Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev Col Gastroenterol vol.26 no.4 Bogotá Oct./Dec. 2011
Osteoporosis in patients with chronic liver disease: An unfamiliar late complication
Hernán David García Botina, MD (1), Nathalia Córdoba Ramírez, MD (1), Juan Ignacio Marín, MD (2), Juan Carlos Restrepo Gutiérrez, MD, MSc PhD. (2)
(1) Gastroenterology and Hepatology Unit, Faculty of Medicine, Universidad de Antioquia. Medellín, Colombia.
(2) Gastroenterology and Hepatology Unit, Faculty of Medicine, Universidad de Antioquia. Hospital Pablo Tobón Uribe. Medellín, Colombia.
Translation from Spanish to English by T.A. Zuur and The Language Workshop
Received: 21-05-11 Accepted: 11-10-11
Abstract
Hepatic osteodystrophy is a frequent late complication in chronic liver diseases in which patients usually present bone mineral density reduction, osteopenia, osteoporosis and fractures. Strategies to decrease incidence, avoid comorbidity and improve patient quality of life have yet to be implemented in clinical practice. Hepatic osteodystrophys pathophysiology is poorly understood. There is controversy about the use of screening tests especially regarding which patients are eligible, at what moment of the disease and with what frequency. Risk factors which are dependent on liver disease and other risk factors which are not liver disease dependent have been identified, all of which affect the natural history of hepatic osteodystrophy and all of which must be taken into account for screening, checkups and treatment. Recommendations for treatment are widely discussed but focus mainly on reduction of risk factors, antiresorptive drugs, calcium supplements and vitamin D.
Key words
Hepatic osteodystrophy, osteoporosis, chronic liver disease, bone mineral density, fractures.
INTRODUCTION
Liver cirrhosis, especially at reaches chronic levels, creates a series of changes in the organism. The term hepatic osteodystrophy usually refers to osteomalacia and osteoporosis in the presence of chronic liver disease (1). A Ber association has been found between cirrhosis and osteoporosis than between cirrhosis and osteomalacia. While osteomalacia is found infrequently among cirrhosis patients (5), 12 to 55% of these patients have been found to have osteoporosis in different studies (2-4): 50% of hepatitis C virus patients, 10% of hepatitis B virus patients and, 30% of patients with alcoholic liver disease and autoimmune disease. Osteomalacia is usually reported to be related to cholestatic diseases such as primary biliary cirrhosis (PBC) and sclerosing cholangitis (6).
Although the pathophysiology of osteoporosis in cirrhotic patients is not completely understood, it primarily affects the trabecular bone and is characterized by diminished bone mineral density (BMD), poor functioning of osteoblasts, increased osteoclastic cell activity and low levels of osteocalcin (6).It is known that osteoporosis is directly related to the type, severity and progression of liver disease.
Cirrhotic patients present lower levels of 25-hydroxyvitamin D and 1,25 dihydroxyvitamin D. They also have diminished bone mineral density, most frequently in the spine.
The diagnosis of osteoporosis in cirrhotic patients requires high clinical expertise because there are no symptoms during the initial stages.
PREVALENCE OF OSTEOPOROSIS IN PATIENTS WITH CHRONIC LIVER DISEASE
The prevalence of osteoporosis in chronic liver diseases is summarized in Table 1 (5), which summarizes results from studies performed mainly on patients with chronic cholestatic diseases, primary biliary cirrhosis and a study of patients with varied liver diseases.
Table 1. Prevalence of osteoporosis and fractures in patients with liver disease.
Four diagnostic categories (9) have been established based on the percentages of osteopenia and osteoporosis reported by the European Foundation for Osteoporosis and Bone Disease, the National Osteoporosis Foundation in the United States and by the World Health Organization WHO (7, 8).
- Normal (T score): mineral bone density (MBD) less than 1 standard deviation (SD) below the T score.
- Low bone mass or osteopenia: MBD greater than 1 but less than 2.5 SD below the T score.
- Osteoporosis: MBD greater than 2.5 SD.
- Severe or established osteoporosis: MBD greater than 2.5 SD and 1 or more vertebral compression fractures.
It is important to mention that there are different densitometric criteria used for osteoporosis in different studies. Consequently Table 1 defines osteoporosis postmenopausal women as T scores greater than 2.5, but defines it as Z scores greater than 2 for men who are less than 50 years old and for premenopausal women.
A series of 185 female Spanish patients with chronic cholestasis in which the lumbar spine was affected more than the femoral neck found that up to 37% of the patients had osteoporosis (10).
A study performed by Joe George and colleagues in India in 2009 found decreased BMD in 68% of the patients diagnosed with cirrhosis. These patients were divided into groups according to etiology: 72% of the group with hepatitis B had decreased BMD, as did 100% of the group with hepatitis C and 56.7% of the alcoholic cirrhosis group. The study also showed that the loss of BMD was the same for all classifications according to the Child score (11). The impact was greater and more severe in the trabecular bone (lumbar spine) than in the cortical bones (femoral neck). The mean of densitometric measurements was -2.28 ± 1.1 in the spine, 1.27 ± 0.74 in the hips, -1.3 ± 0.8 for the trochanter; and 0.75 ± 0.86 in the femoral neck (12).
Similarly, in a large series of patients at the Mayo Clinic, Menon et al. found that up to 20% of patients with primary biliary cirrhosis had osteoporosis (16). Although osteoporosis has been proven to be more frequent among women with PBC than among healthy women (10, 16, 19), there is a still debate on whether osteoporosis is a specific complication of PBC or is simply related to postmenopausal age among women (17, 18).
The data are not yet conclusive (4, 20) for non-cholestatic disorders such as hemochromatosis (25 to 34%) (23, 30) or for disorders which are more frequent causes of chronic liver diseases such as viruses and alcohol ingestion.
Contradictory results have been obtained regarding the relation of chronic viral hepatitis and its treatment with ribavirin and interferon and osteodystrophy. There have been studies that show no significant relation (31) while others show prevalences of nearly 100% (12). Non-conclusive evidence has been found regarding the relation between antiretroviral therapy for hepatitis and the development of unprecedented osteopenia (6, 32-37).
Alcohol consumption leads to abnormal liver function, is directly related to decreased BMD, and doubles risk of fractures. High risk levels of alcohol consumption can be defined by the following criteria (38):
- Men: 280 g/week (40g/day) of pure alcohol
- Women: 168 g/ week (24g/ day) of pure alcohol
- 80 grams/day, at least once a month, whether or not the weekly limit is exceeded
- Any consumption by pregnant women and minors or by people with pathologies for which alcohol consumption is discouraged or whose treatments are incompatible with alcohol consumption.
Various studies have shown that a high percentage of patients with chronic alcoholism have a significantly decreased BMD. Up to 29% have osteoporosis regardless of liver involvement (39-43).
It has been shown that after two or more years of abstinence there is significant bone mass improvement and the reduction of fracture risk (41).
24-38% of patients with chronic liver disease have osteoporosis according to the densitometric criteria reported in Table 1 (5). Guichelaar et al. conducted a study of 360 patients with advanced cholestatic disease (PBC and primary sclerosing cholangitis) and found that 38% had osteoporosis, 39% osteopenia and 23% had normal bone mass.
Other series show percentages from 12% to 55% in patients varying in age, etiologies, degree of liver disease, nutritional state and hypogonadism (1-4).
Many patients with terminal liver disease are transplant candidates (44). Transplantation has been associated with osteoporosis and increased risk of fractures, especially lumbar fractures, for several decades (1, 5). Osteoporosis and fractures occur primarily in the first few months following transplants, a period that is characterized by significant weight loss due to factors such as administration of immunosuppressants and glucocorticoids (reviewed below). It has been proven that BMD decreases by about 6% three months after transplantation. After adequate monitoring, lumbar BMD reaches reference values two years after surgery. However, femoral BMD may take up to 5 years to reach reference values.
Transplantation is an additional cause of osteoporosis and fractures, particularly during the first months after surgery, a period associated with a high rate of bone mass loss (5, 10, 45).
PREVALENCE OF FRACTURES AMONG PATIENTS WITH CHRONIC LIVER DISEASE
Fracture prevalence among patients with chronic liver disease ranges from 7% to 35% in the studies shown in Table 1 (4, 5, 16, 19). A recent study by Parés and Guañábens of 170 patients with primary biliary cirrhosis showed an 11.2% prevalence of vertebral fractures, 12.2% for non-vertebral fractures and 20.8% for general fractures (10). Although prevalence of fractures among patients with alcoholic disease has hardly been evaluated, one study found that fractures were present in 36% of 76 men with alcoholic cirrhosis (22). In effect, this study has elated alcohol consumption to fractures. The appearance of osteoporotic fractures was evaluated in a meta-analysis of patients who did not consume alcohol and those who drank more than two glasses a day. That study found that the latter group had a greater risk of hip fractures and diminished BMD (46). Vertebral fractures are frequent among terminal cirrhosis patients and among transplant patients, especially during the first 6 to 12 months (22, 24, 41) when incidences range from 22% to 65% (16). The highest fracture rates are found in immunosuppressed patients with cholestatic diseases (5).
PATHOPHYSIOLOGY OF HEPATIC OSTEODYSTROPHY
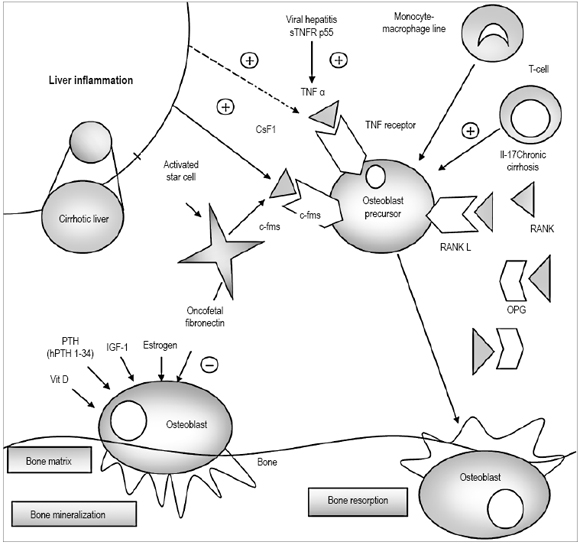
The skeletal system is one of the main systems of the organism. Its active cellular components and bone matrix have several key functions, the most important of which is protection and support of the soft tissues (e.g. the cardiovascular system), support for the muscular system allowing for body movement, provision of a reservoir of ions such as calcium and phosphorous, as an active deposit of bone marrow which plays a leading role in hematopoiesis (47) (Figure 1).
Figure 1. Pathophysiology of hepatic osteodystrophy. Continuous arrows represent established routes, discontinuous lines represent routes that have insufficient evidence.Osteoclast precursors are activated by pro-inflammatory cytokines like TNF a, whose levels increase in patients with viral hepatitis and alcoholic liver disease. Colony stimulating factor 1 (Csf1) binds to its receptor c-fms to induce osteoclastogenesis. An increase in Csf1 levels has been identified in patients with cholestatic liver disease which might be induced by inflammation of this organ. IL-17 is a cytokine produced by a subset of T lymphocytes (Th 17 cells). Their numbers increase in patients with alcohol related liver disease which may induce bone loss. RANK-L is a key regulator for osteoclast functioning. It binds to its RANK receptor to induce osteoclastogenesis. OPG is a decoy receptor that inhibits RANK-L. The RANKL-RANK-OPG axis may or may not have an important role in bone loss associated with chronic liver disease. Vitamin D, PTH, IGF-I and oncofetal fibronectin affect the formation of osteoblasts. The oncofetal fibronectin produced by activated star cells suppresses osteoblasts and bone formation in patients with primary biliary cirrhosis. Intermittent HPTH 1-34 secretion stimulates osteoblasts into functioning but has not been studied in patients with liver disease (50). Modified from: Nakchbandi A, Schalk W. Current Understanding of Osteoporosis Associated with Liver Disease 2009; 6(11): 660-70. Review.
Bone regulation maintenance depends on bone density and bone quality. The latter is understood as architecture, bone renewal, accumulation of injuries, and mineralization.
Any rupture in the balance of bone regulation can lead to fragility and increased risk of fracture. Support for this conclusion is to be found in the statistics which indicate that the probability of a 50 year old person developing a hip fracture is 14% for women and 5% to 6% for men (48)while the probability that postmenopausal women will develop some degree of vertebral deformity is 25% (49).
Osteoclasts are multinucleate cells, whose progenitors are circulating monocytes that have the important function of degrading the bone matrix. Osteoblasts are cells that originate from mesenchyme and are involved in the production of proteins for the skeletal matrix and mineralization of the bone system after the osteoclasts do their job. The activities of osteoclasts and osteoblasts must remain in close balance to maintain constant bone mass. It is believed that factors liberated by osteoclasts represent the activating signals for osteoclasts while osteoblasts in turn liberate substances such as receptor activator of nuclear factor kappa-B ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) which are responsible for stimulating osteoclasts. RANKL is primary cytokine involved in this process because once it has bonded to its receptor in the osteoclast the cell can differentiate and activate (50). Osteoblasts also segregate osteoprotegerin (OPG), a decoy receptor which prevents the RANK RANK-L connection (51). It is also important to mention that other hormones, cytokines and vitamins also act in the microenvironment of osteoblasts and osteoclasts to regulate different aspects of mineralization and bone reabsorption.
OSTEOPOROSIS AND RISK OF FRACTURES
Osteoporosis is defined as "a systemic skeletal disease, characterized by low bone mass and deterioration of the bone tissues microarchitecture which increases bone fragility and consequently increases risk of bone fractures" (48). This definition has also been adopted by the WHO. Its clinical significance lies in the fractures which are its consequences and in the ensuing morbidity and mortality which can result (52).
The etiology of osteoporosis in patients with chronic liver disease has yet to be clarified. Various authors have proposed that many factors can intervene in the formation of liver osteodystrophy. Among them are nutritional state, active alcoholism, physical exercise, toxins such as tobacco, diseases such as diabetes, bone fractures in adults and the severity of liver disease. Nutritional state can be evaluated by skinfold measurement and the severity of cirrhosis can be evaluated with the Child-Pugh classification (11). The other the variables are determined by the patients clinical history (53).
The table 2 presents risk factors for the development of osteoporosis which are independent of bone mineral density (1).
Table 2. Risk factors for osteoporosis other than BMD.
Other recently described factors that have become important are (54)rheumatoid arthritis which it may appear in an autoimmune context such as autoimmune hepatitis and Type 2 diabetes mellitus which is common in patients with fatty liver disease associated with metabolic syndrome.
These factors are generally found in patients with chronic liver disease, as well as malnutrition, excessive alcohol consumption, hypogonadism, and corticosteroid consumption (1).
A study by Joe George et al (12)found that low physical activity, poor exposure to sunlight, vitamin D deficiency and low IGF 1 levels were related to diminished BMD and a greater risk of fractures in patients with cirrhotic diseases.
A study of 76 men who drank more than 216 grams/day for more than 24 years found that only 22% presented abnormal liver histology. Although 30% had vertebral compression fractures, only 4% were symptomatic. Accumulated alcohol consumption in men has also been found to be inversely proportional to bone mineral density (45).
Reduction in serum testosterone levels, which occurs actively in patients with alcoholism or cirrhosis, may contribute to osteoporosis. Low levels of vitamin D have also been reported in one third of alcoholics with low BMD and it has been demonstrated that supplementing diet with vitamin D improves bone mineral density in some patients wrists (55).
Long term hypogonadism in men is associated with an increased remodeling and reduction of bone formation which is reversible with adequate hormone replacement (1). In hypogonadism, free testosterone levels increase up to 75% in patients with cirrhosis who need transplants, but it is not related to the etiology of cirrhosis (56, 57).
DIAGNOSIS
Bone densitometry is a precise procedure for establishing a diagnosis for osteoporosis (58).
Patients with chronic liver disease should be evaluated for osteoporosis and osteopenia, especially those with the following risk factors: chronic alcohol consumption, smoking, early menopause, body mass index (BMI) < 19k/m², male hypogonadism, secondary amenorrhea for more than 6 months, hemochromatosis, family history of fractures and osteoporosis, and glucocorticoid treatment of 5mg/day for more than 3 months (5).
However, there is controversy about what is the right moment to perform bone densitometry. Some authors believe that it should be performed when a patient has had previous fractures, exposure to glucocorticoids and prior to a liver transplant. In addition, BMD evaluation seems appropriate for primary biliary cirrhosis, chronic cholestasis, cirrhosis, some individuals with risks for fractures mentioned above and for those who have received a transplant (19).
Another topic of discussion that arises frequently when defining BMD measurements is that it should be performed every year or two in patients with chronic cholestasis with risk factors, patients who have been treated with glucocorticoids and liver transplants. Measurements every three years should be performed on patients with normal test results and those with low risk of developing osteoporosis (5).
The American Gastroenterological Association guidelines suggest that bone mineral density should be measured in all patients diagnosed with primary biliary cirrhosis and patients with bilirubin three times the upper limit. A study performed at the Mayo Clinic found that 26% of patients with primary sclerosing cholangitis require liver transplants and also met the criteria for osteoporosis. For this reason this group of patients received periodic mineralization tests (1, 3).
Screening tests must be performed to prevent fractures and their recurrence (59) because they are associated with declining quality of life. Lumbar and femoral bone densitometry, lumbar and dorsal x-rays are recommended to rule out vertebral fractures. Measurement of serum calcium, parathyroid hormone, phosphorous and vitamin D is also recommended. Perform of renal function tests and PTH measurements in patients with chronic cirrhosis complications such as type-2 hepatorenal syndrome are also important since this condition could worsen bone mineralization.
Measurement of biochemical markers for bone turnover such as alkaline phosphatase, serum osteocalcin, type-I serum peptide (formation), N-telopeptide, C-telopeptide, Deoxypyridinoline (resorption) can be useful for monitoring individual reactions to treatment for osteoporosis (60). However, there is little information on how this is modified by chronic liver disease given that the degree of hepatic fibrosis can interfere with the markers levels (59, 61). Bone biopsies are only indicated when mineralization disorders are suspected, and these disorders are rare in liver diseases (62).
PREVENTION AND TREATMENT
Most important among measures for prevention of BMD loss and development osteoporosis is decreasing risk factors such as alcohol, body mass index and smoking. Physical exercise is also recommended for patients who do not have other contraindicating pathologies.
A study of 75 cirrhotic men performed in the Basurto Hospital in Bilbao, Spain found that 82.3% of the participants showed bone loss in the lumbar spine and 17.7% in the necks of both femurs. The body fat percentage was 28.92% in non-osteopenic patients and 27.69% in osteopenic patients. The body fat percentage was also slightly lower for the group with osteopenia (9). Even though this study concluded that fat percentages and nutritional states were unrelated to bone mass loss, other sources support the relation between poor nutritional state and BMD.
It is important for patients to receive 1,000mg to 1,500mg daily calcium supplements and either 260mgr of hydroxyvitamin D for two weeks or 800U of oral vitamin D3 daily (5000 U/week). When it is possible to perform periodic tests it is even desirable to set doses at levels required to maintain normal levels (5). Since studies have not yet been performed to establish vitamin D supplement and calcium dosages for patients with chronic liver diseases, a simple scheme could be 800 UI of vitamin D and 1 gram of calcium per day.
A study of 25 patients with alcoholic cirrhosis and low serum vitamin D levels found that administering hydroxyvitamin D3 raised BMD above initial levels. Another study of 76 patients with cirrhosis showed that the use of 1 alpha 25 hydroxyvitamin D3 resulted in brief increases of BMD in the lumbar spine though no data was obtained on whether the supplement really diminishes risk of fracture. Patients with poor absorption may require higher doses (63, 64).
Hormone replacement has been poorly studied in patients with chronic liver diseases since it is considered that high levels of ethinylestradiol may worsen the initial condition especially in women whose hepatic fibrosis is in advanced stages. Nevertheless, several studies have shown that hormone replacement can be used safely in this group of patients. If possible, hormones should be administered subdermally since this minimizes the impact on the diseased liver. The recommended dosage is 50mcg/day, and the duration of treatment should be between 5 and 10 years, even though there are guidelines yet that recommend specific treatment time. Even though the relation between BMD and hormone replacement has not been thoroughly studied, the data which does exist shows increments in bone mineralization. Similarly, even though testosterone replacement has been proven to be beneficial for BMD in male patients with hypogonadism, it has not been studied in patients with chronic liver diseases (5, 59, 61). The percentage of cirrhotic patients with hypogonadism is unknown but believed to be high, especially in the late stages of the disease. It is known that high levels of testosterone can increase the risk of hepatocellular carcinoma which is why the risk-benefit trade-off of replacement therapy should be evaluated for each patient. As with estrogen, the preferred method of administration is subdermal.
Bisphosphonates, widely used in osteoporosis treatment, have had favorable results for BMD and in decreasing vertebral and non-vertebral fracture risk. There are no studies comparing the effectiveness of different bisphosphonates. 10 to 70 mg doses of alendronate may be used weekly, but it must be noted that it can cause esophageal ulcerations which is why it must be used with precaution on patients with portal hypertension that might develop esophageal varices. This complication has not been reported with Risedronate. Cyclic etidronate has been used safely for several years without complications. The use of bisphosphonates has become important in preventive treatment of osteoporosis in patients who receive glucocorticoids for underlying diseases including autoimmune hepatitis and primary biliary cirrhosis and following transplants. This treatment has resulted in BMD improvement in all of these types of patients. Zoledronic acid, a powerful bisphosphonate, significantly decreases acute bone loss following transplants. It is administered 7 days after transplantation and then at the end of the first, third, sixth and ninth months after transplantation (1, 65).
Bisphosphonates must be taken 2 hours before breakfast, and immediate consumption of other medicines or supplements such as calcium which bond with and inactivate bisphosphonates must be avoided. The use of low or pulsatile doses of pulsatile parathyroid hormone (PTH) contributes to bone formation and remodeling, although it has not been studied in patients with chronic liver disease (5, 59, 61).
Calcitonin has been used in postmenopausal women with osteoporosis to prevent vertebral fractures and increase mineralization, but its results have not been entirely satisfactory. Calcitonin administered intramuscularly or subdermally has yet to be evaluated in patients with chronic liver diseases. For this reason its use is not recommended and is limited to a last option when antiresorptive drugs do not have any effect or are counter indicated. The recommendations of the American Gastroenterogical Association about osteoporosis in patients with liver disorders supports the treatments mentioned above (64).
CONCLUSIONS
- Hepatic osteodystrophy is a rarely used term in daily clinical practice. It refers to the presence of osteopenia or osteoporosis in patients affected by chronic liver diseases. Prevalence lies somewhere between 12% and 55% according to various studies. Prevalence is related to the type, evolution and severity of these diseases.
- It was found that the prevalence of osteoporosis among patients with hepatotropic virus infection is up to 100% up to 56.7% among patients with alcoholic cirrhosis.
- Although the pathophysiology of osteoporosis in cirrhotic patients is not completely understood, it mainly affects the trabecular bone and is characterized by decreased bone mineral density, poor functioning of osteoblasts, increased osteoclastic cell activity and low levels of osteocalcin.
- Cirrhotic patients present decreased 25 hydroxyvitamin D and 1.25 dihydroxyvitamin D levels which may lead to osteomalacia.
- There are no specific densitometric criteria for diagnosing hepatic osteodystrophy.
- There are no reported differences in the prevalence of osteoporosis and fractures according to the Child Pugh classification.
- The period after a liver transplant is critical since bone mineral density decreases at an accelerated rate in part due to prolonged use of immunosuppressants and significant loss of body fat. These are important risk factors in the development of osteoporosis.
- The prevalence of fractures in patients with chronic liver diseases ranges from 7% to 35%.
- Alcohol consumption notoriously decreases BMD and doubles risk of fracture, but it has been observed that after two or more years of abstinence there is a significant improvement in bone mass and fracture risk.
- Vertebral fractures are frequent in patients in terminal stages of cirrhosis and after transplantation because of factors such as highly inflammatory cytokines and the use of drugs such as glucocorticoids.
- The deregulation of the equilibrium between bone formation and resorption plays an important role in the pathophysiology of hepatic osteodystrophy because it influences inflammation and hepatic fibrosis (Interleukins, oncofetal fibronectin, tumor necrosis factor). Other factors associated with the disease such as cholestasis, chronic alcohol abuse, and immunosuppressive drugs also contribute to creation of imbalance.
- Bone densitometry is the diagnostic and monitoring procedure of choice for hepatic osteopathy. It should be performed on patients with associated risk factors (BMI <19 kg / m², alcoholism, early menopause, previous fractures, hypogonadism, etc.). Bone densitometry is also recommended prior to and following liver transplantation.
- It is currently being debated if monitoring should be performed every 1 or 2 years for patients with fracture risks and every 3 years if the initial test results are normal or show low risks.
- Lumbar and dorsal x rays are suggested to rule out vertebral fractures. Measurements of serum calcium, parathyroid hormone, phosphorus and vitamin D are also recommended.
- Measurement of bone turnover biomarkers is not standard but may be used according to the opinion of the treating physician.
- All patients require education regarding life style changes.
- All patients should receive 1gram to 1.2 gram doses of elemental Calcium and 400-800 IU of vitamin D daily. Vitamin D deficiency may be corrected by increasing serum levels of 25 hydroxyvitamin D to between 25ng/mL and30ng/mL.
- Hormone replacement (sub dermal administration is preferred) in women younger than 45 y/o with hypogonadism or menopause prevents the development of osteoporosis.
- The use of testosterone is recommended for men with hypogonadism.
- Bisphosphonates should be considered for patients with osteoporosis, vertebral fracture or for patients who must continue using corticosteroids after three months of treatment. They are approved by the Food and Drug Administration (FDA) for treating and preventing fractures, so they may be used safely for patients with chronic liver diseases (special precaution must be taken with patients who have portal hypertension).
- The use of parathormone is FDA approved even though its efficiency has yet to be evaluated in patients with hepatic dystrophy.
REFERENCES
1. Collier J. Bone Disorders in Chronic Liver Disease. Hepatology 2007; 46: 1271-8.
2. Gallejo F, Gonzalez J, Muñoz M, Mundi J, Fernandez R, Perez R, et al. Bone mineral density, serum insulin like growth factor I and bone turnover markers in viral cirrhosis. Hepatology 1998; 28: 695-9.
3. Guichelaar M, Kendall R, Schmoll J, Malinchoc M, Hay J. Bone mineral density before and after OLT; long-term follow-up and predictive factors 2006; 12:1390-402.
4. Diamond T, Stiel D, Lunzer M, Wilkinson M, Roche J, Posen S. Osteoporosis and skeletal fractures in chronic liver disease. Gut 1980; 31: 82-7.
5. Guañabens N, Parés A. Liver and bone. Archives of Biochemistry and Biophysics. 2010; 503: 84-94.
6. Wariaghli G, Mounach A, Achemlal L, Benbaghdadi I, Aouragh A. Osteoporosis in chronic liver disease: a casecontrol study. Rheumatology International. 2010; 30: 893-9.
7. Kanis J, editor. Osteoporosis y sus consecuencias. 1 ed. Londres1996.
8. Valdivia G, Szot J. Epidemiología de la osteoporosis. Boletín de la escuela de medicina 1999; 28(1-2).
9. Escalante M, Vicario F, Cubas L, Goiría J, Zuleta M, Cabarcos A, et al. Nutrición, enfermedad ósea y cirrosis alcohólica. An Med Interna (Madrid) [serial on the Internet]. 2002; 19.
10. Guanabens N, Cerda D, Monegal A, Pons F, Caballeria L, Peris P, et al. Low Bone Mass and Severity of Cholestasis Affect Fracture Risk in Patients With Primary Biliary Cirrhosis. Gastroenterology 2010; 138 (7): 2348-56.
11. Conn H. A peek at the Child-Turcotte classification. Hepatology. 1981;1:673-7.
12. George J, Ganesh H, Acharya S, Bandgar T, Shivane V, Karvat A. Bone mineral density and disorders of mineral metabolism in chronic liver disease. World J Gastroenterol 2009; 15(28): 3516-22.
13. Guanabens N, Pares A, Navasa M. Cyclosporin A increases the biochemical markers of bone remodeling in primary biliary cirrhosis J Hepatol 1994; 21: 24-8.
14. Springer J, Cole D, Rubin L, Dudek C, Harewood L. Vitamin Dreceptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology 2000; 118: 145-51.
15. Pares A, Guanabens N, Alvarez L, Osaba M, Oriola R. Collagen type Ia1 and vitamin D receptor gene polymorphisms and bone mass in primary biliary cirrhosis. Hepatology 2001; 33: 554-60.
16. Menon K, Angulo P, Weston S, Dickson E, Lindor K. Bone disease in primary biliary cirrhosis: independent indicators and rate of progression. J Hepatol 2001; 35: 316-23.
17. Newton J, Francis R, Prince M, James O, Bassendine D, Jones R. Osteoporosis in primary biliary cirrhosis revisited Gut 2001; 49: 282-7.
18. Solerio E, Isaia G, Innarella R, Farina S, Borghesio E, Framarin L. Osteoporosis: still a typical complication of primary biliary cirrhosis? Dig Liver Dis 2003; 35(5): 339-46.
19. Guanabens N, Pares A, Ros I, Caballeria L, Pons F, Vidal S. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol 2005; 42(4): 573-7.
20. Bonkovsky H, Hawkins M, Steinberg K, Hersh T, Galambos J, Henderson J, et al. Prevalence and prediction of osteopenia in chronic liver disease. Hepatology 1990; 12: 273-80.
21. Chen C, Wang S, Jeng F, Lee S. Metabolic bone disease of liver cirrhosis: Is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol 1996; 11: 417-21.
22. Monegal A, Navasa M, Guanabens N, Peris P, Pons F, Martinez de Osaba M. Osteoporosis and bone mineral metabolism disorders in cirrhotic patients referred for orthotopic liver transplantation. Calcif Tissue Int 1997; 60: 148-54.
23. Sinigaglia L, Fargion S, Fracanzani A, Binelli L, Battafarano N, Varenna M, et al. Bone and joint involvement in genetic hemochromatosis: role of cirrhosis and iron overload. J Rheumatol 1997; 24: 1809-13.
24. Ninkovic M, Skingle S, Bearcroft P, Bishop N, Alexander G, Compston J. Incidence of vertebral fractures in the first three months after orthotopic liver transplantation. Eur J Gastroenterol Hepatol 2000;12: 931-5.
25. Ninkovic M, Love S, Tom B, Alexander G, Compston J. High prevalence of osteoporosis in patients with chronic liver disease prior to liver transplantation. Calcif Tissue Int 2001; 69(6): 321-6.
26. Carey E, Balan V, Kremers W, Hay J. Osteopenia and Osteoporosis in Patients With EndStage Liver Disease Caused by Hepatitis C and Alcoholic Liver Disease: Not Just a Cholestatic Problem. Liver Transpl 2003; 9(11): 1166-73.
27. Sokhi R, Anantharaju A, Kondaveeti R, Creech S, Islam K, Van Thiel D. Bone Mineral Density among Cirrhotic Patients Awaiting Liver Transplantation. Liver Transpl 2004; 10(5): 648-53.
28. Guggenbuhl P, Deugnier Y, Boisdet J, Rolland Y, Perdriger A, Pawlotsky Y, et al. Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int 2005; 16(12): 1809-14.
29. Gonzalez J, Mundi J, Casado F, Abadia A, Ibanez J. Bone Mineral Density and Serum Levels of Soluble Tumor Necrosis Factors, Estradiol, and Osteoprotegerin in Postmenopausal women with Cirrhosis after Viral Hepatitis. Clin Endocrinol Metab 2009; 94(12): 4844-50.
30. Valenti L, Varenna M, Fracanzani A, Rossi V, Fargion S, Sinigaglia L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int 2009; 20(4): 549-55.
31. Nanda K, Ryan E, Murray B, Brady J, McKenna M, Nolan N, et al. Effect of chronic hepatitis C virus infection on bone disease in postmenopausal women. Clin Gastroenterol Hepatol 2009; 7(8): 894-9.
32. Yenice N, Gumrah M, Mehtap O, Kozan A, Turkmen S. Assessment of bone metabolism and mineral density in chronic viral hepatitis. Turk J Gastroenterol 2006; 17: 260-6.
33. Schiefke I, Fach A, Wiedmann M, Aretin A, Schenker E, Borte G, et al. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol 2005; 11: 1843-7.
34. Hofmann W, Kronenberger B, Bojunga J, Stamm B, Herrmann E, Bucker A, et al. Prospective study of bone mineral density and metabolism in patients with chronic hepatitis C during pegylated interferon alpha and ribavirin therapy. J Viral Hepat 2008; 15(11): 790-6.
35. Solis J, Castellano G, Fernandez I, Munoz R, Hawkins F. Decreased bone mineral density after therapy with alpha interferon in combination with ribavirin for chronic hepatitis C. J Hepatol 2000; 33: 812-7.
36. Schnitzler C, Solomon L. Bone changes after alcohol abuse. S Afr Med J 1984; 66(19): 730-4.
37. Lalor B, France M, Powell D, Adams P, Counihan T. Bone and mineral metabolism and chronic alcohol abuse. Q J Med 1986; 59(229): 497-511.
38. González J. Tipos de intervención en el consumo de riesgo de alcohol. Revista de la Sociedad Española de Salud Laboral en la Administración Pública 2003; 1: 1-6.
39. Peris P, Parés A, Guañabens N, Pons F, Martínez de Osaba M, Caballería J, et al. Reduce spinal and femoral bone mass and deranged bone mineral. Alcohol and Alcoholism 1992; 27(6): 619-25.
40. Gonzalez J, García A, Bellot V, Muñoz M, Raya E, Salvatierra D. Mineral metabolism, osteoblastic funtion and bone mass in cronic alcoholism. Alcohol and Alcoholism 1993; 28(5): 571-9.
41. Peris P, Parés A, Guañabens N, Del Río R, Pons F, Martínez de Osaba M, et al. Bone mass improves in alcoholics after two years of abstinence. J Bone Miner Res 1994; 9: 1607-12.
42. Peris P, Guañabens N, Parés A, Pons F, Del Río R, Monegal A, et al. Vertebral fractures and osteopenia in chronic alcoholic patients. Calcif Tissue Int 1995; 57(2): 111-4.
43. Malik P, Gasser R, Kemmler G, Moncayo R, Finkenstedt G, Kurz M, et al. Low bone mineral density and impaired bone metabolism in young alcoholic patients without liver cirrhosis: a cross-sectional study. Alcohol Clin Exp Res 2009; 33: 375-81.
44. Hígado AEpeEd. Tratamiento de las enfermedades hepáticas y biliares. 1 ed. Berenguer J, Bruguera M, editors. Barcelona: ELBA, S.A.; 2007.
45. Peris P, Guanabens N, Pares S, Pons F, Monegal A. Vertebral fracture and osteopaenia in chronic alcoholic patients. Calcif Tissue Int 1995; 57: 111-4.
46. Berg K, Kunins H, Jackson J, Nahvi S, Chaudhry A, Harris K, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 2008; 121(5): 406-18.
47. Lafita J. Fisiología y fisiopatología ósea. Anales del Sistema Sanitario de Navarra 2003; 26: 7-15.
48. Bouillon R, Fujita T, Burckhardt P, Switzerland C, Christiansen C, Fleisch H. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. En Osteoporosis EFf, editor. Osteoporosis International; Copenhague: Am J Med 2003; p. 646-50.
49. Klibanski A, Adams L, Bassford T, Blair S, Boden S, Dickersin K, et al. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consens Statement Online; 2000 [cited 2010 noviembre 1 de 2010] http://consensus.nih.gov/2000/Osteoporosis111html.htm].
50. Nakchbandi A, Schalk W. Current understanding of osteoporosis associated with liver disease. Nat Rev Gastroenterol Hepatol 2009; 6: 660-70.
51. Caetano Lopes J, Canhao H, Fonseca J. Osteoimmunology-the hidden immune regulation of bone. Autoimmun Rev 2009; 8: 250-5.
52. WHO. Prevention and management of osteoporosis. In: Geneva, editor. Switzerland: Report of a WHO scientific group; 2003.
53. Escalante M, Franco R, Cubas L, Goiría J, Zulueta M. Cirrosis alcohólica y osteodistrofia hepática: ¿cuáles son los principales factores implicados? Gaceta Médica de Bilbao 2003; 100(2): 47-9.
54. Hippisley J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of fracture scores. BMJ 2009; 339: 1-17.
55. Mobarhan SA, Russell RM, Recker RR, Posner DB, Iber FL, Miller P. Metabolic bone disease in alcoholic cirrhosis: a comparison of the effects of vitamin D2, 25 hydroxyvitamin D or supportive treatment. Hepatology 1984; 4: 266-73.
56. Guichelaar M, Malinchoc M, Sibongo J, Clarke B, Hay J. Immunosuppressive and postoperative effects of orthotopic liver transplantation on bone metabolism. Liver Transpl 2004; 10: 638-47.
57. Kaymakoglu S, Okten A, Cakalogu Y, Boztas G, Besisik F, Tascioglu C. Hypogonadism is not related to the etiology of liver cirrhosis. J Gastroenterol 1995; 30: 745-50.
58. Parés A, Guañabens N. Osteoporosis in Primary Biliary Cirrhosis: Pathogenesis and Treatment. Clin Liver Dis 2008; 12: 407-24.
59. Gasser R. Cholestasis and metabolic bone disease a clinical review. Wien Med Wochenschr 2008; 19: 553-7.
60. Fauci A, Braunwald E, Kasper D, Hauser S, Longo D. Harrison, principios de medicina interna. 2009.
61. Collier J, Ninkovic M, Compston J. Guidelines on the management of osteoporosis associated with chronic liver disease. BMJ 2002; 50: 1-19.
62. Szulc P, Delmas P. Biochemical markers of bone turnover in osteoporosis. American Society of Bone and Mineral Research 2008: 174-9.
63. Shiomi S, Masaki K, Habu D, Takeda T, Nishiguchi S, Kuroki T, et al. Calcitriol for bone loss in patients with primary biliary cirrhosis. Journal of Gastroenterology. 1999; 34(2): 241-5.
64. Association AG. Osteoporosis in Hepatic Disorders. J Gastroenterol 2003; 125: 937-40.
65- Woo S, Hande K, Richardson P. Osteonecrosis of the Jaw and Bisphosphonates. N Engl J Med 2005; 353: 99-102.
1. Collier J. Bone Disorders in Chronic Liver Disease. Hepatology 2007; 46: 1271-8. [ Links ]
2. Gallejo F, Gonzalez J, Muñoz M, Mundi J, Fernandez R, Perez R, et al. Bone mineral density, serum insulin like growth factor I and bone turnover markers in viral cirrhosis. Hepatology 1998; 28: 695-9. [ Links ]
3. Guichelaar M, Kendall R, Schmoll J, Malinchoc M, Hay J. Bone mineral density before and after OLT; long-term follow-up and predictive factors 2006; 12:1390-402. [ Links ]
4. Diamond T, Stiel D, Lunzer M, Wilkinson M, Roche J, Posen S. Osteoporosis and skeletal fractures in chronic liver disease. Gut 1980; 31: 82-7. [ Links ]
5. Guañabens N, Parés A. Liver and bone. Archives of Biochemistry and Biophysics. 2010; 503: 84-94. [ Links ]
6. Wariaghli G, Mounach A, Achemlal L, Benbaghdadi I, Aouragh A. Osteoporosis in chronic liver disease: a case–control study. Rheumatology International. 2010; 30: 893-9. [ Links ]
7. Kanis J, editor. Osteoporosis y sus consecuencias. 1 ed. Londres1996. [ Links ]
8. Valdivia G, Szot J. Epidemiología de la osteoporosis. Boletín de la escuela de medicina 1999; 28(1-2). [ Links ]
9. Escalante M, Vicario F, Cubas L, Goiría J, Zuleta M, Cabarcos A, et al. Nutrición, enfermedad ósea y cirrosis alcohólica. An Med Interna (Madrid) [serial on the Internet]. 2002; 19. [ Links ]
10. Guanabens N, Cerda D, Monegal A, Pons F, Caballeria L, Peris P, et al. Low Bone Mass and Severity of Cholestasis Affect Fracture Risk in Patients With Primary Biliary Cirrhosis. Gastroenterology 2010; 138 (7): 2348-56. [ Links ]
11. Conn H. A peek at the Child-Turcotte classification. Hepatology. 1981;1:673-7. [ Links ]
12. George J, Ganesh H, Acharya S, Bandgar T, Shivane V, Karvat A. Bone mineral density and disorders of mineral metabolism in chronic liver disease. World J Gastroenterol 2009; 15(28): 3516-22. [ Links ]
13. Guanabens N, Pares A, Navasa M. Cyclosporin A increases the biochemical markers of bone remodeling in primary biliary cirrhosis J Hepatol 1994; 21: 24-8. [ Links ]
14. Springer J, Cole D, Rubin L, Dudek C, Harewood L. Vitamin D–receptor genotypes as independent genetic predictors of decreased bone mineral density in primary biliary cirrhosis. Gastroenterology 2000; 118: 145-51. [ Links ]
15. Pares A, Guanabens N, Alvarez L, Osaba M, Oriola R. Collagen type Ia1 and vitamin D receptor gene polymorphisms and bone mass in primary biliary cirrhosis. Hepatology 2001; 33: 554-60. [ Links ]
16. Menon K, Angulo P, Weston S, Dickson E, Lindor K. Bone disease in primary biliary cirrhosis: independent indicators and rate of progression. J Hepatol 2001; 35: 316-23. [ Links ]
17. Newton J, Francis R, Prince M, James O, Bassendine D, Jones R. Osteoporosis in primary biliary cirrhosis revisited Gut 2001; 49: 282-7. [ Links ]
18. Solerio E, Isaia G, Innarella R, Farina S, Borghesio E, Framarin L. Osteoporosis: still a typical complication of primary biliary cirrhosis? Dig Liver Dis 2003; 35(5): 339-46. [ Links ]
19. Guanabens N, Pares A, Ros I, Caballeria L, Pons F, Vidal S. Severity of cholestasis and advanced histological stage but not menopausal status are the major risk factors for osteoporosis in primary biliary cirrhosis. J Hepatol 2005; 42(4): 573-7. [ Links ]
20. Bonkovsky H, Hawkins M, Steinberg K, Hersh T, Galambos J, Henderson J, et al. Prevalence and prediction of osteopenia in chronic liver disease. Hepatology 1990; 12: 273-80. [ Links ]
21. Chen C, Wang S, Jeng F, Lee S. Metabolic bone disease of liver cirrhosis: Is it parallel to the clinical severity of cirrhosis? J Gastroenterol Hepatol 1996; 11: 417-21. [ Links ]
22. Monegal A, Navasa M, Guanabens N, Peris P, Pons F, Martinez de Osaba M. Osteoporosis and bone mineral metabolism disorders in cirrhotic patients referred for orthotopic liver transplantation. Calcif Tissue Int 1997; 60: 148-54. [ Links ]
23. Sinigaglia L, Fargion S, Fracanzani A, Binelli L, Battafarano N, Varenna M, et al. Bone and joint involvement in genetic hemochromatosis: role of cirrhosis and iron overload. J Rheumatol 1997; 24: 1809-13. [ Links ]
24. Ninkovic M, Skingle S, Bearcroft P, Bishop N, Alexander G, Compston J. Incidence of vertebral fractures in the first three months after orthotopic liver transplantation. Eur J Gastroenterol Hepatol 2000;12: 931-5. [ Links ]
25. Ninkovic M, Love S, Tom B, Alexander G, Compston J. High prevalence of osteoporosis in patients with chronic liver disease prior to liver transplantation. Calcif Tissue Int 2001; 69(6): 321-6. [ Links ]
26. Carey E, Balan V, Kremers W, Hay J. Osteopenia and Osteoporosis in Patients With EndStage Liver Disease Caused by Hepatitis C and Alcoholic Liver Disease: Not Just a Cholestatic Problem. Liver Transpl 2003; 9(11): 1166-73. [ Links ]
27. Sokhi R, Anantharaju A, Kondaveeti R, Creech S, Islam K, Van Thiel D. Bone Mineral Density among Cirrhotic Patients Awaiting Liver Transplantation. Liver Transpl 2004; 10(5): 648-53. [ Links ]
28. Guggenbuhl P, Deugnier Y, Boisdet J, Rolland Y, Perdriger A, Pawlotsky Y, et al. Bone mineral density in men with genetic hemochromatosis and HFE gene mutation. Osteoporos Int 2005; 16(12): 1809-14. [ Links ]
29. Gonzalez J, Mundi J, Casado F, Abadia A, Ibanez J. Bone Mineral Density and Serum Levels of Soluble Tumor Necrosis Factors, Estradiol, and Osteoprotegerin in Postmenopausal women with Cirrhosis after Viral Hepatitis. Clin Endocrinol Metab 2009; 94(12): 4844-50. [ Links ]
30. Valenti L, Varenna M, Fracanzani A, Rossi V, Fargion S, Sinigaglia L. Association between iron overload and osteoporosis in patients with hereditary hemochromatosis. Osteoporos Int 2009; 20(4): 549-55. [ Links ]
31. Nanda K, Ryan E, Murray B, Brady J, McKenna M, Nolan N, et al. Effect of chronic hepatitis C virus infection on bone disease in postmenopausal women. Clin Gastroenterol Hepatol 2009; 7(8): 894-9. [ Links ]
32. Yenice N, Gumrah M, Mehtap O, Kozan A, Turkmen S. Assessment of bone metabolism and mineral density in chronic viral hepatitis. Turk J Gastroenterol 2006; 17: 260-6. [ Links ]
33. Schiefke I, Fach A, Wiedmann M, Aretin A, Schenker E, Borte G, et al. Reduced bone mineral density and altered bone turnover markers in patients with non-cirrhotic chronic hepatitis B or C infection. World J Gastroenterol 2005; 11: 1843-7. [ Links ]
34. Hofmann W, Kronenberger B, Bojunga J, Stamm B, Herrmann E, Bucker A, et al. Prospective study of bone mineral density and metabolism in patients with chronic hepatitis C during pegylated interferon alpha and ribavirin therapy. J Viral Hepat 2008; 15(11): 790-6. [ Links ]
35. Solis J, Castellano G, Fernandez I, Munoz R, Hawkins F. Decreased bone mineral density after therapy with alpha interferon in combination with ribavirin for chronic hepatitis C. J Hepatol 2000; 33: 812-7. [ Links ]
36. Schnitzler C, Solomon L. Bone changes after alcohol abuse. S Afr Med J 1984; 66(19): 730-4. [ Links ]
37. Lalor B, France M, Powell D, Adams P, Counihan T. Bone and mineral metabolism and chronic alcohol abuse. Q J Med 1986; 59(229): 497-511. [ Links ]
38. González J. Tipos de intervención en el consumo de riesgo de alcohol. Revista de la Sociedad Española de Salud Laboral en la Administración Pública 2003; 1: 1-6. [ Links ]
39. Peris P, Parés A, Guañabens N, Pons F, Martínez de Osaba M, Caballería J, et al. Reduce spinal and femoral bone mass and deranged bone mineral. Alcohol and Alcoholism 1992; 27(6): 619-25. [ Links ]
40. Gonzalez J, García A, Bellot V, Muñoz M, Raya E, Salvatierra D. Mineral metabolism, osteoblastic funtion and bone mass in cronic alcoholism. Alcohol and Alcoholism 1993; 28(5): 571-9. [ Links ]
41. Peris P, Parés A, Guañabens N, Del Río R, Pons F, Martínez de Osaba M, et al. Bone mass improves in alcoholics after two years of abstinence. J Bone Miner Res 1994; 9: 1607-12. [ Links ]
42. Peris P, Guañabens N, Parés A, Pons F, Del Río R, Monegal A, et al. Vertebral fractures and osteopenia in chronic alcoholic patients. Calcif Tissue Int 1995; 57(2): 111-4. [ Links ]
43. Malik P, Gasser R, Kemmler G, Moncayo R, Finkenstedt G, Kurz M, et al. Low bone mineral density and impaired bone metabolism in young alcoholic patients without liver cirrhosis: a cross-sectional study. Alcohol Clin Exp Res 2009; 33: 375-81. [ Links ]
44. Hígado AEpeEd. Tratamiento de las enfermedades hepáticas y biliares. 1 ed. Berenguer J, Bruguera M, editors. Barcelona: ELBA, S.A.; 2007. [ Links ]
45. Peris P, Guanabens N, Pares S, Pons F, Monegal A. Vertebral fracture and osteopaenia in chronic alcoholic patients. Calcif Tissue Int 1995; 57: 111-4. [ Links ]
46. Berg K, Kunins H, Jackson J, Nahvi S, Chaudhry A, Harris K, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 2008; 121(5): 406-18. [ Links ]
47. Lafita J. Fisiología y fisiopatología ósea. Anales del Sistema Sanitario de Navarra 2003; 26: 7-15. [ Links ]
48. Bouillon R, Fujita T, Burckhardt P, Switzerland C, Christiansen C, Fleisch H. Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis. En Osteoporosis EFf, editor. Osteoporosis International; Copenhague: Am J Med 2003; p. 646-50. [ Links ]
49. Klibanski A, Adams L, Bassford T, Blair S, Boden S, Dickersin K, et al. Osteoporosis Prevention, Diagnosis, and Therapy. NIH Consens Statement Online; 2000 cited 2010 noviembre 1 de 2010 http://consensus.nih.gov/2000/Osteoporosis111html.htm. [ Links ]
50. Nakchbandi A, Schalk W. Current understanding of osteoporosis associated with liver disease. Nat Rev Gastroenterol Hepatol 2009; 6: 660-70. [ Links ]
51. Caetano Lopes J, Canhao H, Fonseca J. Osteoimmunology-the hidden immune regulation of bone. Autoimmun Rev 2009; 8: 250-5. [ Links ]
52. WHO. Prevention and management of osteoporosis. In: Geneva, editor. Switzerland: Report of a WHO scientific group; 2003. [ Links ]
53. Escalante M, Franco R, Cubas L, Goiría J, Zulueta M. Cirrosis alcohólica y osteodistrofia hepática: ¿cuáles son los principales factores implicados? Gaceta Médica de Bilbao 2003; 100(2): 47-9. [ Links ]
54. Hippisley J, Coupland C. Predicting risk of osteoporotic fracture in men and women in England and Wales: prospective derivation and validation of fracture scores. BMJ 2009; 339: 1-17. [ Links ]
55. Mobarhan SA, Russell RM, Recker RR, Posner DB, Iber FL, Miller P. Metabolic bone disease in alcoholic cirrhosis: a comparison of the effects of vitamin D2, 25 hydroxyvitamin D or supportive treatment. Hepatology 1984; 4: 266-73. [ Links ]
56. Guichelaar M, Malinchoc M, Sibongo J, Clarke B, Hay J. Immunosuppressive and postoperative effects of orthotopic liver transplantation on bone metabolism. Liver Transpl 2004; 10: 638-47. [ Links ]
57. Kaymakoglu S, Okten A, Cakalogu Y, Boztas G, Besisik F, Tascioglu C. Hypogonadism is not related to the etiology of liver cirrhosis. J Gastroenterol 1995; 30: 745-50. [ Links ]
58. Parés A, Guañabens N. Osteoporosis in Primary Biliary Cirrhosis: Pathogenesis and Treatment. Clin Liver Dis 2008; 12: 407-24. [ Links ]
59. Gasser R. Cholestasis and metabolic bone disease a clinical review. Wien Med Wochenschr 2008; 19: 553-7. [ Links ]
60. Fauci A, Braunwald E, Kasper D, Hauser S, Longo D. Harrison, principios de medicina interna. 2009. [ Links ]
61. Collier J, Ninkovic M, Compston J. Guidelines on the management of osteoporosis associated with chronic liver disease. BMJ 2002; 50: 1-19. [ Links ]
62. Szulc P, Delmas P. Biochemical markers of bone turnover in osteoporosis. American Society of Bone and Mineral Research 2008: 174-9. [ Links ]
63. Shiomi S, Masaki K, Habu D, Takeda T, Nishiguchi S, Kuroki T, et al. Calcitriol for bone loss in patients with primary biliary cirrhosis. Journal of Gastroenterology. 1999; 34(2): 241-5. [ Links ]
64. Association AG. Osteoporosis in Hepatic Disorders. J Gastroenterol 2003; 125: 937-40. [ Links ]
65. Woo S, Hande K, Richardson P. Osteonecrosis of the Jaw and Bisphosphonates. N Engl J Med 2005; 353: 99-102. [ Links ]











 text in
text in