIDs), H. pylori is the most important cause of peptic ulcers. Outside of the intestines two clinical entities are clearly related to H. Pylori: iron deficiency anemia (10) and idiopathic thrombocytopenic purpura (itp) (11). According to the Maastricht III consensus report current indications for H. Pylori and its eradication are those listed in table 1 (12).
Table 1. Indications for treatment of Helicobacter pylori.
Eradicating H. pylori, has been one of gastroenterology's most important challenges since this microorganism was discovered. Various circumstances make H. Pylori particularly difficult to eliminate with antimicrobials. Some of these circumstances are inherent in the microorganism while others are characteristic of bacterial infections in general.
One problem has been that antibiotics commonly used were designed for the treatment of infections throughout the body rather than for delivering high concentrations within the stomach. Another factor of particular importance is the fact that H. pylori is protected by the acid of the mucous lining of the stomach in which it is immersed. This acid acts as a barrier contact between the microorganisms and the antibiotics within the stomach (13). Adding to the difficulty of treatment, upon reaching the stomach antibiotics naturally continue on through the intestine, losing their topical effect, leaving only their overall systemic effects on the infection (14). An additional problem is that various antibiotics lose their effectiveness in the acid of the stomach (15). Clarithromycin is particularly sensitive to acid which causes it to degrade. At ph2 its life is just one half hour (17). (Nevertheless, the efficacy of many other antibiotics is not influenced by acid (16). Among these are bismuth salts, tetracycline and methronidazolee, which explains the efficacy of the classic triple therapy using these three medications.
Two other important difficulties are the inoculum effect and the effects of biofilms (18, 19). The inoculum effect occurs in highly concentrated populations of bacteria, including populations of H. pylori, in which individual cells do not reproduce, or are dormant. As a result they can survive treatment with antibiotics without being resistant to the antibiotic (s) being used (19). Biofilm is a population of microorganisms which adhere to each other and/or to a surface which are embedded within a self-produced matrix of extracellular polymeric substance (EPS) which protects the microorganisms from the action of antibiotics (19). It has recently been demonstrated that H. pylori can form a living biofilm which could be an important mechanism for persistence of the infection and for protecting the infection against antimicrobials (19).
Since H. pylori does not replicate at pH levels lower than 6 large populations of bacteria can exist without reproducing in this type of micro-environment. However, once pH levels increase, proliferation begins again, and antibiotics again become effective (20).
Two basic therapeutic strategies have been used against this infection. The first uses two antibiotics with ranitidine bismuth citrate. A more soluble form of bismuth is generated which allows the release of the antibiotics bringing them into contact with the microorganisms and into action (13, 20). The other strategy is to use a proton pump inhibitors (PPI), which reduces gastric acid production, in combination with two antibiotics. Amoxicillin is usually combined with clarithromycin or methronidazolee, although their synergies have not yet been proven experimentally (20).
Theoretically there are many arguments which favor elevated pH levels:
They result in lower production of HCl, which in turn diminishes the volume of gastric liquid thus increasing the concentration of antibiotics both within the gastric lumen and within the mucus (21)
Higher pH levels also diminish the minimum inhibitory concentration (MIC) of amoxicillin and clarithromycin, and increases the stability of these molecules which are affected by acid pH levels (21, 22)
An individual's immune system functions most efficiently at higher pH levels (20).
Inhibition of Cytochrome P450 3A4 (CYP3A4) due to omeprazole combined with alteration of the metabolism of other substrates of this enzymatic system by agents such as clarithromycin increase the area under the curve by 15% in both plasma and gastric juice. The inverse is also true, clarithromycin elevates the area under the curve of omeprazole by 95%, while it doubles the area under the curve of esomeprazole (23).
Modification of pH induces important changes in the biology of H. pylori. It can survive at pH levels between 4.0 and 8.0, but it can synthesize proteins only when pH levels are between 6.0 and 8.0. Consequently it can not multiply at pH levels between 4.0 and 6.O, and thus is not susceptible to antibiotics such as amoxicillin and clarithromycin which can not affect the bacteria unless it is multiplying (20).
Upon elevation of the pH in the stomach through the use of proton pump inhibitors, the H. pylori population begins to reproduce. This allows bactericides like amoxicillin, and bacteriostatics like clarithromycin to work. If the pH continues to rise to the point of neutrality, the PPI itself will eliminate many of the remaining microorganisms through inhibition of the urease enzyme system. For this reason rapid urease tests can give false negatives in the presence of a PPI (12, 20).
Recently the hypothesis that increasing pH levels increases efficacy was corroborated by demonstrating that the efficacy of triple therapy is higher when the average pH in patients' stomachs was 6.4 than when the average pH in patients' stomachs was 5.2 (24). Similarly a meta-analysis showed that for application of triple therapy over seven days, high doses of omeprazole (40 mg two times per day) are more effective for curing H. pylori infections than standard doses of omeprazole (25). In addition, when rapid PPI metabolizers are encountered, the inhibitory effect of the PPI is reduced (26), and the standard triple therapy becomes less effective (27). On the other hand high levels of eradication of H. Pylori (over 90%) continue to be observed among slow PPI metabolizers, such as found in certain Asian populations. This is true for both standard triple therapy and for dual therapy combining a PPI and high doses of amoxicillin (28). (Dual therapy has been largely discontinued due to low efficacy.) In Japan some researchers believe that the rate of H. Pylori eradication through standard dosage triple therapy depends as much on CYP2C19 polymorphisms in individual patients as it depends on bacterial sensitivity to clarithromycin (29). The majority of PPIs are metabolized in the liver by Cytochrome P450 through the CYP2C19 enzyme (30), however Rabeprazole is a PPI which is not involved in this enzyme system (31). PPIs which are metabolized through CYP219 are affected by polymorphisms of this enzyme system.
While 19 alleles have been encountered, the majority of them can be classified into three types: slow metabolizers, intermediate metabolizers, and rapid metabolizers. Recently a fourth category, ultra-rapid metabolizers, has been described (32-34). Rapid metabolizers are dominant allele homozygotes while slow metabolizers are recessive allele homozygotes and intermediate metabolizers are heterozygotes expressing the dominant allele (32-34). Differences in frequencies of occurrence of slow metabolizers exist among ethnic groups: 2.5% to 3.5% among Caucasians, 2% among Afro-Americans, 13 to 20% among Chinese and 18 to 22% among Japanese (32).
Slow metabolizers have higher biological availability which allows PPIs to be more effective than in fast metabolizers who need higher doses of both PPIs and antibiotics (33). A study of patients who were rapid or intermediate metabolizers and who were not infected with H. Pylori showed that a combination of 10mg. Rabeprazole, 30 mg. Lanzoprazole 30 mg and 20 mg. omeprazole 20mg acted more while Rabeprazole alone led to greater increases in gastric pH (34). Another recent study showed that Lanzoprazole doses personalized according to the patients' CYP2C19, and administered without Clarithromycine if the patient's H. Pylori infection was resistant to it, resulted in a much higher eradication rate (96%) than did triple therapy using the usual dosage of Lanzoprazole (70%). Resistance was measured through a cost effective pharmacogenomic analysis of punctual mutations in the RNAr 23S gene (29). Different genotypes of CYP2C19 influence the magnitude and duration of inhibition of acid secretion by PPIs. Treatment results differ in Asian populations in which slow metabolizers are numerous and in occidental populations in which slow metabolizers are uncommon (16).
According to some authors, knowledge of the greater efficacy of currently abandoned dual therapy to profoundly suppress acid justifies reconsideration. New studies would test the hypothesis that at higher doses of PPIs and amoxicillin, administered more frequently, patients with CYP2C19 polymorphism who are rapid PPI metabolizers should be similar to patients with slow metabolizing polymorphisms. For two weeks PPI would be administered four times a day and 500 mg of amoxicillin would be administered every six hours (16). Although resistance to amoxicillin is very rare (13, 16, 35), smoking has been shown to diminish the efficacy of treatment schemes which contain it (36). The cause of this link is unknown but it could be related to the fact that cigarette smoking induces higher production of hydrochloric acid HCl (16).
Methronidazolee does not require cellular replication to eliminate the microorganism. It enters the cell through diffusion and is reduced. The resulting composite damages the macromolecules and degrades the DNA of the bacteria (35).
From the beginning of the search for treatments to eradicate H. pylori the antimicrobial approach has differed from that classically used against other infectious bacteria. Treatment for the majority of bacterial infections has been based on tests of the bacteria's susceptibility to different antibiotics with the objective of finding a treatment which will be successful in all cases, or in almost all cases (16). Physicians are attentive to the patterns of resistance by the most common microorganisms in their community (12, 16), knowledge which they use to select initial treatments. Those initial treatments are then adjusted in accord with results from cultures and the patterns of resistance which emerge. Treatment schemes can be adjusted very rapidly in order to maintain high levels of success.
This contrasts with what happens with H. pylori today. Gastroenterologists generally are unaware of the prevalence of the microorganism's primary resistances to different antibiotics which are used in a hit or miss fashion. Nevertheless they expect to obtain high rates of success. Assuming their prescribed treatment has cured everything, gastroenterologists rarely verify whether or not the bacteria has in fact been eradicated.
When a physician decides to treat a patient infected with H. Pylori, the ideal would be to verify eradication using non-invasive techniques. Tests such as the breath test for urea or ELISA testing for fecal antigens should be administered four to six weeks after the end of treatment, although when these are not indicated an endoscopy should be performed (9, 12). Testing to determine whether or not the bacteria have been eradicated is fundamental. If the infection continues after treatment, an adequate new treatment must be administered.
To the problems already mentioned can be added the fact that clinical studies rarely look at antimicrobial susceptibility prior to treatment, If undertaken this would allow adequate evaluation of the efficacy of the prescribed treatment. Moreover it would also allow the physician to prepare a fall back treatment should the first prescribed treatment fail (37). This method would allow physicians to individualize treatment for each patient.
To eradicate H. pylori treatment should be based on the results of clinical tests which include tests of susceptibility. Whenever possible biopsies of the patient's gastric mucus should be tested. When this is not possible, local rates of success of different treatments and the experience of the attending physician should be taken into account. Absent or insufficient pre-treatment information on the microorganism's resistance is an obstacle which impedes evaluation of the impact of the resistance, impedes evaluation of the efficacy of the clinical testing, and impedes any understanding of secondary resistance and of the reasons for the failure of a given treatment (37).
When this data is absent, treatment strategies for eradicating H. Pylori must be chosen on a trial and error basis. Some experts recommend pilot studies, or rehearsals, in which a new treatment scheme is administered to a small number of patients to identify whether or not a reasonable rate of success can be obtained with that treatment. If successful with the small group, a larger rehearsal in which more patients are treated, can then be organized (38).
One concept which is fundamental for confronting H. pylori is that it is an infection, and as such it is understood that it is curable (37). Recently, experts have suggested that the minimum expected success rate for any treatment should be 95% to be considered excellent. They suggested a grading system for the success rate of H. Pylori treatments: A for 95% or better success rates; B for 90% to 94% success rates; C for 85% to 89% success rates; D for 81% to 84% success rates; and F for success rates of 80% or less (39). Experts consider that clinical testing of a treatment on a sufficient number of patients should be measured in two different ways to determine if it achieves even the minimum level of success (37) The first measure compares the difference between the new treatment's rate of eradication with the lower limit of the 95% confidence interval (delta). Delta should be less than 10% to consider treatments equivalent. In the second method the lower limit of the 95% confidence interval should be superior to 80% to consider the new therapy effective. For a 90% point estimate a minimum sample of 80 patients is necessary to determine if the lower limit of the 95% confidence interval is superior to 80% (37).
The first treatment scheme for eradication of H. Pylori to convincingly demonstrate a success rate over 90% was the classic triple therapy. Its components are bismuth salts, methronidazolee, and tetracycline. It is administered without a PPI or other acid secretion inhibitor over a period of 14 days (40). However, its efficacy has been less than 90% in areas where resistance to methronidazolee has been high.
The first Maastricht consensus report, issued in 1997 (41), recommended that treatments to eradicate H. pylori should achieve a minimum success rate above 80%. The report's treatment of choice was a triple therapy which used a PPI and two of three antibiotics: Methronidazolee (or tinidazole) or clarithromycin and amoxicillin. This became the standard triple therapy. It had been described first by the Italian researchers Barzola and colleagues (42). After Maastricht I various scientific associations and experts in every part of the world paid considerable attention to the raggedy stone that was the treatment of this infection. (43-46). In the last ten years the treatment recommended by the first Maastricht consensus has not changed: it was ratified again by Maastricht III (12) and more recently by the American College of Gastroenterology (47). Nevertheless, the Maastricht III consensus recognizes that resistances to Clarithromycine and methronidazolee are becoming ever more frequent problems in many countries, even though treatment success rates in other countries continue to be high. The therapy has not been abandoned for this latter reason (12) even though initial success rates higher than 90% have declined notoriously and progressively in diverse parts of the world. They have fallen to levels between 57% and 73% for seven day treatments, and to levels between 67% and 79% for ten day treatments (48). This notable decline in efficacy has not only found in studies in the United States, studies in many other countries have found similar declines. For example a recent study in Italy found that 10 day treatments had success rates of 77% (49), statistically similar to the 81.7% success rate for ten day treatments and the 80% success rate for 7 day treatments encountered in another study (50). Despite triple therapy's loss of efficacy, primary care physicians and gastroenterologists in many parts of the world continue to prescribe this treatment (51, 52). The reason for this decline in efficacy has been attributed to growing resistance to Clarithromycine and methronidazolee (12).
Consistently high levels of resistance to methronidazolee have been documented in Colombia (53-55), and recently resistance rates to Clarithromycine around 20% have been reported (55). A decade ago, using the E-test, resistance to methronidazolee was measured at over 80%. More recently two different studies using the same test measured it at over 70%. Taking into account the fact that this test overestimates resistance to methronidazolee (54), and reducing these figures by the probable magnitude of the overestimation, we can conclude that resistance levels are above the 40% (54) maximum resistance level indicated in Maastricht III for the use of methronidazolee (12). The maximum resistance allowed for the use of Clarithromycine is 15-20% (12). An additional consideration is that primary resistance to Clarithromycine increases dramatically after the failure of standard triple therapy (secondary resistance) (56, 57). Consequently, under these circumstances, Clarithromycine can not be used in any rescue therapy after it has already been used as part of the initial treatment (12, 58).
In the face of growing resistance to Clarithromycine and increasing failure of standard triple therapy, other therapies which have been second choices or rescue therapies are now being given consideration. Among these are quadruple therapies in which a PPI is added to the classic trio of bismuth salts, tetracycline and methronidazolee for either seven or fourteen days (12, 13, 16). Success rates for this therapy have varied between 57% and 91%, with an average rate of 77% (59). When resistance to Clarithromycine is less than 15%, the efficacy of seven day quadruple therapy is similar to seven day standard triple therapy's success rate. Eradication rates are 82% and 78% respectively (60). As a rescue therapy after failure of initial treatment, quadruple therapy's success rates are similar for different durations of administration: 74% for seven day treatments; 72% for ten day treatments; and 81% for fourteen day treatments (59). One problem in the administration of quadruple therapy is the large numbers of tablets a patient must take every day which diminishes adherence to the treatment program. Another problem is that in some areas bismuth is not available. To overcome these problems a new form of quadruple therapy has been developed. It consists of a capsule which contains bismuth biskalcitrate, methronidazolee and tetracycline which is taken three times a day, plus a PPI which is taken twice a day (61). In the United States the efficacy of this treatment has been 87.7% (61), while in Europe it has been 93% (62).
Taking into account the high resistance level to methronidazolee in Colombia, a different quadruple therapy, a ten day treatment using a combination of Omeprazole, Tetracycline, Amoxicillin and Furazolidone (OTAF 10), has been tested here. It has achieved an 80% eradication rate (63). Because of low resistance to Furazolidone and because of its efficacy in treatment schemes, there have been frequent recommendations in studies in different parts of the world for its inclusion in eradication therapies when other therapies have failed (12, 13,16, 64, 65, 66-77). Nevertheless recent works have emphasized its toxicity which includes the capacity to cause tumors in lower animals, and to produce genotoxicity (78-80). For these reasons we believe that it should not be used until it is determined whether or not it produces similar results in human beings. Nevertheless, work is continuing with this medication. In the most recent work reviewed (77), it produced adverse effects at a rate of 85%. Its success rate was 100% when the initial treatment which failed did not contain Furazolidone, but was only 75% when the failed initial treatment did contain Furazolidone.
Because of the problems which currently exist with the use of standard triple therapy, particularly resistance to methronidazolee and Clarithromycine, the world wide community of gastroenterologists faces a new challenge in the eradication of H. pylori: finding other treatment schemes to use which are well tolerated, economical, and highly effective. Philosophically, achievement of such a scheme is the "Holy Grail" of the search for therapies against H. pylori (37). In this quest, Italian authors have developed a sequential therapy which is superior to standard triple therapy. Its efficacy is 89% versus 77% when an intention to treat (ITT) analysis is done (81). Recent meta-analyses have concluded that this therapy has a raw efficacy rate of 93.4% versus 76.9% for standard triple therapy (82). Sequential therapy consists of administration of a PPI for ten days. During the first five days 1 gram doses of amoxicillin are administered twice a day. During the next five days a combination of 500 mg of Clarithromycine and 500 mg of tinidazole administered twice a day replaces the amoxicillin (81). One disadvantage of this scheme is that it includes amoxicillin which can not be administered to patients who are allergic to penicillin. By the same token experts recommend that Clarithromycine not be used when there is high resistance to it (16, 81-83). In a pioneer study of this therapy H. pylori was not eradicated in all four patients who had shown resistance to both antibiotics (81). Since the number of patients was so small further studies are needed. In cases in which patients are resistant only to Clarithromycine, this therapy eradicated the infection in 90% of the cases studied, a far higher rate than the 28.6% success rate of standard triple therapy for this type of case (81). The exact mechanism through which this therapy works remains unknown. It is believed that the initial use of amoxiciline reduces the bacterial load while simultaneously weakening the bacterial walls of H. pylori. This produces an osmotic disequilibrium which affects the bacteria especially Bly during the growth phase impeding the development of bacterial efflux pumps. These pumps normally remove noxious chemicals including antibiotics from the bacteria, thus constituting an important resistance mechanism to Clarithromycine, preventing it from combining with bacterial ribosomes (81, 84). A recent study has ratified this hypotheses (85). A group of punctual mutations occurring in RNAr 23S are the changes most frequently related to resistance. Cytosine or guanine is substituted for adenine at position 2142 (A2142C, A2142G) or guanine is substituted at position 2143 (A2143G)(35). In sum, the damage to the bacterial wall caused by the amoxicillin prevents the bacterial efflux pump from removing the Clarithromycine when it arrives during the second phase of the treatment, thus allowing it to bind to the bacterial ribosomes.
Although the most important experience to date with this therapy has been in Italy, Sánchez Delgado and colleagues in Spain (86) have had a success rate of 84% measured by intention to treat and 90% when measured by protocol. In Korea the therapy has been used with a success rate of 80.8% (87). These results outside of Italy, whereas poor results were obtained in cases of dual resistance, indicate that studies of this treatment's efficacy are needed in every country rather than simple empirical use of the therapy.
Prior to the introduction of sequential therapy various concomitant quadruple rescue therapies without bismuth and lasting from three to six days were introduced (16, 88-91). Various combinations of antibiotics are used in these therapies. They include amoxicillin, clarithromycine, and methronidazolee; tinidazole and amoxicillin; and methronidazolee and roxithromycin.
A recent meta-analysis demonstrated that concomitant therapy, measured by intention to treat, was more effective than standard triple therapy. Its success rate was 90% compared to 79% for standard triple therapy, while its 95% confidence interval was 86.8% to 93.6% compared to a 95% confidence interval for standard triple therapy of only 67.8% to 87.1% (83). A recent randomized study done in Taiwan compared 10 day sequential therapy with a 7 day concomitant therapy and found similar rates of eradication. Measured by intention to treat, both therapies had 87% rates, whereas when they were measured by protocol the rate for sequential treatment was 93% and that for concomitant therapy was 91% (92). Based on these results the authors of the meta-analysis concluded that concomitant therapy should be included among treatment alternatives because of its efficacy, short treatment duration and relative simplicity (83). These authors also concluded that more studies are needed, and that consideration should be given to extending duration of treatment in an effort to reach the 95% success rate which is the objective for treatment of all bacterial infections in general. Like sequential therapy this treatment may encounter poor results in cases of simultaneous resistance to methronidazolee and Clarithromycine. When there is adequate adherence to the treatment regime, pretreatment resistance of H. pylori to antibiotics is the single most important reason for therapeutic failure (93).
Levofloxacin is another antibiotic which has emerged as a potential replacement for clarithromycine in triple therapies. It is combined with amoxicillin in the usual doses and with a PPI which is administered twice a day (12). Levofloxacin, a quinolone antibiotic, is an isomer of ofloxacin and a topoisomerase type II inhibitor with wide spectrum action against both Gram positive and Gram negative bacteria (94-97). Administered orally, it is rapid and is absorbed almost completely with bioavailability of 100%. It has a half life of between 9 and 16 hours, and most is renally excreted. It has few side effects (98, 99). Treatment schemes which have included this antibiotic have achieved high success rates: an initial seven to ten day treatment scheme achieved a rate over 85% (98), while a 4 day scheme achieved an eradication rate over 90% (99).
Triple therapy schemes using levofloxacin have been used as first (100-102), second line (103-106) and third line therapies (107,108). In first line schemes it has achieved an average efficacy rate of 90%, ranging from lows of 84% (100) and 87% (101) to a high of 96 % (102). Combined with Clarithromycine it has demonstrated similar rates when resistance to Clarithromycine is not high (101, 102). In second line rescue therapies success rates of 75% (104) and 77 % (105) have been published for populations in which H. pylori is susceptible and only 33% for a population in which the bacteria are resistant (104). In one study of its use in a second line therapy it achieved a success rate of 67% in a 7 day scheme, but reached an 87.5% rate in a 10 day scheme (p=0.004) (106).
In second line therapy 500 mg doses once a day have shown to be nearly as effective as the same dosage given twice a day, although they had failed earlier in standard triple therapy with clarithromycine (103). Administration of this dose once a day achieved a 79.6% success rate measured by intention to treat, while twice a day administration achieved an 80% rate by the same measure. In third line rescue therapies 60% (97) and 70% (108) rates of eradication have been reported.
Treating patients who are allergic to penicillin is particularly difficult given the importance of amoxicillin in the eradication of H. pylori. There are very few therapeutic options for these patients. One good option is a 10 day triple therapy which substitutes Levofloxacin for amoxicillin. In addition to a PPI, 500 mg doses of Levofloxacin and 500 mg doses of clarithromycine are administered twice a day. This triple therapy has achieved an efficacy rate of 87%, in the general population (98). Another possibility is a triple therapy combining clarithromycine, methronidazolee and a PPI (109), although this treatment is subject to limitations due to resistance to clarithromycine and methronidazolee. In places were bismuth is available, another therapeutic alternative is a quadruple therapy combining a PPI, bismuth salts, methronidazolee and tetracycline (109).
Despite the efficacy of Levofloxacin and its increasing use for the treatment of other infections, high levels of resistance to it exist in some countries. In Japan the resistance level is 15%, while in France it is 17% and in Alaska in the United States it is 8.8%. As with clarithromicine, high levels of resistance to quinolone antibiotics have been observed after a treatment fails to eradicate the bacteria (113).
Controversy exists over whether and when it is necessary to culture bacteria, and over whether or not tests of susceptibility are necessary to guide future treatments. The question is, "To culture, or not to culture" (114).
Unlike the case of functional dyspepsia, other conditions require the definitive eradication of H. pylori. They include MALT lymphomas, peptic ulcers and gastric cancer antecedents (partial gastroectomy or endoscopic resection for early cancer) (93). In these situations the various rescue therapies should be used in a progressive manner until eradication is achieved. After the failure of the second empirical treatment the Maastricht III consensus calls for culturing bacteria and for tests of anti-microbial susceptibility (12). Some authors have compared the strategy of a second line treatment scheme based on culturing with empirical treatment and found that treatment guided by information provided by the culture had an 86% success rate versus a 63% success rate for empirical second line treatment (115). Other studies have had similar results with success rates as high as 95% (116). Nevertheless various authors have empirically prescribed three or four different rescue treatments in succession with cumulative results of nearly complete success in all patients (98-99%) (117,118). A prospective study of 500 patients at one center used four consecutive treatment schemes empirically and achieved a cure rate of 99.5%. With these results the authors consider the need for bacterial cultures to be marginal (119).
If bacteria are cultured and susceptibility tests are done after failure of the second empirical treatment, the third treatment based on these test results has a 99% success rate (120). Recently a study was conducted in Greece where facilities for culturing bacteria were not readily available (108). Maastricht III recommendations were followed in part, taking into account that resistance to clarithromycine was less than 20%. Standard triple therapy was administered for ten days, followed empirically by quadruple rescue therapy including bismuth for ten more days. The third rescue therapy, chosen empirically due to absence of facilities for culturing bacteria, was a ten day triple therapy substituting 500 mg doses of Levofloxacin twice a day for clarithromycine. An initial eradication rate of 70% increased to a final cumulative success rate of 90% of 540 patients measured by the intention to treat method, and to 98% when measured by protocol (108). This study demonstrates that without recourse to bacterial cultures an final excellent final rate of eradication can be achieved, effectively challenging the Maastricht III recommendations (12). Nevertheless, it will be necessary to conduct studies in which the recommendations of the consensus for a scheme based on susceptibility testing are followed and then compared with this empirical scheme.
Whether or not susceptibility tests should be conducted before the first treatment should also be considered. So far the evidence is contradictory. One study of 240 patients compared the efficacy of treatment guided by susceptibility tests to treatment without prior testing. There were no significant differences between the two methods (121). However two other studies have shown important differences (122,123), demonstrating that susceptibility testing prior to treatment was useful for choosing the best treatment.
Although various experts consider the utility of culturing H. Pylori to be marginal or minimal (98,108), others consider culturing to be cost effective (122,124). Based on the publications reviewed above we consider that antimicrobial susceptibility testing has limitations, especially in terms of cost and availability. Many hospitals, and even some countries, do not have facilities to culture H. pylori. Variable rates of success of obtaining H. pylori from gastric samples, plus the necessity of performing an endoscopy to obtain samples from the stomach are additional limits. On top of these, in vitro resistance to methronidazolee is not a good predictor for resistance within the living body (125). Despite these limitations it is necessary for every region to undertake studies to determine local resistance characteristics in order to choose the best initial empirical treatment. More randomized and double blind studies are needed to compare the usefulness of culturing under different controversial circumstances and to define the place of this classical tool used in fighting other bacterial infections. We consider that it is necessary to continue investigating and improving culturing methods and methods of taking H. pylori samples. Considering how wide spread H. pylori infection is, and how serious are its consequences, it should be clear that we need to have a therapeutic and diagnostic arsenal at our disposal at least the equal of those available to confront other infectious diseases. We should not have to continue investigating treatments using the trial and error method.
As mentioned Furazolidone has also been used in rescue therapies. Another antibiotic which has been used (93,126), although there are many clear arguments against its use in H. pylori eradication therapies. Schemes which include it have efficacy rates lower than schemes using Levofloxacin, 85% vs. 45% (126), moreover it can produce irreversible mielotoxicity iand is expensive. It is the current stone in the shoe of Mycobacterium tuberculosis, especially n treatment of HIV positive patients. Wide spread usage can produce resistant strains of Mycobacterium tuberculosis (127,128).
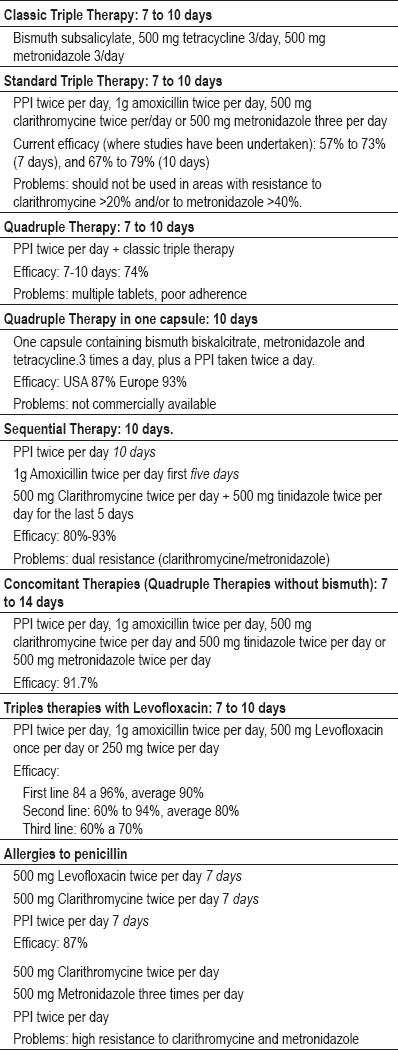
The various therapeutic options and their components are shown in table 2.
Table 2. Therapeutic options for eradicating H. pylori.
Numerous research studies on the treatment of H. pylori reflect the serious difficulty gastroenterologists in eradicating it. Initially effective treatments have progressively lost effectiveness as microorganisms have acquired resistance to antimicrobials. We have reached the point where there are reports of cases of H. pylori which has not been eradicated after four different treatments have failed (129).
An interesting approach for improving the efficacy of antibiotics in the treatment of H. pylori infections is the use of gastro-retentive systems. These systems use nano particles in a mucous-adhesive system to prevent the antibiotics from passing rapidly through the stomach, thus allowing them to have a much greater topical effect (130). Using particles between 550 nm and 900 nm in diameter the amount of amoxicillin, clarithromycine and omeprazole trapped within the stomach was between 60% and 90% (130). This system of drug release offers hope for optimizing H. pylori treatment.
CONCLUSIONS
H. pylori infections should be treated like any other infectious disease, taking into account identification of resistance and susceptibility in each region. We consider that it is necessary to verify eradication in every patient four to six weeks after treatment ends. This is especially true for patients with defined organic pathologies other than functional dyspepsia.
Empirical treatment options for H. pylori include standard triple therapy, triple therapy with Levofloxacin, sequential therapies, concomitant therapies, and quadruple therapies. The order of use of these therapies depends on the situation and upon local experience. All of these therapies may be used as first line therapies with the exception of quadruple therapies which to date have been reserved for use as rescue therapies.
Currently eradication rates for H. pylori treatment schemes which are in use are lower than 90%.
Standard triple therapy combining a PPI with amoxicillin and either clarithromycine or metronidazole has notably lost efficacy in the majority of countries where recent research has been done. It continues to be useful in populations with low levels of resistance to clarithromycine and/or metronidazole and in populations like that of Japan in which slow metabolizing polymorphisms of CYP2C19 are common. Whether or not this therapy is effective must be determined on a country to country basis.
The efficacy of the currently most used sequential therapy (ten days PPI, first five days amoxicillin, second five days clarithromycine and tinidazole) is not high in every situation and is not a good choice where high resistance to clarithromycine and/or metronidazole exists.
Dual therapy using high doses of amoxicillin and a PPI has regained favor in countries like Japan which have proportionally high numbers of slow metabolizers in the population, and where the new dual therapy has achieved acceptable eradication rates.
Clinical trials should always be used to determine H. pylori's pretreatment resistance to antibiotics in order to determine the usefulness of prescribed antibiotics for future treatments.
In general, and according to the Maastricht III consensus, empirical strategies of eradication should be used as first and second line therapies, but thereafter therapy should be based on the results of a culture. However, this recommendation has recently been challenged by the achievement of adequate eradication rates using a third empirical rescue therapy including Levofloxacin and without the guidance of a culture. As of yet Colombia has no definitively established treatment scheme based on local studies. Results of clinical trials now underway (Otero, Trespalacios) are needed to establish such a definitive treatment. Our therapeutic approach today consists of starting with standard triple therapy, verifying eradication by testing for fecal antigens, proceeding to triple rescue therapy with Levofloxacin when initial therapy fails, verifying whether eradication has been successful, and finally either proceeding to concomitant quadruple therapy or to bacterial culture (depending on availability and economic resources). Schemes using Levofloxacin have demonstrated their efficacy as first line therapies and as second, third and fourth line rescue therapies. They should also be seen as options for treating patients who are allergic to penicillin.
Every region should adopt locally chosen sequential therapies the components of which are chosen in accordance with knowledge of local susceptibility tests and antimicrobial pharmacokinetics.
Specific contributions of the authors
William Otero MD developed the original idea, designed the organization of the article, and critically reviewed the literature. Trespalacios AA MSc, and Otero E MD, revised and completed the manuscript. All of the authors have approved the final content.
Disclosure
None.
Acknowledgements
Thanks to Doctor Héctor Sandoval and Doctor Catalina Torres for critically reading the manuscript and for their suggestions.
REFERENCES
1. Fallush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, et al. Traces of human migrations in Helicobacter pylori populations. Science 2003; 299: 1582-5.
2. Linz B, Balloux F, Moodley Y, Manica A, Liu HA Roumagnac P, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 2007; 445: 915-18.
3. Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983; 1: 1273-5.
4. Malaty HM. Epidemiology of Helicobacter pylori. Best Pract Res Clin Gastroenterol 2007; 21: 205-14.
5. Suebaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002; 347: 1175-86.
6. Report of the Digestive Health Initiative International Update. Conference on Helicobacter pylori. Gastroenterology 1997; 113 (Suppl): S4-S8.
7. Saad R, Chey W. A clinician's guide to managing Helicobacter pylori infection. Clev Clin J Med 2005; 72: 109-124.
8. Parsonnet J. Helicobacter pylori: the size of the problem. Gut 1998; 43: S6-S9.
9. Otero W, Gómez M, Trespalacios AA. Helicobacter pylori: después de todo. Temas escogidos de gastroenterología. Asociación Colombiana de Gastroenterología 2007: 43-56.
10. Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and metatanalysis. Helicobacter 2008; 13: 323-40.
11. Stasi R, Satparwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, et al. Effects of eradication Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood 2009; 113: 1231-40.
12. Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection - The Maastricht III consensus report. Gut 2007; 56: 772-81.
13. Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology 2007; 133: 985-1001.
14. Ishack RAH, Awad GAS, Mortada ND, Nour SAK. Preparation in vitro and in vivo evaluation of stomach specific metronidazole loaded alginate beads as local anti Helicobacter pylori therapy. J Control release 2007; 119: 207-14.
15. Graham DY, More MP. Variability in the outcome of treatment of Helicobacter pylori infection: a critical analysis. En: Hunt RH, Tytgat GNJ (edit) Helicobacter pylori: basic mechanisms to clinical cure Dordrcht Kluwer Academic Publisher 1994. p. 426-40.
16. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nature Clin Pract Gastroenterol Hepatol 2008; 5: 321-31.
17. Erah P, Goddard A, Barrett D, Shaw PN, Spiller RC. The stability of amoxicillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrobial Cemetery 1997; 39: 5-12.
18. Lewis K. Persisted cells, dormancy and infectious disease. Nature Rev. Microbiol 2007; 5: 48-56.
19. Coticchia JM, Sugawa C, Tran VR, Gurrola J, Wowalski E, Carron MA. Presence and density of Helicobacter pylori biofilms in human gastric mucosa in patients with peptic ulcer. J Gastrointest Surg 2006; 10: 883-889.
20. Scott D, Weeks D, Melchers K, Sachs G. The life and death of Helicobacter pylori. Gut 1998; 43: S56-S60.
21. Midolo PD, Turnidge JD, Lambert JR. Bactericidal activity and synergy studies of proton pump inhibitors and antibiotics against Helicobacter pylori in vitro. J Antimicrob Chemother 1997; 39: 331-7.
22. Goddard AF, Jessa MJ, Barrett DA, Shaw PN, Idstrom JP, Cederberg C, et al. Effect of omeprazole on the distribution of metronidazole, amoxicillin and clarithromycin in lumen gastric juice. Gastroenterology 1996; 111: 358-67.
23. Treiber G, Malfertheiner P, Klotz U. Treatment and dosing of Helicobacter pylori infection: when pharmacology metes clinic. Expert Opin 2007; 8: 329-50.
24. Sugimoto M, Furuta T, Shirai N, Chise K, Masafumi N, Mutsuhiro I, et al. Evidence that the degree and duration ofacid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter 2007; 12: 317-23.
25. Villoria A, García P, Clavet X, Gisbert JP, Vergara EM. Meta-analysis: high-dose proton pump inhibitors vs. Standard dose in triple therapy for Helicobacter pylori eradication. Aliment Phramacol Ther 2008; 28: 868-77.
26. Horai Y, Kimura M, Furuie H, Matzuguma K, Irie S, Koga Y, et al. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther 2001; 15: 793-803.
27. Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH. The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta-analysis. Am J Gastroenterol 2006; 101: 1467-75.
28. Shirai N, Sugimoto M, Kodaira C, Nishino M, Ikuma M, Kajimura M, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after standard triple therapy. Eur J Clin Pharmacol 2007; 63: 743-9.
29. Furuta T, Shirai N, Kodaira M, Sugimoto M, Nogaki A, Kutiyama S, et al. Pharmacogenomics-based tailored versus standard therapeutics regimen for eradication of H. pylori. Clin Pharmacol Ther 2007; 81: 521-8.
30. Oasim A, O'Morain O, O'Connor HJ. Helicobacter pylori eradication: role of individual therapy constituents and therapy. Fun Clin Pharmacol 2008; 43-52.
31. Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors-emphasis on rabeprazole. Aliment Pharmacol Ther 1999; 13(Suppl 3): 27-36.
32. Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Yamade M, et al. Treatment strategy to eradicate Helicobacter pylori: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert Opin Pharmacother 2007; 8: 2701-17.
33. Klotz U. Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem. Int J Clin Pharmacol 2006; 44: 297-302.
34. Saitoh T, Fukusshima Y, Otsuka H, et al. Effects of rabeprazole, lanzoprazole, and omeprazole on intragastric pF in CYP2C19 extensive metabolizers. Aliment Phrmacol Ther 2002; 16: 1811-7.
35. Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007; 20: 280-322.
36. Suzuki T, Matsuo K, Sawaki A, Kawai K, Hirose K, Hito H, et al. Influence of smoking and CYP2C19 genotypes on H. pylori eradication success. Epidemiol Infect 2007; 135: 171-6.
37. Hopkins RJ. In search of the Holy Grail of Helicobacter remedies. Helicobacter 2001; 6:81-3.
38. Graham DY, Lu H, Yamaoka Y. Therapy for Helicobacter pylori infection can be improved. Sequential therapy and beyond. Drugs 2008; 68: 725-36.
39. Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007; 4: 257-8.
40. George LL. Cure of duodenal ulcer after eradication of Helicobacter pylori. Med J Aust 1990; 153: 145-9.
41. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter pylori Study Group. Gut 1997; 41: 8-13.
42. Bazzoli F, Zagari RM, Fossi S, Pozzato P, Roda A, Roda E. Short term low-dose triple therapy for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol 1994; 6: 773-77.
43. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori. Ad Hoc Committee on practice parameters of the American College of Gastroenterology. Am J Gastroenterol 1998; 93: 2330-8.
44. Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol 1998; 13: 1-12.
45. Bytzer P, O'Morain C. Treatment of Helicobacter pylori Helicobacter 2005; 10(Suppl 1):40-46.
46. Wolle K, Malfertheiner P. Treatment of Helicobacter pylori. Best Pract Res Clin Gastroenterol 2007; 21: 315-24.
47. Chey WD, Wong BCY. American College of Gastroenterology Guideline on the Management of Helicobacter pylori Infection. Am J Gastroenterol 2007; 102: 1808-25.
48. Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology 2007; 133: 985-1001.
49. Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, et al. Sequential therapy versus standard tripledrug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med 2007; 146: 556-63.
50. Zagari RM, Bianchi-Porro G, Fiocca R, Gasbarrini G, Roda E, Bazzoli F. Comparison of 1 and 2 weeks of omeprazole, amoxicillin and clarithromycin treatment for Helicobacter pylori eradication: The HYPER study. Gut 2007; 56: 475-9.
51. Sharma VK, Howden CW. A national survey of primary care physician's perceptions and practice related to Helicobacter pylori infection. J Clin Gastroenterol 2004; 38: 326-31.
52. Shirin H, Birkenfeld S, Shevah O, Levine A, Epstein J, Boaz M, et al. Application of Maastricht 2-2000 guidelines for the management of Helicobacter pylori among specialists and primary care physicians in Israel: are we missing the malignant potential of Helicobacter pylori. J Clin Gastroenterol 2004; 38: 322-5.
53. Gutiérrez O, Otero W. Resistencia de Helicobacter pylori al Metronidazol en Colombia. Rev Col Gastroenterol 1998; 12: 31-5.
54. Henao S, Otero W, Ángel LA, Martínez J. Resistencia Primaria a metronidazol en aislamientos de Helicobacter pylori en pacientes adultos de Bogotá Colombia Rev Col Gastroenterol 2009; 24: 10-5.
55. Trespalacios AA, Díaz G, Mercado M, Muñoz A, Navarro J. Helicobacter 2007; 12: 452 Abstract No.: P130.
56. Adamek RJ, Suerbaum S, Pfaffenbach B, Opferkuch W. Primary and acquired Helicobacter pylori resistance to clarithromycin, metronidazole, and amoxicillin-influence on treatment outcome. Am J Gastroenterol 1998; 93: 386-9.
57. Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxicillin reduces selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents 2002; 19: 67-70.
58. Gisbert JP, Pajares JM. Review article: Helicobacter pylori "rescue" regimen when proton pump inhibitor-based triple therapy fails. Aliment Pharmacol Ther 2002; 16: 1047-57.
59. Gisbert JP. "Rescue" regimens after Helicobacter pylori treatment failure. World J Gastroenterol 2008; 14: 5385-5402.
60. Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication. The QUADRATE study. Gastroenterology 2002; 123: 1763-9.
61. Laine L, Hunt R, El-Zimaity H, B. Nguyen, Osato M, Spenard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole and tetracycline given with omeprazole versus omeprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American Trial. Am J Gastroenterol 2003; 98: 562-7.
62. O'Morain C, Borody T, Farley A, de Boer WA, Dallaire C, Schuman R, et al. Efficacy and safety of single triple capsules of bismuth biskalcitrate, metronidazole, and tetracycline, given with omeprazole. For the eradication of Helicobacter pylori: an international multicenter study. Aliment Pharmacol Ther 2003; 17: 415-20.
63. Gutiérrez O, Otero W, Cardona H, Quintero F. Terapia cuádruple con furazolidona como tratamiento de rescate para la infección por Helicobacter pylori Rev. Col Gastroenterol 2003; 18: 222-8.
64. Daghaghzadeh, H, Hasan M, Karimi S, Raisi M. One-week versus two-week furazolidone-based quadruple therapy as the first-line treatment for Helicobacter pylori. J Gastroenterol Hepatol 2007; 22: 1399-1403.
65. Zaterka S, Eisig JN. Quadruple therapy with furazolidone for retreatment in patients with peptic ulcer disease. World J Gastroenterol 2008; 14: 6224-6227.
66. Akheri H, Merat S, Hosseini V, Malekzadeh R. Low-dose furazolidone in triple and quadruple regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther 2004; 19:8993.
67. Isakov V, Domareva I, Koudryavtseva L, Maev I, Ganskaya Z. Furazolidone-based triple rescue therapy' vs. quadruple rescue therapy' for the eradication of Helicobacter pylori resistant to metronidazole. Aliment. Pharmacol. Ther. 2002; 16: 1277-82.
68. Sotoudehmanesh R, Malekzadeh R, Vahedi H, Dariani NE, Asgari AA, Massarrat S. Second-line Helicobacter pylori eradication with a furazolidone-based regimen in patients who have failed a metronidazole-based regimen. Digestion 2001; 64: 222-5.
69. Treiber G, Ammon S, Malfertheiner P, Klotz U. Impact of furazolidone-based quadruple therapy for eradication of Helicobacter pylori after previous treatment failures. Helicobacter 2002; 7: 225-31.
70. Lu H, Zhang DZ, Hu PJ Li ZS, Lu XH, Fang XC, et al. One-week regimens containing ranitidine bismuth citrate, furazolidone and either amoxicillin or tetracycline effectively eradicates Helicobacter pylori: a multicentre, randomized, double-blind study. Aliment Pharmacol Ther 2001; 15: 1975-9.
71. Liu WZ, Xiao SD, Hu PJ, Lu H, Cui Y, Tytgat GN. A new quadruple therapy for Helicobacter pylori using tripotassium dicitrato bismuthate, furazolidone, josamycin and famotidine. Aliment Pharmacol Ther 2000; 14: 1519-22.
72. Wong WM, Wong BC, Lu H, Gu Q, Yin Y, Wang WH, et al. One-week omeprazole, furazolidone and amoxicillin rescue therapy after failure of Helicobacter pylori eradication with standard triple therapies. Aliment Pharmacol Ther 2002; 16: 793-8.
73. Malekzadeh R, Ansari R, Vahedi H Shiavoshi F, Alizadeh BZ, Eshraghian MR, et al. Furazolidone versus metronidazole in quadruple therapy for eradication of Helicobacter pylori in duodenal ulcer disease. Aliment Pharmacol Ther 2000; 14: 299-303.
74. Graham DY, Osato MS, Hoffman J, Opekun AR, Anderson SY, El Zimaity HM. Furazolidone combination therapies for Helicobacter pylori infection in the United States. Aliment Pharmacol Ther 2000; 14: 211-15.
75. Segura AM, Gutiérrez O, Otero W, Ángel A, Genta RM, Graham DY. Furazolidone, amoxicillin, bismuth triple therapy for Helicobacter pylori infection. Aliment Pharmacol Ther 1997; 11: 529-32.
76. Dani R, Queiroz DM, Díaz MG Franco JM, Magalhaes LC, Méndez GS, et al. Omeprazole, clarithromycin and furazolidone for the eradication of Helicobacter pylori in patients with duodenal ulcer. Aliment Pharmacol Ther 1999; 13: 1647-52.
77. Eisig JN, Silva FM, Navarro Rodríguez T, Malfertheiner P, Filho MJP, Saterka S. Efficacy of a 7-day course of furazolidone, levofloxacin, and lansoprazole after failed Helicobacter pylori eradication. BMC Gastroenterol 2009; 9: 38-43.
78. Tatsuta M, Lash H, Baba M, Taniguchi H. Attenuating effect of the monoamine oxidase inhibitor furazolidone on the anticarcinogenetic effect of cysteamine on gastric carcinogenesis induced by N-methyl-N'-nitro-N-nitrosoguanidine in Wistar rats. Int J Cancer 1991; 48: 605-608.
79. Ahmed HH, El-Aziem SH, Abdel-Wahhab MA. Potential role of cysteine and methionine in the protection against hormonal imbalance and mutagenicity induced by furazolidone in female rats. Toxicology 2008; 243: 31-42.
80. De Francesco V, Lerardi E, Hassan C, Zullo A. Furazolidone Therapy for Helicobacter pylori: Is effective and safe? World J Gastroenterol 2009; 15; 1915-16.
81. Vaira D, Zulo A, Vakil N, Gatta L, Ricci C, Perna F, et al. Sequential therapy versus standard triple drug therapy for Helicobacter pylori eradication. Ann Intern Med 2007; 146: 556-63.
82. Jaffri N, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med 2008; 148: 1-9.
83. Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four drug, three-antibiotic, non bismuth containing "concomitant therapy" versus triple therapy for Helicobacter pylori eradication. Helicobacter 2009; 14: 109-18.
84. De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani I, Bertini O, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med 2006; 144: 94-100.
85. Nakagawua I, Matsuzaki J, Nishizawa T, Tsugawa H, Muraoka H, Nakagawua I, et al. Efflux pumps contribute to claritromycin resistance in H.pylori. AGA 2009; Gastroenterology Suppl 1: Abstract M1051.
86. Sánchez-Delgado J, Calvet X, Bujanda L, Gisbert J, Titó L, Castro M, Ten Day sequential treatment for Helicobacter pylori eradication in clinical practice. Am J gastroenterol 2008; 103: 1-4.
87. Park S, Chun HJ, Kim ES, Patk SC, Jung ES, Lee SD, et al. The 19-day sequential therapy for Helicobacter pylori eradication in Korea: Less effective than expected. AGA 2009, Gastroenterología Suppl 1: Abstract M1053.
88. Nagahara A, Miwa H, Ogawa K, Yamada T, Kurosawa A, Ohkura R, et al. Addition of metronidazole to rabeprazole-amoxicillin-clarithromycin regimen for Helicobacter pylori infection provides an excellent cure rate with five day therapy. Helicobacter 2000; 5: 88-93.
89. Neville PM, Everett S, Langworthy H, Tompkins D, Mapstone NP, Axon ATR, et al. The optimal antibiotic combination in a 6-day Helicobacter eradication regimen. Aliment Pharmacol Ther 1999; 13: 497-501.
90. Okada M, Nishimura H, Kawashima M, Okabe N, Maeda K, Deo M, et al. A new quadruple therapy for Helicobacter pylori: influence of resistant strains on treatment outcome. Aliment Pharmacol Ther 1999; 13: 769-74.
91. Treiber G, Ammon S, Schneider E, Klotz U. Amoxicillin, metronidazole, omeprazole clarithromycin: a new, short quadruple therapy for Helicobacter pylori eradication. Helicobacter 1998; 3: 54-68.
92. Wu DC, Hsu PI, Wu JY, Opekun AR, Graham DY. Randomized controlled comparison of sequential and quadruple (concomitant) therapies for H. pylori infection. Gastroenterology 2008; 134: A24.
93. Moayyedi P, Soo, Deeks J, Delaney B, Harris A, Innes M, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev 2006; CD002096.
94. Schito AM, Schito GC. Levofloxacin, a broad spectrum anti infective: from Streptococcus pneumoniae to Pseudomonas aeruginosa. J Chemoter 2004;16 (Suppl 2): 3-7.
95. Candelli M, Nista EC, Carloni E, Pignataro G, Zocco MA, Cazzato A, et al. Treatment of H. pylori infection: a review. Curr Med Chem 2005; 12: 375-84.
96. Just PM. Overview of the fluoroquinolone antibiotics. Pharmacotherapy 1993; 13:4S-17S.
97. Croom KF, Goa KL. Levofloxacin: a review of its use in the treatment of bacterial infections in the United States. Drugs 2003; 63: 2769-802.
98. Nista EC, Candelli M, Zocco MA, Cremonini F, Ojetti V, Finizio R, et al. Levofloxacin-Based Triple Therapy in FirstLine Treatment for Helicobacter pylori eradication. Am J Gastroenterol 2006; 101: 1985-90.
99. Giannini EG, Bilarde C, Dulbecco P, Mamone M, Santi ML, Testa R, et al. Can Helicobacter pylori eradication regimens be shortened in clinical practice? An opel-label, randomized, pilot study of 4 and 7day triple therapy with rabeprazole, high dose levofloxacin and tinidazole. J Clin Gastroenterol 2006; 40: 515-520.
100. Gisbert JP, Fernández M, Molina J, Pérez A, Prieto B, Matos JM, et al. First line triple therapy with levofloxacin for Helicobacter pylori eradication. Alimen Pharmacol Ther 2007; 26: 495-500.
101. Nista EC, Candelli M, Zocco MA, Cremonini F, Ojeti M, Finizio R, et al. Levofloxacin-based triple therapy in first line treatment for Helicobacter pylori eradication. Am J Gastroenterol 2006; 101: 1985-90.
102. Schrauwen RWM, Jannssen MJR, de Boer WA. Seven-day PPI triple therapy with levofloxacin is very effective for Helicobacter pylori infection. J Med 2009; 67: 96-101.
103. Cheng HC, Chang WL, Chen WY, Yang HB, WU JJ, Sheu BS. Levofloxacincontaining triple therapy to eradicate the persistent H. pylori after a failed conventional triple therapy. Helicobacter 2007; 12: 359-63.
104. Perna F, Zullo A, Ricci C, Hassan C, Morini S, Vaira D. Levofloxacin based triple therapy for Helicobacter pylori re-treatment: role of bacterial resistance. Dig Liv Dis 2007; 39: 1001-5ç.
105. Gisbert JP, Bermejo F, Castro M, Aisa A, Fernández M, Tomas A, et al. Second Line therapy with levofloxacin after H.pylori treatment failure: a Spanish multicenter study of 300 patients. Am J Gastroenterol 2008; 103: 71-6.
106. Di Caro S, Franceschi F, Mariani A, Thompson F, Raimondo D, Masci E, Testoni A, et al. Second line levofloxacin based triple schemes for Helicobacter pylori eradication. Dig Liv Dis 2009; 41: 480-85.
107. Gisbert JP, Castro M, Bermejo F, Pérez A, Ducons J, Fernández M, Bory F, et al. Third line rescue therapy with levofloxacin after two H. pylori treatment failures. Am J Gastroenterol 2006; 101: 243-7.
108. Rokkas T, Sechopoulos P, Robotis I, Margantinis G, Pistiolas D. Cumulative H. pylori eradication rates in clinical practice by adopting first and second line regimens proposed by the Maastricht III consensus and a third line empirical regimen. Am J Gastroenterol 2009; 104: 21-5.
109. Kwok A, Lam P, Katelaris P, Leong RWL. Helicobacter pylori eradication therapy: indications, efficacy and safety. Expert Opin Drug Saf 2008; 7: 271-81.
110. Miyachi H, Miki I, Aoyama N, Shirasaka D, Matsumoto Y, Toyoda M, et al. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter 2006; 11: 243-9.
111. Carothers JJ, Bruce MG, Hennessy TW, Bensler M, Morris JM, Reasonover AL, et al. The relationship between previous fluoroquinolone use and levofloxacin resistance in Helicobacter pylori infection. Clin Infect Dis 2007; 44:e5-8.
112. Cattoir V, Nectoux J, Lascols C, Deforges L, Delchier JC, Megraud F, et al Update on fluroquinolones resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int J Antimicrob Agents 2007; 29: 389-96.
113. Nishizawa T, Suzuki H, Kurabayashi K, Masaoka T, Muraoka H, Mori M, et al. Gatifloxacin resistance and mutations in gyrA after unsuccessful Helicobacter pylori eradication in Japan. Antimicrob Agents Chemoter 2006; 50: 1538-40.
114. Zullo A, Hassan C, Lorenzetti R, Wins S, Morini S. A clinical practice viewpoint: to culture or not to culture Helicobacter pylori? Dig Liver Dis 2003; 35: 357-61.
115. Yahav J, Samra Z, Niv Y, Evans CT, Passaro DJ, Dinari G, Shmuly H. Susceptibility-guided vs. empiric retreatment of Helicobacter pylori infection after treatment failure. Dig Dis Sci 2006; 51: 2316-2.
116. Lamouliatte H, Megraud F, Delchier JC, Bretagne JF, Courillon A, DE Korwin JD, et al. Second line treatment for failure to eradicate Helicobacter pylori: a randomized trial comparing four treatment strategies. Alimen Pharmacol Ther 2003; 18: 791-7.
117. Gisbert JP, Gisbert JL, Marcos S, Pajares JM. Empirical Helicobacter pylori "rescue" therapy after failure of two eradication treatments. Dig Liver Dis 2004; 36: 7-12.
118. Treiber G, Ammon S, Malfertheiner P, Klotz U. Impact of furzolidone-based quadruple therapy for eradication of Helicobacter pylori after previous treatment failures. Helicobacter 2002; 7: 225-31.
119. Gisbert JP, Gisbert JL, Marcos S, Jimenez-Alonso I, Moreno-Otero R, Pajares JM. Empirical rescue therapy after Helicobacter pylori treatment failure: a 10 year single-centre study of 500 patients. Alimen Pharmacol Ther 2008; 27: 346-54.
120. Vicente R, Sicilia B, Gallego S, Revillo MJ, Ducons J, Gomollón F. Helicobacter pylori eradication in patients with peptic ulcer after two treatment failures: a prospective culture guided study. Gastroenterol Hepatol 2002; 25: 438-42.
121. Neri M, Milano A, Laterza F, Di Bonaventura G, Piccolomini R, Caldarella MP, et al. Role of antibiotic sensitivity testing before first-line Helicobacter pylori eradication treatments. Aliment Pharmacol Ther 2003; 18: 821-7.
122. Romano M, Mamo R, Cuomo A, DE Simone T, Mucherino C, Iovene MR, et al. Pretreatment antimicrobial susceptibility testing is cost saving in the eradication of Helicobacter pylori. Clin Gastroenterol Hepatol 2003; 1: 273-8.
123. Toracchio S, Cellini L, Di Campli E, Cappello G, Malatesta MG, Ferri A, et al. Role of antimicrobial susceptibility testing on efficacy of triple therapy in Helicobacter pylori eradication. Aliment Pharmacol Ther 2000; 14: 1639-43.
124. Breuer T, Graham DY. Costs of diagnosis and treatment of Helicobacter pylori infection: when does choosing the treatment regimen based on susceptibility testing become cost effective? Am J Gastroenterol 1999; 94: 725-9.
125. Fischbach IA, Van Zanten SV, Dickason J. Meta-analysis: The efficacy adverse events and the adherence related to first-line anti Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther 2004; 20: 1071-82.
26. Gisbert JP, Gisbert JL, Marcos S, Moreno-Otero R, Pajares JM. Third line rescue therapy with levofloxacin is more effective tan rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment Pharmacol Ther 2006; 24: 1469-74.
127. Luccio F, Laterza L, Zagari RM; Cennamo V, Grilli D, Bazzoli F. Treatment of Helicobacter pylori infection. BMJ 2008; 337: 746-50.
128. Fuccio L, Zagari RM, Bazzoli F. What is the best salvage for patients with Helicobacter pylori infection? Nature Clin Pract Gastroenterol Hepatol 2008; 5: 608-9.
129. Dore MP, Marras I, Maragkoudakis E, Nieddu S, Manca A, Graham DY. Salvage therapy after two or none prior Helicobacter pylori treatment failures: the super salvage regimen. Helicobacter 2003; 8: 307-9.
130. Ramteke S, Ganeh N, Bhattachrya S, Hain NK. Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J Drugs Targ 2009; 17: 225-34.
1. Fallush D, Wirth T, Linz B, Pritchard JK, Stephens M, Kidd M, et al. Traces of human migrations in Helicobacter pylori populations. Science 2003; 299: 1582-5. [ Links ]
2. Linz B, Balloux F, Moodley Y, Manica A, Liu HA Roumagnac P, et al. An African origin for the intimate association between humans and Helicobacter pylori. Nature 2007; 445: 915-18. [ Links ]
3. Warren JR, Marshall BJ. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983; 1: 1273-5. [ Links ]
4. Malaty HM. Epidemiology of Helicobacter pylori. Best Pract Res Clin Gastroenterol 2007; 21: 205-14. [ Links ]
5. Suebaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002; 347: 1175-86. [ Links ]
6. Report of the Digestive Health Initiative International Update. Conference on Helicobacter pylori. Gastroenterology 1997; 113 (Suppl): S4-S8. [ Links ]
7. Saad R, Chey W. A clinician's guide to managing Helicobacter pylori infection. Clev Clin J Med 2005; 72: 109-124. [ Links ]
8. Parsonnet J. Helicobacter pylori: the size of the problem. Gut 1998; 43: S6-S9. [ Links ]
9. Otero W, Gómez M, Trespalacios AA. Helicobacter pylori: después de todo. Temas escogidos de gastroenterología. Asociación Colombiana de Gastroenterología 2007: 43-56. [ Links ]
10. Muhsen K, Cohen D. Helicobacter pylori infection and iron stores: a systematic review and metatanalysis. Helicobacter 2008; 13: 323-40. [ Links ]
11. Stasi R, Satparwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, et al. Effects of eradication Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood 2009; 113: 1231-40. [ Links ]
12. Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection - The Maastricht III consensus report. Gut 2007; 56: 772-81. [ Links ]
13. Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology 2007; 133: 985-1001. [ Links ]
14. Ishack RAH, Awad GAS, Mortada ND, Nour SAK. Preparation in vitro and in vivo evaluation of stomach specific metronidazole loaded alginate beads as local anti Helicobacter pylori therapy. J Control release 2007; 119: 207-14. [ Links ]
15. Graham DY, More MP. Variability in the outcome of treatment of Helicobacter pylori infection: a critical analysis. En: Hunt RH, Tytgat GNJ (edit) Helicobacter pylori: basic mechanisms to clinical cure Dordrcht Kluwer Academic Publisher 1994. p. 426-40. [ Links ]
16. Graham DY, Shiotani A. New concepts of resistance in the treatment of Helicobacter pylori infections. Nature Clin Pract Gastroenterol Hepatol 2008; 5: 321-31. [ Links ]
17. Erah P, Goddard A, Barrett D, Shaw PN, Spiller RC. The stability of amoxicillin, clarithromycin and metronidazole in gastric juice: relevance to the treatment of Helicobacter pylori infection. J Antimicrobial Cemetery 1997; 39: 5-12. [ Links ]
18. Lewis K. Persisted cells, dormancy and infectious disease. Nature Rev. Microbiol 2007; 5: 48-56. [ Links ]
19. Coticchia JM, Sugawa C, Tran VR, Gurrola J, Wowalski E, Carron MA. Presence and density of Helicobacter pylori biofilms in human gastric mucosa in patients with peptic ulcer. J Gastrointest Surg 2006; 10: 883-889. [ Links ]
20. Scott D, Weeks D, Melchers K, Sachs G. The life and death of Helicobacter pylori. Gut 1998; 43: S56-S60. [ Links ]
21. Midolo PD, Turnidge JD, Lambert JR. Bactericidal activity and synergy studies of proton pump inhibitors and antibiotics against Helicobacter pylori in vitro. J Antimicrob Chemother 1997; 39: 331-7. [ Links ]
22. Goddard AF, Jessa MJ, Barrett DA, Shaw PN, Idstrom JP, Cederberg C, et al. Effect of omeprazole on the distribution of metronidazole, amoxicillin and clarithromycin in lumen gastric juice. Gastroenterology 1996; 111: 358-67. [ Links ]
23. Treiber G, Malfertheiner P, Klotz U. Treatment and dosing of Helicobacter pylori infection: when pharmacology metes clinic. Expert Opin 2007; 8: 329-50. [ Links ]
24. Sugimoto M, Furuta T, Shirai N, Chise K, Masafumi N, Mutsuhiro I, et al. Evidence that the degree and duration ofacid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter 2007; 12: 317-23. [ Links ]
25. Villoria A, García P, Clavet X, Gisbert JP, Vergara EM. Meta-analysis: high-dose proton pump inhibitors vs. Standard dose in triple therapy for Helicobacter pylori eradication. Aliment Phramacol Ther 2008; 28: 868-77. [ Links ]
26. Horai Y, Kimura M, Furuie H, Matzuguma K, Irie S, Koga Y, et al. Pharmacodynamic effects and kinetic disposition of rabeprazole in relation to CYP2C19 genotypes. Aliment Pharmacol Ther 2001; 15: 793-803. [ Links ]
27. Padol S, Yuan Y, Thabane M, Padol IT, Hunt RH. The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: a meta-analysis. Am J Gastroenterol 2006; 101: 1467-75. [ Links ]
28. Shirai N, Sugimoto M, Kodaira C, Nishino M, Ikuma M, Kajimura M, et al. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after standard triple therapy. Eur J Clin Pharmacol 2007; 63: 743-9. [ Links ]
29. Furuta T, Shirai N, Kodaira M, Sugimoto M, Nogaki A, Kutiyama S, et al. Pharmacogenomics-based tailored versus standard therapeutics regimen for eradication of H. pylori. Clin Pharmacol Ther 2007; 81: 521-8. [ Links ]
30. Oasim A, OMorain O, OConnor HJ. Helicobacter pylori eradication: role of individual therapy constituents and therapy. Fun Clin Pharmacol 2008; 43-52. [ Links ]
31. Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors-emphasis on rabeprazole. Aliment Pharmacol Ther 1999; 13(Suppl 3): 27-36. [ Links ]
32. Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Yamade M, et al. Treatment strategy to eradicate Helicobacter pylori: impact of pharmacogenomics-based acid inhibition regimen and alternative antibiotics. Expert Opin Pharmacother 2007; 8: 2701-17. [ Links ]
33. Klotz U. Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: a review of a special problem. Int J Clin Pharmacol 2006; 44: 297-302. [ Links ]
34. Saitoh T, Fukusshima Y, Otsuka H, et al. Effects of rabeprazole, lanzoprazole, and omeprazole on intragastric pF in CYP2C19 extensive metabolizers. Aliment Phrmacol Ther 2002; 16: 1811-7. [ Links ]
35. Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev 2007; 20: 280-322. [ Links ]
36. Suzuki T, Matsuo K, Sawaki A, Kawai K, Hirose K, Hito H, et al. Influence of smoking and CYP2C19 genotypes on H. pylori eradication success. Epidemiol Infect 2007; 135: 171-6. [ Links ]
37. Hopkins RJ. In search of the Holy Grail of Helicobacter remedies. Helicobacter 2001; 6:81-3. [ Links ]
38. Graham DY, Lu H, Yamaoka Y. Therapy for Helicobacter pylori infection can be improved. Sequential therapy and beyond. Drugs 2008; 68: 725-36. [ Links ]
39. Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007; 4: 257-8. [ Links ]
40. George LL. Cure of duodenal ulcer after eradication of Helicobacter pylori. Med J Aust 1990; 153: 145-9. [ Links ]
41. Current European concepts in the management of Helicobacter pylori infection. The Maastricht Consensus Report. European Helicobacter pylori Study Group. Gut 1997; 41: 8-13. [ Links ]
42. Bazzoli F, Zagari RM, Fossi S, Pozzato P, Roda A, Roda E. Short term low-dose triple therapy for the eradication of Helicobacter pylori. Eur J Gastroenterol Hepatol 1994; 6: 773-77. [ Links ]
43. Howden CW, Hunt RH. Guidelines for the management of Helicobacter pylori. Ad Hoc Committee on practice parameters of the American College of Gastroenterology. Am J Gastroenterol 1998; 93: 2330-8. [ Links ]
44. Lam SK, Talley NJ. Report of the 1997 Asia Pacific Consensus Conference on the management of Helicobacter pylori infection. J Gastroenterol Hepatol 1998; 13: 1-12. [ Links ]
45. Bytzer P, O'Morain C. Treatment of Helicobacter pylori Helicobacter 2005; 10(Suppl 1):40-46. [ Links ]
46. Wolle K, Malfertheiner P. Treatment of Helicobacter pylori. Best Pract Res Clin Gastroenterol 2007; 21: 315-24. [ Links ]
47. Chey WD, Wong BCY. American College of Gastroenterology Guideline on the Management of Helicobacter pylori Infection. Am J Gastroenterol 2007; 102: 1808-25. [ Links ]
48. Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology 2007; 133: 985-1001. [ Links ]
49. Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, et al. Sequential therapy versus standard triple–drug therapy for Helicobacter pylori eradication: a randomized trial. Ann Intern Med 2007; 146: 556-63. [ Links ]
50. Zagari RM, Bianchi-Porro G, Fiocca R, Gasbarrini G, Roda E, Bazzoli F. Comparison of 1 and 2 weeks of omeprazole, amoxicillin and clarithromycin treatment for Helicobacter pylori eradication: The HYPER study. Gut 2007; 56: 475-9. [ Links ]
51. Sharma VK, Howden CW. A national survey of primary care physicians perceptions and practice related to Helicobacter pylori infection. J Clin Gastroenterol 2004; 38: 326-31. [ Links ]
52. Shirin H, Birkenfeld S, Shevah O, Levine A, Epstein J, Boaz M, et al. Application of Maastricht 2-2000 guidelines for the management of Helicobacter pylori among specialists and primary care physicians in Israel: are we missing the malignant potential of Helicobacter pylori. J Clin Gastroenterol 2004; 38: 322-5. [ Links ]
53. Gutiérrez O, Otero W. Resistencia de Helicobacter pylori al Metronidazol en Colombia. Rev Col Gastroenterol 1998; 12: 31-5. [ Links ]
54. Henao S, Otero W, Ángel LA, Martínez J. Resistencia Primaria a metronidazol en aislamientos de Helicobacter pylori en pacientes adultos de Bogotá Colombia Rev Col Gastroenterol 2009; 24: 10-5. [ Links ]
55. Trespalacios AA, Díaz G, Mercado M, Muñoz A, Navarro J. Helicobacter 2007; 12: 452 Abstract No.: P130. [ Links ]
56. Adamek RJ, Suerbaum S, Pfaffenbach B, Opferkuch W. Primary and acquired Helicobacter pylori resistance to clarithromycin, metronidazole, and amoxicillin-influence on treatment outcome. Am J Gastroenterol 1998; 93: 386-9. [ Links ]
57. Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxicillin reduces selection of clarithromycin resistance during Helicobacter pylori eradication therapy. Int J Antimicrob Agents 2002; 19: 67-70. [ Links ]
58. Gisbert JP, Pajares JM. Review article: Helicobacter pylori "rescue" regimen when proton pump inhibitor-based triple therapy fails. Aliment Pharmacol Ther 2002; 16: 1047-57. [ Links ]
59. Gisbert JP. "Rescue" regimens after Helicobacter pylori treatment failure. World J Gastroenterol 2008; 14: 5385-5402. [ Links ]
60. Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication. The QUADRATE study. Gastroenterology 2002; 123: 1763-9. [ Links ]
61. Laine L, Hunt R, El-Zimaity H, B. Nguyen, Osato M, Spenard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole and tetracycline given with omeprazole versus omeprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American Trial. Am J Gastroenterol 2003; 98: 562-7. [ Links ]
62. OMorain C, Borody T, Farley A, de Boer WA, Dallaire C, Schuman R, et al. Efficacy and safety of single triple capsules of bismuth biskalcitrate, metronidazole, and tetracycline, given with omeprazole. For the eradication of Helicobacter pylori: an international multicenter study. Aliment Pharmacol Ther 2003; 17: 415-20. [ Links ]
63. Gutiérrez O, Otero W, Cardona H, Quintero F. Terapia cuádruple con furazolidona como tratamiento de rescate para la infección por Helicobacter pylori Rev. Col Gastroenterol 2003; 18: 222-8. [ Links ]
64. Daghaghzadeh, H, Hasan M, Karimi S, Raisi M. One-week versus two-week furazolidone-based quadruple therapy as the first-line treatment for Helicobacter pylori. J Gastroenterol Hepatol 2007; 22: 1399-1403. [ Links ]
65. Zaterka S, Eisig JN. Quadruple therapy with furazolidone for retreatment in patients with peptic ulcer disease. World J Gastroenterol 2008; 14: 6224-6227. [ Links ]
66. Akheri H, Merat S, Hosseini V, Malekzadeh R. Low-dose furazolidone in triple and quadruple regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther 2004; 19:89–93. [ Links ]
67. Isakov V, Domareva I, Koudryavtseva L, Maev I, Ganskaya Z. Furazolidone-based triple rescue therapy vs. quadruple rescue therapy for the eradication of Helicobacter pylori resistant to metronidazole. Aliment. Pharmacol. Ther. 2002; 16: 1277-82. [ Links ]
68. Sotoudehmanesh R, Malekzadeh R, Vahedi H, Dariani NE, Asgari AA, Massarrat S. Second-line Helicobacter pylori eradication with a furazolidone-based regimen in patients who have failed a metronidazole-based regimen. Digestion 2001; 64: 222-5. [ Links ]
69. Treiber G, Ammon S, Malfertheiner P, Klotz U. Impact of furazolidone-based quadruple therapy for eradication of Helicobacter pylori after previous treatment failures. Helicobacter 2002; 7: 225-31. [ Links ]
70. Lu H, Zhang DZ, Hu PJ Li ZS, Lu XH, Fang XC, et al. One-week regimens containing ranitidine bismuth citrate, furazolidone and either amoxicillin or tetracycline effectively eradicates Helicobacter pylori: a multicentre, randomized, double-blind study. Aliment Pharmacol Ther 2001; 15: 1975-9. [ Links ]
71. Liu WZ, Xiao SD, Hu PJ, Lu H, Cui Y, Tytgat GN. A new quadruple therapy for Helicobacter pylori using tripotassium dicitrato bismuthate, furazolidone, josamycin and famotidine. Aliment Pharmacol Ther 2000; 14: 1519-22. [ Links ]
72. Wong WM, Wong BC, Lu H, Gu Q, Yin Y, Wang WH, et al. One-week omeprazole, furazolidone and amoxicillin rescue therapy after failure of Helicobacter pylori eradication with standard triple therapies. Aliment Pharmacol Ther 2002; 16: 793-8. [ Links ]
73. Malekzadeh R, Ansari R, Vahedi H Shiavoshi F, Alizadeh BZ, Eshraghian MR, et al. Furazolidone versus metronidazole in quadruple therapy for eradication of Helicobacter pylori in duodenal ulcer disease. Aliment Pharmacol Ther 2000; 14: 299-303. [ Links ]
74. Graham DY, Osato MS, Hoffman J, Opekun AR, Anderson SY, El Zimaity HM. Furazolidone combination therapies for Helicobacter pylori infection in the United States. Aliment Pharmacol Ther 2000; 14: 211-15. [ Links ]
75. Segura AM, Gutiérrez O, Otero W, Ángel A, Genta RM, Graham DY. Furazolidone, amoxicillin, bismuth triple therapy for Helicobacter pylori infection. Aliment Pharmacol Ther 1997; 11: 529-32. [ Links ]
76. Dani R, Queiroz DM, Díaz MG Franco JM, Magalhaes LC, Méndez GS, et al. Omeprazole, clarithromycin and furazolidone for the eradication of Helicobacter pylori in patients with duodenal ulcer. Aliment Pharmacol Ther 1999; 13: 1647-52. [ Links ]
77. Eisig JN, Silva FM, Navarro Rodríguez T, Malfertheiner P, Filho MJP, Saterka S. Efficacy of a 7-day course of furazolidone, levofloxacin, and lansoprazole after failed Helicobacter pylori eradication. BMC Gastroenterol 2009; 9: 38-43. [ Links ]
78. Tatsuta M, Lash H, Baba M, Taniguchi H. Attenuating effect of the monoamine oxidase inhibitor furazolidone on the anticarcinogenetic effect of cysteamine on gastric carcinogenesis induced by N-methyl-N-nitro-N-nitrosoguanidine in Wistar rats. Int J Cancer 1991; 48: 605-608. [ Links ]
79. Ahmed HH, El-Aziem SH, Abdel-Wahhab MA. Potential role of cysteine and methionine in the protection against hormonal imbalance and mutagenicity induced by furazolidone in female rats. Toxicology 2008; 243: 31-42. [ Links ]
80. De Francesco V, Lerardi E, Hassan C, Zullo A. Furazolidone Therapy for Helicobacter pylori: Is effective and safe? World J Gastroenterol 2009; 15; 1915-16. [ Links ]
81. Vaira D, Zulo A, Vakil N, Gatta L, Ricci C, Perna F, et al. Sequential therapy versus standard triple drug therapy for Helicobacter pylori eradication. Ann Intern Med 2007; 146: 556-63. [ Links ]
82. Jaffri N, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med 2008; 148: 1-9. [ Links ]
83. Essa AS, Kramer JR, Graham DY, Treiber G. Meta-analysis: four drug, three-antibiotic, non bismuth containing "concomitant therapy" versus triple therapy for Helicobacter pylori eradication. Helicobacter 2009; 14: 109-18. [ Links ]
84. De Francesco V, Margiotta M, Zullo A, Hassan C, Troiani I, Bertini O, et al. Clarithromycin-resistant genotypes and eradication of Helicobacter pylori. Ann Intern Med 2006; 144: 94-100. [ Links ]
85. Nakagawua I, Matsuzaki J, Nishizawa T, Tsugawa H, Muraoka H, Nakagawua I, et al. Efflux pumps contribute to claritromycin resistance in H.pylori. AGA 2009; Gastroenterology Suppl 1: Abstract M1051. [ Links ]
86. Sánchez-Delgado J, Calvet X, Bujanda L, Gisbert J, Titó L, Castro M, Ten Day sequential treatment for Helicobacter pylori eradication in clinical practice. Am J gastroenterol 2008; 103: 1-4. [ Links ]
87. Park S, Chun HJ, Kim ES, Patk SC, Jung ES, Lee SD, et al. The 19-day sequential therapy for Helicobacter pylori eradication in Korea: Less effective than expected. AGA 2009, Gastroenterología Suppl 1: Abstract M1053. [ Links ]
88. Nagahara A, Miwa H, Ogawa K, Yamada T, Kurosawa A, Ohkura R, et al. Addition of metronidazole to rabeprazole-amoxicillin-clarithromycin regimen for Helicobacter pylori infection provides an excellent cure rate with five day therapy. Helicobacter 2000; 5: 88-93. [ Links ]
89. Neville PM, Everett S, Langworthy H, Tompkins D, Mapstone NP, Axon ATR, et al. The optimal antibiotic combination in a 6-day Helicobacter eradication regimen. Aliment Pharmacol Ther 1999; 13: 497-501. [ Links ]
90. Okada M, Nishimura H, Kawashima M, Okabe N, Maeda K, Deo M, et al. A new quadruple therapy for Helicobacter pylori: influence of resistant strains on treatment outcome. Aliment Pharmacol Ther 1999; 13: 769-74. [ Links ]
91. Treiber G, Ammon S, Schneider E, Klotz U. Amoxicillin, metronidazole, omeprazole clarithromycin: a new, short quadruple therapy for Helicobacter pylori eradication. Helicobacter 1998; 3: 54-68. [ Links ]
92. Wu DC, Hsu PI, Wu JY, Opekun AR, Graham DY. Randomized controlled comparison of sequential and quadruple (concomitant) therapies for H. pylori infection. Gastroenterology 2008; 134: A24. [ Links ]
93. Moayyedi P, Soo, Deeks J, Delaney B, Harris A, Innes M, et al. Eradication of Helicobacter pylori for non-ulcer dyspepsia. Cochrane Database Syst Rev 2006; CD002096. [ Links ]
94. Schito AM, Schito GC. Levofloxacin, a broad spectrum anti infective: from Streptococcus pneumoniae to Pseudomonas aeruginosa. J Chemoter 2004;16 (Suppl 2): 3-7. [ Links ]
95. Candelli M, Nista EC, Carloni E, Pignataro G, Zocco MA, Cazzato A, et al. Treatment of H. pylori infection: a review. Curr Med Chem 2005; 12: 375-84. [ Links ]
96. Just PM. Overview of the fluoroquinolone antibiotics. Pharmacotherapy 1993; 13:4S-17S. [ Links ]
97. Croom KF, Goa KL. Levofloxacin: a review of its use in the treatment of bacterial infections in the United States. Drugs 2003; 63: 2769-802. [ Links ]
98. Nista EC, Candelli M, Zocco MA, Cremonini F, Ojetti V, Finizio R, et al. Levofloxacin-Based Triple Therapy in First–Line Treatment for Helicobacter pylori eradication. Am J Gastroenterol 2006; 101: 1985-90. [ Links ]
99. Giannini EG, Bilarde C, Dulbecco P, Mamone M, Santi ML, Testa R, et al. Can Helicobacter pylori eradication regimens be shortened in clinical practice? An opel-label, randomized, pilot study of 4 and 7–day triple therapy with rabeprazole, high dose levofloxacin and tinidazole. J Clin Gastroenterol 2006; 40: 515-520. [ Links ]
100. Gisbert JP, Fernández M, Molina J, Pérez A, Prieto B, Matos JM, et al. First line triple therapy with levofloxacin for Helicobacter pylori eradication. Alimen Pharmacol Ther 2007; 26: 495-500. [ Links ]
101. Nista EC, Candelli M, Zocco MA, Cremonini F, Ojeti M, Finizio R, et al. Levofloxacin-based triple therapy in first line treatment for Helicobacter pylori eradication. Am J Gastroenterol 2006; 101: 1985-90. [ Links ]
102. Schrauwen RWM, Jannssen MJR, de Boer WA. Seven-day PPI triple therapy with levofloxacin is very effective for Helicobacter pylori infection. J Med 2009; 67: 96-101. [ Links ]
103. Cheng HC, Chang WL, Chen WY, Yang HB, WU JJ, Sheu BS. Levofloxacin–containing triple therapy to eradicate the persistent H. pylori after a failed conventional triple therapy. Helicobacter 2007; 12: 359-63. [ Links ]
104. Perna F, Zullo A, Ricci C, Hassan C, Morini S, Vaira D. Levofloxacin –based triple therapy for Helicobacter pylori re-treatment: role of bacterial resistance. Dig Liv Dis 2007; 39: 1001-5ç. [ Links ]
105. Gisbert JP, Bermejo F, Castro M, Aisa A, Fernández M, Tomas A, et al. Second Line therapy with levofloxacin after H.pylori treatment failure: a Spanish multicenter study of 300 patients. Am J Gastroenterol 2008; 103: 71-6. [ Links ]
106. Di Caro S, Franceschi F, Mariani A, Thompson F, Raimondo D, Masci E, Testoni A, et al. Second line levofloxacin based triple schemes for Helicobacter pylori eradication. Dig Liv Dis 2009; 41: 480-85. [ Links ]
107. Gisbert JP, Castro M, Bermejo F, Pérez A, Ducons J, Fernández M, Bory F, et al. Third line rescue therapy with levofloxacin after two H. pylori treatment failures. Am J Gastroenterol 2006; 101: 243-7. [ Links ]
108. Rokkas T, Sechopoulos P, Robotis I, Margantinis G, Pistiolas D. Cumulative H. pylori eradication rates in clinical practice by adopting first and second line regimens proposed by the Maastricht III consensus and a third line empirical regimen. Am J Gastroenterol 2009; 104: 21-5. [ Links ]
109. Kwok A, Lam P, Katelaris P, Leong RWL. Helicobacter pylori eradication therapy: indications, efficacy and safety. Expert Opin Drug Saf 2008; 7: 271-81. [ Links ]
110. Miyachi H, Miki I, Aoyama N, Shirasaka D, Matsumoto Y, Toyoda M, et al. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter 2006; 11: 243-9. [ Links ]
111. Carothers JJ, Bruce MG, Hennessy TW, Bensler M, Morris JM, Reasonover AL, et al. The relationship between previous fluoroquinolone use and levofloxacin resistance in Helicobacter pylori infection. Clin Infect Dis 2007; 44:e5-8. [ Links ]
112. Cattoir V, Nectoux J, Lascols C, Deforges L, Delchier JC, Megraud F, et al Update on fluroquinolones resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. Int J Antimicrob Agents 2007; 29: 389-96. [ Links ]
113. Nishizawa T, Suzuki H, Kurabayashi K, Masaoka T, Muraoka H, Mori M, et al. Gatifloxacin resistance and mutations in gyrA after unsuccessful Helicobacter pylori eradication in Japan. Antimicrob Agents Chemoter 2006; 50: 1538-40. [ Links ]
114. Zullo A, Hassan C, Lorenzetti R, Wins S, Morini S. A clinical practice viewpoint: to culture or not to culture Helicobacter pylori? Dig Liver Dis 2003; 35: 357-61. [ Links ]
115. Yahav J, Samra Z, Niv Y, Evans CT, Passaro DJ, Dinari G, Shmuly H. Susceptibility-guided vs. empiric retreatment of Helicobacter pylori infection after treatment failure. Dig Dis Sci 2006; 51: 2316-2. [ Links ]
116. Lamouliatte H, Megraud F, Delchier JC, Bretagne JF, Courillon A, DE Korwin JD, et al. Second line treatment for failure to eradicate Helicobacter pylori: a randomized trial comparing four treatment strategies. Alimen Pharmacol Ther 2003; 18: 791-7. [ Links ]
117. Gisbert JP, Gisbert JL, Marcos S, Pajares JM. Empirical Helicobacter pylori "rescue" therapy after failure of two eradication treatments. Dig Liver Dis 2004; 36: 7-12. [ Links ]
118. Treiber G, Ammon S, Malfertheiner P, Klotz U. Impact of furzolidone-based quadruple therapy for eradication of Helicobacter pylori after previous treatment failures. Helicobacter 2002; 7: 225-31. [ Links ]
119. Gisbert JP, Gisbert JL, Marcos S, Jimenez-Alonso I, Moreno-Otero R, Pajares JM. Empirical rescue therapy after Helicobacter pylori treatment failure: a 10 year single-centre study of 500 patients. Alimen Pharmacol Ther 2008; 27: 346-54. [ Links ]
120. Vicente R, Sicilia B, Gallego S, Revillo MJ, Ducons J, Gomollón F. Helicobacter pylori eradication in patients with peptic ulcer after two treatment failures: a prospective culture guided study. Gastroenterol Hepatol 2002; 25: 438-42. [ Links ]
121. Neri M, Milano A, Laterza F, Di Bonaventura G, Piccolomini R, Caldarella MP, et al. Role of antibiotic sensitivity testing before first-line Helicobacter pylori eradication treatments. Aliment Pharmacol Ther 2003; 18: 821-7. [ Links ]
122. Romano M, Mamo R, Cuomo A, DE Simone T, Mucherino C, Iovene MR, et al. Pretreatment antimicrobial susceptibility testing is cost saving in the eradication of Helicobacter pylori. Clin Gastroenterol Hepatol 2003; 1: 273-8. [ Links ]
123. Toracchio S, Cellini L, Di Campli E, Cappello G, Malatesta MG, Ferri A, et al. Role of antimicrobial susceptibility testing on efficacy of triple therapy in Helicobacter pylori eradication. Aliment Pharmacol Ther 2000; 14: 1639-43. [ Links ]
124. Breuer T, Graham DY. Costs of diagnosis and treatment of Helicobacter pylori infection: when does choosing the treatment regimen based on susceptibility testing become cost effective? Am J Gastroenterol 1999; 94: 725-9. [ Links ]
125. Fischbach IA, Van Zanten SV, Dickason J. Meta-analysis: The efficacy adverse events and the adherence related to first-line anti Helicobacter pylori quadruple therapies. Aliment Pharmacol Ther 2004; 20: 1071-82. [ Links ]
126. Gisbert JP, Gisbert JL, Marcos S, Moreno-Otero R, Pajares JM. Third line rescue therapy with levofloxacin is more effective tan rifabutin rescue regimen after two Helicobacter pylori treatment failures. Aliment Pharmacol Ther 2006; 24: 1469-74. [ Links ]
127. Luccio F, Laterza L, Zagari RM; Cennamo V, Grilli D, Bazzoli F. Treatment of Helicobacter pylori infection. BMJ 2008; 337: 746-50. [ Links ]
128. Fuccio L, Zagari RM, Bazzoli F. What is the best salvage for patients with Helicobacter pylori infection? Nature Clin Pract Gastroenterol Hepatol 2008; 5: 608-9. [ Links ]
129. Dore MP, Marras I, Maragkoudakis E, Nieddu S, Manca A, Graham DY. Salvage therapy after two or none prior Helicobacter pylori treatment failures: the super salvage regimen. Helicobacter 2003; 8: 307-9. [ Links ]
130. Ramteke S, Ganeh N, Bhattachrya S, Hain NK. Amoxicillin, clarithromycin, and omeprazole based targeted nanoparticles for the treatment of H. pylori. J Drugs Targ 2009; 17: 225-34. [ Links ]











 text in
text in