Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev Col Gastroenterol vol.24 no.3 Bogotá July/Sept. 2009
Utility of fibrin glue in therapeutic endoscopy
John Ospina Nieto, MD, MACC, MACG, MSCED, MACH, MACCP, MFELAC, MALHE (1), John Villamizar Suárez MD, MSCG, MSCC (2), Ángela Paola Rodríguez González, MD (3)
(1) Gastrointestinal Surgeon and Digestive Endoscopist. Gastrointestinal Surgery and Endoscopy Coordinator at the Hospital Cardiovascular del Niño (Soacha), Dispensario Central del Ejército (Bogotá), Member of gastrointestinal surgery committee ACC. Member of Latin-American single balloon enteroscopy group. Johnosni@ yahoo.com.
(2) Gastrointestinal Surgeon and Digestive Endoscopist. Hospital Cardiovascular del Niño de Cundinamarca (Soacha) SALUDCOOP Liga contra el cáncer. Bogotá, Colombia.
(3) General surgeon. Hospital Militar Central. Bogotá, Colombia.
Received: 10-12-08 Accepted: 12-08-09
Summary
Fibrin glues, often used in surgery, are hemostatic, adhesive agents derived principally from products of human plasma. In use for more than 20 years, they have been associated with a variety of applications that seek to reduce the incidence of surgical complications such as hemorrhage and leakage. However, in spite of more than 20 years of use, the literature about the usefulness of these glues in endoscope procedures remains scant. This article presents four clinical cases in which endoscopic use of this agent improved the therapeutic development and outcome of the patient. It also presents a brief review of the literature regarding endoscopic employment of these products.
Key words
Fibrin glue, digestive endoscopy, digestive hemorrhage.
INTRODUCTION AND HISTORICAL BACKGROUND
Fibrin glues are hemostatic and adhesive agents used in surgery. They are derived principally from products of human plasma. Composed of fibrinogen, thrombin, factor XIII, antifibrinolytic agent (aprotinin) and calcium chloride, they reproduce the final steps in the coagulation cascade, generating a stable fibrin-clotting, stopping loss of blood flow and helping in the normal healing process (1-3).
Clots formed by these agents are similar to normal clots and thus are naturally degraded by corporal enzymes. This glue has mainly been used in surgery for hemostasis, suture support and tissue adhesion. It is also used to obtain hemostasis in hemorrhagic fields, especially in situations where sutures are not appropriate, to reduce blood flow of solid organs, to seal anastomoses or filtrations of hollow organs, and to replace sutures in surgical procedures, especially when these are difficult or impossible (1-3, 7, 8).
In the present work, four clinical cases are presented, where endoscopic use of this agent improved the evolution and patients therapeutic conduct. In addition, a brief literature review of these products and their endoscopic usage is presented in this work.
CLINICAL CASES
Patient 1
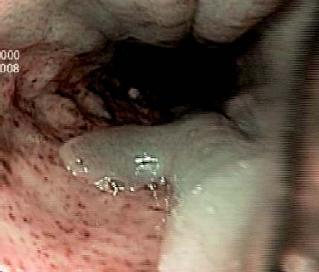
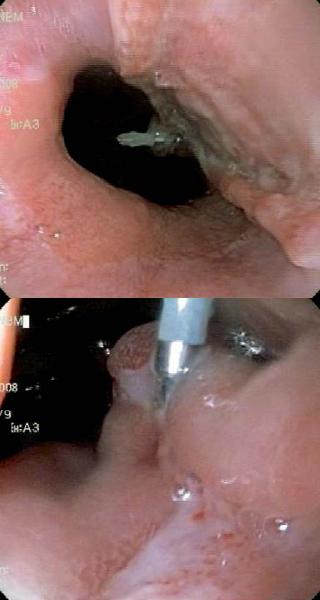
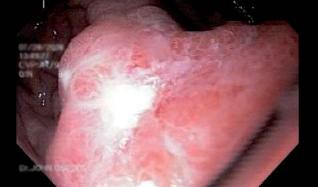
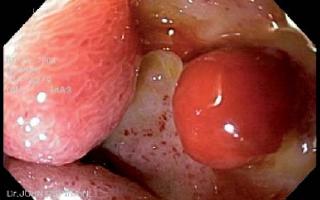
Patient 1, a 23 year old HIV positive person, was sent to the endoscopy service of the HCC with a diagnosis of thoracic pain. The endoscopy service documented giant esophageal ulcers caused by citomegalovirus (figure 1). 8 days after the diagnosis patient 1 entered the institution with upper digestive tract hemorrhaging and stage II hypovolemia. A digestive tract endoscopy was performed, and it revealed a Forrest 1B ulcer with hemorrhaging near the distal end of the gastroesophageal junction. Endoscopic injection sclerosis with 1:10.000 adrenaline was performed prior to initiating medical treatment. 30 hours after the endoscopic therapy, the patient showed renewed bleeding. A new digestive tract endoscopy was performed, and it revealed renewed bleeding at the same level. To stop the bleeding an endoscopic clip was placed (figure 2). The case was presented at a surgical meeting. Due to the high risk of renewed bleeding and concomitant surgical risk the decision was made to apply a fibrin glue sealant (Tissucol ®) to the lesion. The evolution was adequate, with good oral tolerance and no overt signs of bleeding. For this reason removal was authorized 72 hours after the sealant application. 20 days later, an endoscopic examination showed that the ulcer was healing (figure 3).
Figure 1. Esophageal ulcer N.B.I technique.
Figure 2. Clip in esophageal ulcer.
Figure 3. Endoscopic examination.
Patient 2
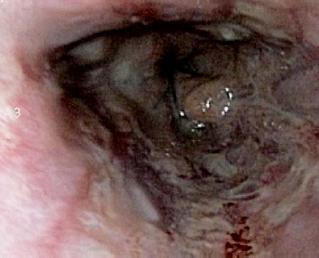
Patient 2, an 82 year old patient with hypertension, diabetes, and a history of coronary heart disease, was sent to the emergency services of the Hospital Cardiovascular del Niño de Cundinamarca (HCC) with a diagnosis of hematemesis and stage II hypovolemia. The patient was stabilized and transferred to the endoscopy service, where a Forrest 1B ulcer with layered hemorrhaging was discovered. It was located at approximately 2cm along the lesser curvature, near the fundus of the stomach. Endoscopic injection sclerosis with 1:10.000 adrenaline was performed resulting in adequate control. During hospitalization, the patient experienced three episodes of renewed bleeding which were controlled with sclerosis procedures and thermal treatment (monopolar coagulation). Nevertheless, the patient experienced a fourth episode of renewed bleeding condition while hospitalized. Consequently it was decided that another sclerosis procedure should be performed and fibrin glue sealant should be applied (Tissucol®). The patient showed adequate evolution, oral tolerance and healing (figures 4 and 5).
Figure 4. Ulcer with layered hemorrhaging in lesser curvature.
Figure 5. Endoscopic examination 28 hours after fibrin glue application.
Patient 3
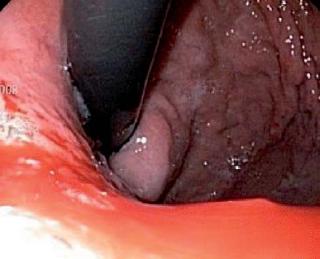
Patient 3, a 91 year old patient with hypertension a history of coronary heart disease and myocardial revascularization, was sent to the emergency services of the Hospital Cardiovascular del Niño de Cundinamarca (HCC) with a diagnosis of hematemesis and stages II and III hypovolemia. The patient was stabilized and transferred to the endoscopy service where a Forrest 1B ulcer was discovered. The ulcer had adhered clotting and was jetting blood. It was located on the posterior wall of the duodenal bulb. Its diameter was more than 25 mm (figure 6). Because of the high risks of renewed bleeding, morbidity and mortality posed by surgery, we decided to perform endoscopic injection sclerosis with 1:10.000 adrenaline and apply fibrin glue sealant (Tissucol ®). The patient showed adequate evolution and removal was 72 hours later. An endoscopic examination performed 20 days later revealed no evidence of any active duodenal ulcer.
Figure 6. Giant duodenal ulcer.
Patient 4
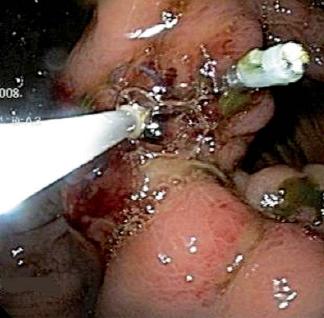
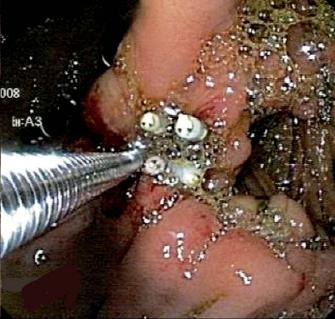
Patient 4, a 52 year old patient post gastric surgery patient, was transferred from a Departmental Hospital in Cundinamarca. Because the patient showed sharp abdominal pain the patient was rehospitalized in the Hospital Cardiovascular del Niño de Cundinamarca. The patient showed biliary peritonitis with partially frozen abdomen in the supramesocolic sector that did not allow exploration of the stomach and duodenum. Drains were placed left in the laparotomy. Later, a digestive tract endoscopy was performed. It revealed leaking sutures toward the right lateral antrum wall and lesser curvature, with bubbles in the laparotomy.
The fistula orifice was closed with clips, and the procedure was reinforced with the application of fibrin glue sealant (Tissucol®). The treatment showed adequate evolution, diminution of drainage, and later the development of oral tolerance (figures 7 and 8).
Figures 7 and 8. Clips and fibrin sealant applied to leaking gastric sutures.
DISCUSSION
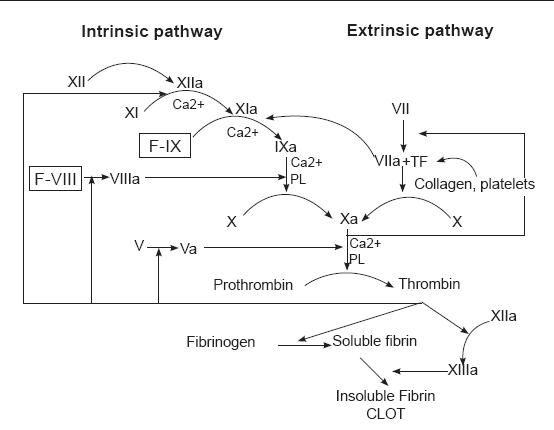
Every method which stops bleeding involves contact with tissue factor (TF), which triggers a series of events, commonly known as "coagulation cascade". This cascade culminates with a fibrin-polymer formation (figure 9).
Figure 9. Coagulation cascade.
After fibrin is deposited around the periphery of the injury, blocking the area, the first stage of healing begins with exudation, autolysis and cytolisis. This stage constitutes a catabolic state that prepares and cleans the zone. Subsequently, capillary growth and fibroblast formation, responsible for collagen production, create a granulated tissue which begins third stage of clot formation. This stage develops from the peripheral epithelium and moves towards the center, supported within the conjunctive tissue formed to cover the wound. Finally, from the second week, the fourth stage of collagen maturation stage provides greater tensile strength to the scar (1, 4, 14).
Knowledge of these stages brought to light the role of fibrin production in the final coagulation stage and in maintenance of hemostasis, decreasing the possibility of new hemorrhaging and accelerate healing.
From this knowledge fibrin glue sealants were born. Now they have been in use for more than 20 years. They have been associated with good tolerance through a variety of applications.
Since they contain human plasma products, modern and commercially available fibrin glue sealants undergo a variety of purification processes that vary from product to product. All commercial fibrin glue sealants have essentially the same composition. There are differences among them in terms of the simplicity of preparation, storage and utility. For example, some preparations need to be stored in a freezer and must be defrosted before use. Other sealants require heating to properly mix the components before use (2).
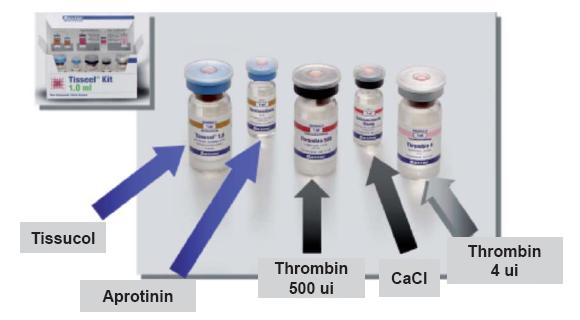
This adhesive system consists of two human origin components: freeze-dried adhesive protein concentrate to be dissolved with aprotinin solution, and freeze-dried thrombin to be reconstituted with calcium chloride solution. Both components are derived from a human plasma mixture (figure 10) (5).
Figure 10. Fibrin adhesive "Kit".
It is important to note that each plasma unit is inspected and checked for the absence of Hbs antigen, anti HIV 1, anti HIV2, anti HVC antibodies, and for ALT levels. An additional security measure to reduce the potential risk of virus transmission is steam treatment of the protein concentrate and human thrombin.
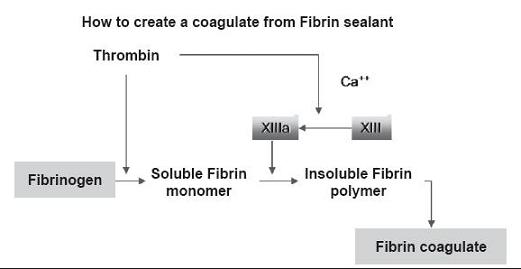
As we mentioned before, the mechanism of this adhesive corresponds to the last phase of blood coagulation. Human Fibrinogen molecule is a glycoprotein composed of three pairs of polypeptide chains (Aa, Bb, g). Two of them form a molecule with two symmetrical halves. Through the action of thrombin fibrinogen is converted into fibrin (a,b,g). Two of these release two fibrin-peptides A and B. Fibrin monomers are polymerased into dimers and are later joined with covalent bonds by the action of factor XIII (which was previously activated by thrombin and calcium ions.)
The fibrin produced adheres to the tissues exposed by the lesion, especially to collagen fibers. The fibrin mesh formed works as support for fibroblast and capillary proliferation produced in the healing process. The process depends on many factors. Among them are thrombin, fibrin and factor XIII which stimulate fibroblast proliferation (figure 11).
Figure 11. Formation of a fibrin clot.
The following stage of the healing process of a wound consists of proteolysis and fibrin-mesh phagocytosis. Fibrinolysis depends upon tissue plasminogen activator, the concentration of which may vary from one tissue to another. Thus, the final stage is the substitution of conjunctive tissue for fibrin-mesh followed by the healing of the tissue. In process, the solidified fibrin adhesive is completely absorbed (6).
THERAPEUTIC INDICATIONS
In addition to the therapeutic endoscopic procedures discussed here, fibrin glue sealants can be used in many surgical procedures, including cardiovascular, thoracic, vascular, abdominal surgery, and neurosurgery.
It has been demonstrated that these agents can reduce the incidence of surgical complications including hemorrhaging and dehiscence. They can also reduce the time of surgery and hospital stay. They can reduce fluid secretion, the necessity for insertion of thorax drainage and the complications related to procedures such as thoracic surgery for treatment of pneumothorax. They also encourage hemostasia in patients with hemorrhaging ulcers. In addition, they reduce the incidence of infection and filtration of cerebrospinal fluid fistula in neurosurgical procedures (1-3, 7).
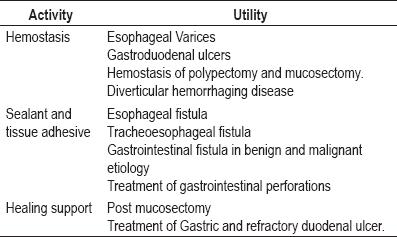
The use of these sealants is indicated in almost every surgical specialty including without exception endoscopic procedures where hemostatic activity is required such as cavity sealing, tissue adhesion, and support for healing wounds (1, 2, 5,7). Various works have supported the use of these agents in gastrointestinal hemorrhage control caused by stomach and duodenal ulcers. The utility of these agents has also been demonstrated in hemorrhages caused by complicated diverticular disease, as well as in therapeutic support for fistula cases, complex treatment of gastrointestinal perforations, and benign and malignant etiologies (9-13, 15).
Its use in treatment for esophageal varices, stimulating healing of chronic ulcers and even in the treatment of Mallory Weiss syndrome has also been reported (9-13,16) (table 1). Our cases included a patient with gastric perforation and three patients with complicated digestive hemorrhaging recommended for surgery, but with high surgical risks (for which the use of this product was decided upon).
Table 1. Indications for endoscopic use of fibrin sealants.
In conclusion, the use of fibrin glue sealants is safe and effective when appropriately indicated. However, these products cannot be administrated in hypersensitive cases associated with bovine proteins (aprotinin). Usage precautions or restrictions must be taken into account during pregnancy and nursing.
An anaphylactic reaction risk exists in cases of injection into tissue or blood vessels. There is also a risk of thromboembolic complications during intravascular injection (1, 5, 7, 8, 14).
It is important to remember that to avoid the excessive granulation tissue formation and assure gradual absorption of fibrin adhesive, a thin layer of solution should be applied.
On the other hand, the adhesive solidification speed depends on thrombin concentration. The adhesive takes a minute to solidify if a 4 ulK/ml thrombin concentration is used, but solidification occurs in just a few seconds if a 500ulK/ml concentration is used.
A greater thrombin concentration is used to obtain local hemostasia, whereas a 4ul/ml concentration is more appropriate for sealing tissue wounds because it gives more time for the edges of the wound (5, 7-9).
Finally it should be noted that these agents have became an additional tool for treating complex hemorrhaging and gastrointestinal fistula in selected patients. However, they are not the first option for gastric and duodenal ulcer treatment for which sclerosants and mechanical and thermal methods are most common.
REFERENCES
1. Brett Reece T, Thomas S. Maxey TS, Kron IL. A prospectus on tissue adhesives. Am J Surg 2002; 187: 40-44.
2. Singer AJ, Thode HC. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg 2004; 182: 40-44.
3. Sachdeva AK, Loiacano LA, Amiel GE, et al. Variability of clinical (residents entering training programs in surgery. Surgery 1995; 118: 300-308.
4. Holcomb JB, Pusateri AE, Hess JR, et al. Implications of new fibrin sealant technology for trauma surgery. Surg Clin North Am 1999; 77: 943-952.
5. Spotnitz WD, Welker RL. Clinical uses of fibrin sealant. En: Mintz PD, ed. Transfusion Therapy: Clinical Principles and Practice. Bethesda: AABB Press 2000. p. 199-221.
6. Joch C, Witzke G, Groner A, et al. Clinical safety of fibrin sealants. Presented at the IXth World Conference of Cardio-Thoracic Surgeons, November 14-17, 1999, Lisboa, Portugal.
7. Pankaj S, Mankad M, et al. The role of fibrin sealants in hemostasis. The American journal of surgery 2001; 182: 21s-28s.
8. Mackie IJ. The biology of haemostasis and thrombosis. En: weatherall DJ, Ledingham JGG, Warrell DA. Eds Oxford Textbook of medicine, 3rd ed. Oxford: Oxford University press 1996. p. 3613-27.
9. Herold G, Stange EF. Sclerotherapy of duodenal varices using a fibrin tissue sealant. Endoscopy 1993; (25)5: 371-372.
10. Andress HJ, Mewes A, Lange V. Endoscopic hemostasis of a bleeding diverticulum of the sigma with fibrin sealant Endoscopy 1993; (25)2: 193.
11. Kubba AK, Lessells A, Palmer KR. Experimental studies of injection therapy for ulcer haemorrhage in rabbits Br J Surg 1997; (84)4: 551-554.
12. Lange V, Maiwald G, Souvatzi T, Meyer G. Endoscopic approaches for occlusion of fistulas. En Schlang G, Wayand W (eds). Fibrin Sealing in Surgical and Nonsurgical Fields: Endoscopy. Berlin, Springer 1995. p. 58-64.
13. Akhras J, Tobi M, Zagnoon A. Endoscopic fibrin sealant injection with application of hemostatic clips: a novel method of closing a refractory gastrocutaneous fistula. Dig Dis Sci 2005; (50)10: 1872-1874.
14. Biblioteca Cochrane Plus (ISSN 1745-9990). De La Biblioteca Cochrane Plus, número 2, 2008. Oxford, Update Software Ltd.
15. Goh P, Kum M, Toh E. Endoscopic patch closure of malignant esophagotracheal fistula using Histoacryl glue. Surg Endosc 1994; 1434-1435.
16. Lucantoni R, Cicconi M, Mestichelli A, Moretti V, Colombati M. Use of fibrin tissue adhesive (Tissucol) in Mallory-Weiss syndrome. G Chir 1989; 10(6): 330-2.
1. Brett Reece T, Thomas S. Maxey TS, Kron IL. A prospectus on tissue adhesives. Am J Surg 2002; 187: 40-44. [ Links ]
2. Singer AJ, Thode HC. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg 2004; 182: 40-44. [ Links ]
3. Sachdeva AK, Loiacano LA, Amiel GE, et al. Variability of clinical (residents entering training programs in surgery. Surgery 1995; 118: 300-308. [ Links ]
4. Holcomb JB, Pusateri AE, Hess JR, et al. Implications of new fibrin sealant technology for trauma surgery. Surg Clin North Am 1999; 77: 943-952. [ Links ]
5. Spotnitz WD, Welker RL. Clinical uses of fibrin sealant. En: Mintz PD, ed. Transfusion Therapy: Clinical Principles and Practice. Bethesda: AABB Press 2000. p. 199-221. [ Links ]
6. Joch C, Witzke G, Groner A, et al. Clinical safety of fibrin sealants. Presented at the IXth World Conference of Cardio-Thoracic Surgeons, November 14-17, 1999, Lisboa, Portugal. [ Links ]
7. Pankaj S, Mankad M, et al. The role of fibrin sealants in hemostasis. The American journal of surgery 2001; 182: 21s-28s. [ Links ]
8. Mackie IJ. The biology of haemostasis and thrombosis. En: weatherall DJ, Ledingham JGG, Warrell DA. Eds Oxford Textbook of medicine, 3a. ed. Oxford: Oxford University press 1996. p. 3613-27. [ Links ]
9. Herold G, Stange EF. Sclerotherapy of duodenal varices using a fibrin tissue sealant. Endoscopy 1993; (25)5: 371-372. [ Links ]
10. Andress HJ, Mewes A, Lange V. Endoscopic hemostasis of a bleeding diverticulum of the sigma with fibrin sealant Endoscopy 1993; (25)2: 193. [ Links ]
11. Kubba AK, Lessells A, Palmer KR. Experimental studies of injection therapy for ulcer haemorrhage in rabbits Br J Surg 1997; (84)4: 551-554. [ Links ]
12. Lange V, Maiwald G, Souvatzi T, Meyer G. Endoscopic approaches for occlusion of fistulas. En Schlang G, Wayand W (eds). Fibrin Sealing in Surgical and Nonsurgical Fields: Endoscopy. Berlin, Springer 1995. p. 58-64. [ Links ]
13. Akhras J, Tobi M, Zagnoon A. Endoscopic fibrin sealant injection with application of hemostatic clips: a novel method of closing a refractory gastrocutaneous fistula. Dig Dis Sci 2005; (50)10: 1872-1874. [ Links ]
14. Biblioteca Cochrane Plus (ISSN 1745-9990). De La Biblioteca Cochrane Plus, número 2, 2008. Oxford, Update Software Ltd. [ Links ]
15. Goh P, Kum M, Toh E. Endoscopic patch closure of malignant esophagotracheal fistula using Histoacryl glue. Surg Endosc 1994; 1434-1435. [ Links ]
16. Lucantoni R, Cicconi M, Mestichelli A, Moretti V, Colombati M. Use of fibrin tissue adhesive (Tissucol) in Mallory-Weiss syndrome. G Chir 1989; 10(6): 330-2. [ Links ]











 text in
text in