Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev Col Gastroenterol vol.27 no.1 Bogotá Jan./Mar. 2012
State of the art liver transplantation in adults
Óscar Santos, MD, (1,2) Juan Marín, MD, (1,2) Octavio Muñoz, MD, (1,2) Álvaro Mena, MD, (1) Carlos Guzmán, MD, (1) Sergio Hoyos, MD, MSc, (1,2) Juan C. Restrepo, MD, MSc, PhD, (1,2) Gonzalo Correa, MD. (1,2)
(1) Liver Transplant and Hepatology Unit at the Universidad de Antioquia and the Hospital Pablo Tobón Uribe in Medellín, Antioquia
(2) Gastro-hepatology Group at the Universidad de Antioquia in Medellín, Antioquia
Received: 22-12-11 Accepted: 21-02-12
Abstract
Liver transplantation has become the best treatment option for cirrhosis, acute liver failure and some tumors. The great advances of recent years have yielded very good results in the long-term survival of these patients. The main complications that occur are vascular and bile duct alterations in the liver graft, relapses of the underlying disease, renal failure, opportunistic infections, and in the long term development of metabolic syndrome, cardiovascular disease, and new malignancies. Currently, several highly complex medical centers in this country perform liver transplants. For this reason it is important for scientists to know the most important aspects in the care of these patients.
Key words
Liver transplantation, cirrhosis, MELD, rejection, liver graft.
INTRODUCTION
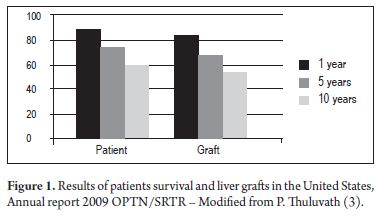
Liver transplantation (LT) is a treatment which has been accepted for treatment of advanced liver disease all over the world-wide since the 1980s (1). It is currently the therapy of choice for acute liver failure in patients with poor prognoses, for chronic liver insufficiency, for treatment of primary liver tumors and for some metabolic diseases. Great advances have been made since 1963 when Dr. Th omas Starzl performed the first successful LT (2). Currently long term patient survival and liver graft survival rates are very good (Figure 1) and provide good patient quality of life including restoration of normal daily activities (3). Th ese favorable results are due to improved surgical techniques, better perioperative care, the use of new and more eff ective immunosuppressants, and careful selection of suitable patients. There has been a progressive depuration of surgical techniques over the last 40 years which has lead to safer procedures for the patient. With the introduction of the piggyback technique which preserves the vena cava we have obtained shorter surgical times, shorter hot ischemia times with shorter anhepatic phases, and decreased blood loss without the need for venovenous bypasses. This has further decreased complications and costs making the piggyback technique the current surgical technique of choice (4). Choledocholithotomies are preferable to biliary anastomosis because they allow for physiological bile flow to the duodenum because they allow the sphincter of Oddi to continue functioning. This avoids intestinal contamination of the bile ducts and allows performance of diagnostic and therapeutic interventions (5). When the recipient's bile ducts present problems, it is possible to choose a Rouxen-Y hepaticojejunostomy. These cases include abnormal ducts such as in primary sclerosing cholangitis (PSC), absence of bile ducts such as in bile atresia, bile ducts aff ected by earlier surgery, and cases of inadequate agreement in the sizes of the donor and the recipient. Perioperative care has shown better results with rational protocols of transfusions, appropriate coagulopathy and acidosis correction, hypothermia prevention and maintenance of low central venous pressure (6). All these changes have allowed for the possibility of early extubation for a large number of patients which is associated with fewer complications including infections and renal injuries and has also resulted in shorter hospital stays and lower resource requriements (7).
INDICATIONS AND CONTRAINDICATIONS
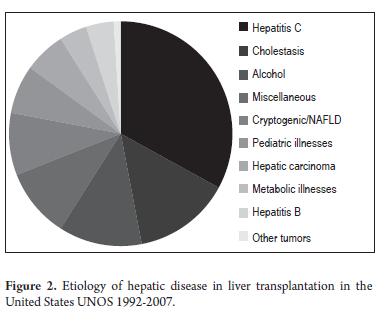
The main indication for LT is hepatic cirrhosis. Chronic hepatitis C virus (HCV) infections are the most frequent cause in the western world followed by alcohol and nonalcoholic fatty liver disease (NAFLD). In Asia and Africa the main cause of cirrhosis is chronic infection by hepatitis B Virus (HBV) (8). Other liver disease etiologies and their frequencies among liver transplant patients appear in Figure 2. In our field the main indication for transplantation is liver disease resulting from alcohol use. In the case of patients with cirrhosis, those classified B or C on the Child-Turcotte-Pugg (CTP) scale who have complications such as spontaneous bacterial peritonitis, refractory ascites, persistent/recurrent hepatic encephalopathy and persistent variceal bleeding are candidates for transplantation. For patients with cholestatic diseases transplants are indicated in moderate to severe cases hyperbilirubinemia, untreatable pruritus, and in persistent cholangitis (specifically in primary sclerosing cholangitis when cholangiocarcinoma has been ruled out and when there is a dominant stenosis that cannot be solved with interventional treatment). Transplants for hepatocellular carcinoma have had very good long term results and a low percentage of relapse. They are accepted everywhere in the world as long as the Milan criteria are fulfilled (9). This means that a single lesion must be smaller than 5 cms, or 3 lesions must be smaller than 3 cms.
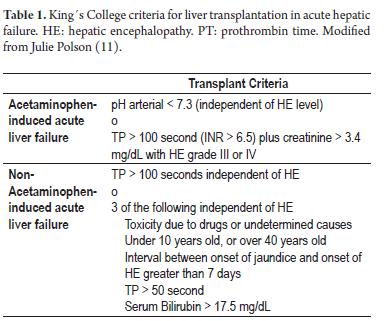
There have been multiple attempts to expand the Milan criteria to include patients with more advanced diseases for transplants. To date, the San Francisco criteria have shown good results for transplants. To date, the San Francisco criteria have shown good results for patients with a single lesion smaller than 6.5 cms or 3 injuries smaller than 4.5 cms when the sum of these lesions is less than 8 cms (10). Nevertheless these criteria have not been accepted by the entire community of medical specialists. For acute liver failure, a liver transplant is the only definitive therapy for those patients who would otherwise have poor life expectancies. Th e King´s College criteria (Table 1) list various factors which can lead to adverse prognoses and which can be used as reference points for selection of transplant patients (11). Patients who have progressively developing cirrhosis, usually those with a CTP score greater than 7 points or a MELD score greater than 11, must be referred to a liver transplantation center for early evaluation and follow-up so that transplantation can be done under the best possible conditions rather than waiting for the appearance of complications that might prevent a transplant.
Contraindications include advanced cardiopulmonary disease which has a prohibitive risk for this surgical procedure. This includes symptomatic coronary disease, severe ventricular dysfunction, advanced cardiomyopathy and severe pulmonary hypertension (SPHT) with an average pulmonary artery pressure over 50 mmHg. In cases of moderate pulmonary hypertension right catheterization must be performed with measurement of pulmonary vascular resistance (PVR). A transplant can only be considered when PVR is less than 240 dynes/cms/sec. Other contraindications are uncontrolled or cured extrahepatic malignancies (except for superficial skin cancer) according to oncological criteria, uncontrolled infectious systemic processes, lack of adequate psycho-social support and active alcohol/psycho-toxin abuse. In order to consider a patient with alcoholic cirrhosis as candidate for LT the majority of centers demand a 6 month minimum abstinence time. In addition to a determined time of abstinence, many experts currently think that adhesion to a rehabilitation program with a good social and family support is required. Obesity is not always a contraindication; only patients in morbid states of obesity with corporal mass indices over 40 are contraindicated for transplants. In recent years patients over 65 years (12) and those with HIV infections (13) have begun to be considered for liver transplantation, but only after exhaustive multidisciplinary evaluations of each particular case. Renal failure is not considered to be an LT contraindication, but patients with chronic renal disease with creatinine depuration under 30 ml/min, renal biopsy with glomerulosclerosis over 30% or hepatorenal syndrome on dialysis for more than 12 weeks should be undergo combined liver and kidney transplant.
SELECTION FOR TRANSPLANT AND ORGAN ALLOCATION
Recent continuing good results have led to a large increase in the number of LT centers around the world and, consequently, in the number of patients referred to these programs. As a result a worsening shortage of organs related to this rising demand has developed, and waiting lists are becoming longer and longer.
The process of distribution of organs from deceased donors has been one of the most controversial issues in liver transplantation. Patients were initially selected to receive donated organs on the basis of their CTP scores and the length of time they had been on the waiting list. Th is method had the disadvantages of subjective evaluations of symptoms for CTP scores, failure to incorporate renal functions in the CPT, and too little weight given to time on the waiting list. Patients had a 12% annual risk of dying while on the waiting list (14). Organ allocation must fulfill the following characteristics: transparency, objectivity and justice, plus the patient must benefit from the transplant.
This is why the MELD score (Model for End Stage Liver Disease) began to be implemented in the United States in 2002. This score was originally developed to evaluate the prognoses of patients who were being considered for transjugular intrahepatic portosystemic shunts (15). Th e MELD score is based on a logarithmic calculation of 3 variables: bilirubin level, plasma concentration of creatinine and the INR. This calculation is used to objectively predict the risk of death within 3 months. Various studies within and outside the United States validate the utility of the MELD score for allocation of organs for LT. High scores ranging from 6 to 40 points indicate greater risks of death and therefore a need for an early transplant. It has been demonstrated that since MELD began to be used, the annual number of deaths of patients on waiting lists has greatly diminished. In addition, even though very ill patients have received transplants, there is no proof that post LT survival has decreased (16). Th e use of MELD has also allowed evaluation of post transplant results in more objective way and has resulted in the finding that low MELD scores are associated with a greater risk of death for patients who undergo LT than is merely being on a waiting list (17). Of course, criticisms of this distribution system exist, especially of the fact that patients with hepatocellular carcinoma are assigned extra points allowing them an advantage over patients with other etiologies for obtaining transplantation. Moreover, sometimes there have been serious questions about the exact diagnosis of the neoplasia and its stratification (18). The fact that the MELD includes serum creatinine has led to increases in the number of transplants for patients with renal failure and in the number of combined liver-kidney procedures. This fact is critical in light of the shortage of available organs (19). Serum sodium levels below 126 mq/L at the moment a patient is placed on a waiting list are associated with 6.3 to 7.8 times increased risk of death risk (20). Consequently, the addition of serum sodium to the MELD score is considered to improve the prognostic quality especially for patients with portal hypertension and its complications. As expected, the MELD score is not appropriate for all patients and therefore some exceptions exist. For some of these such as hepatocellular carcinoma, hepatopulmonary syndrome, portopulmonary hypertension, familial amyloidosis polyneuropathy and primary hyperoxaluria, extra points are added to the MELD score to obtain a suitable degree of priority for a transplant. Other exceptions are untreatable pruritus and recurrent cholangitis.
When an organ becomes available the recipient is chosen from patients on the waiting list by looking at blood type and RH compatibility, compatibility of patient and donor's weight and height and by looking at the patients with the highest MELD scores. Several strategies have ari-sen to optimize the small number of donors compared to the number of recipients on waiting lists. One is to extend the criteria for use of organs (21) to include organs from donors over 50 years old and organs from donors who have suffered cardiac arrest. These extensions have been associated with greater risks of graft dysfunction and greater needs for re-transplantation (22). Another strategy is to use livers from live donors. This has received its greatest acceptance in Asia. It has the advantages of appropriate donor screening, minimum cold ischemia time and the possibility of choosing the optimal time for the transplant. In addition, organs are now being divided so that the left lateral segment can be used for transplantation into a child and the rest of the liver can be transplanted into an adult. Alternately, the left and right hepatic lobes can be separately transplanted into 2 adults, but with results that are not easily reproduced (23). Finally, domino LTs using livers donated by patients with metabolic diseases have been considered (24).
PRE-TRANSPLANT AND POST TRANSPLANT CONSULTATION
The objective of consultation prior to transplantation is to verify whether the patient is in suffi ciently good condition to tolerate surgery, the immune suppressants and the post transplant care. First, the absence of contraindications must be confi rmed. Hidden viral infections must be ruled out, and the patient's cytomegalovirus (CMV) status must be verified. It is vitally important to perform a CAT scan and/or MRI to rule out the presence of lesions indicating hepatocellular carcinoma or thrombosis of the portalmesenteric axis. The patients must be evaluated by a dentist, a nutritionist, a social worker, a psychologist and finally by the anesthesiologist before the procedure is approved and the patient can be included on the waiting list. During the time that the patient is on the waiting list she or he must attend routine appointments with the transplant group to evaluate her status, to rule out any appearance of new complications from cirrhosis and to treat these opportunely. Sometimes it is necessary to temporarily suspend a patient from the transplantation waiting list or even to definitively remove the patient from the list. At each medical appointment a new MELD score is calculated which can change the patient's priority for transplantation relative to other patients on the list.
Post transplant consultations are commonly done every eight to fifteen days for the first 2 months after the transplant and then every month for the rest of the first year. Th ereafter patients return for check-ups every 3 months. During these evaluations liver graft functioning is verified and the physician checks for short and long term complications which will discussed later in this article. Th is consultation is also very important for confirmation of patient adhesion to the treatment regime. At these times the physician can insist on patients adhering to good lifestyles with proper diet and exercise to avoid metabolic complications. Finally the physician can stimulate patients to restore their family, social and work activities.
IMMUNOSUPPRESSION
Since the introduction of cyclosporin to the world of transplants in 1979 (25) new immunosuppressant medicines have appeared on the scene. All of these medications have made it possible to increase graft and patient survival times and rates by reducing the number and intensity of acute and chronic rejection episodes. Current schemes use a combination of different steroids: a calcineurin inhibitor (cyclosporine or tacrolimus), and a purine synthesis inhibitor (azathioprine or mycophenolate). A strict balance between drug toxicity and drug effectiveness must be achieved.
The calcineurin inhibitors inhibit transcription of Interleukin 2 (IL 2) and are the backbone of immunosuppression. Tacrolimus is 100 times more powerful than cyclosporine. Even though tacrolimus has been shown to result in superior survival and graft loss rates as well as in fewer numbers of acute rejections, the differences shown in various clinical studies have not been great (26), and tacrolimus is related to increased frequency of diabetes mellitus and neurotoxicity. Other important complications with these immunosuppressants are renal insuffi ciency, arterial hypertension and dyslipidemia. In addition they have significant relations to tumor promoters.
Azathioprine and mycophenolate inhibit purine synthesis and block proliferation of lymphocytes. Th e main advantage of mycophenolate is the absence of toxic renal effects. Nevertheless, when it was evaluated for use as a monotherapy, higher rates of acute rejection were found. The primary adverse effects of azathioprine and mycophenolate are gastrointestinal and hematological. Although at this moment azathioprine is not widely used in the United States, it has advantages including low cost and confirmed utility for autoimmune hepatopathies.
Serious questions have arisen regarding mTOR inhibitors such as sirolimus and everolimus which block activation of T cells. These doubts are related to the relation of sirolimus to hepatic artery thrombosis (27) and to increases in mortality rates among stable patients who had been receiving calcineurin inhibitors. Other important adverse eff ects include delays in the healing of wounds, dyslipidemia, lower limb edema, proteinuria, oral pneumonia and ulcers. Initially, it was thought that the profiles of these drugs might be better for renal failure, but this assumption has not been demonstrated in clinical studies. Th eir anti-tumor and antifibrotic properties have also been spoken of, but clinical tests to determine if such advantages exist have yet to be published (28).
Immunosuppression with steroids is intended to last three to six months after LT. After this time they are normally suspended to avoid long term adverse eff ects. An exception is made in cases of basic autoimmune pathologies for which low doses of steroids are commonly continued. Other options for immunosuppression include are treatments based on antibodies and small molecules such as the anti-lymphocyte - anti-thymocyte globulins, basiliximab (antibodies against interleukin-2 receptors) and alemtuzumab (a lymphocyte depleting agent). Although these are not routinely used in LTs, they are useful for certain specific cases (especially for patients with renal failure) as agents for saving calcineurin inhibitors in the immediate post transplant period (28).
VASCULAR COMPLICATIONS
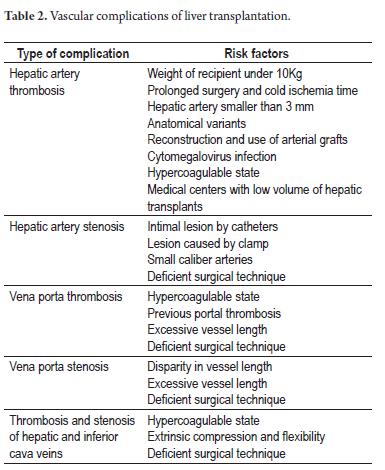
Although vascular complications have been appearing with decreasing frequency, they always generate preoccupation about the status of the patient and the hepatic graft because they can lead to graft loss, re-transplantation and death. Th e main diagnostic tool for detecting this type of complication is Doppler ultrasonography which - according to protocol - should be performed on the day following surgery. In case of doubts the diagnosis can be confirmed with computerized tomography, magnetic resonance imaging or interventional radiology studies. This is especially important when further surgical intervention is planned. Arterial complications include thrombosis, stenosis, hepatic and splenic artery pseudo-aneurisms, and gastroduodenal arterial steal syndrome. All of these can lead to graft dysfunction and biliary tree complications because they depend exclusively on arterial blood flow for irrigation. Risk factors for vascular complications are found in Table 2. Although thrombosis of the hepatic artery occurs in less than 5% of adult patients following LT, it is a catastrophic event which is related to 53% of graft losses and 30% of mortality cases (29). Thrombosis of the hepatic artery is characterized by sudden elevation of transaminases, biliary prolongation of prothrombin time, changes in the patient's mental state, stenosis and sepsis with a biliary source. Up to 30% of patients may be asymptomatic. Treatment options are surgical re-intervention, thrombolysis/angioplasty/stent by means of interventional radiology and liver retransplantation. Retransplantation is necessary for more than 50% of these patients. Hepatic artery stenosis appears in 3 to 5% of LTs (30). It occurs primarily at the location of the anastomosis. The treatment of choice is angioplasty and endoscopic placement of a stent. Currently complications of venous structures are rare: thrombosis and stenosis of the portal vein occur in less than 2% of adult patients following LT (31). These complications are characterized by abdominal pain, intestinal venous congestion, ascites, variceal hemorrhaging and hepatic biochemical alterations. Th rombosis is treated surgically or by percutaneous thrombectomy combined with anticoagulants. The primary treatment for stenosis is angioplasty with endoscopic placement of a prosthesis, although it must be recognized that for some patients the stenoses are not hemodynamically significant and do not require treatment. Complications of the hepatic veins and inferior vena cava occur in less than 1% of LT patients. They are often associated with ascites and inferior member edema, but sometimes they can lead to severe dysfunction of the liver graft as a result of obstruction of the vein leaving the biliary tract (32). Interventional radiology is the treatment of choice, but sometimes surgical reconstruction is necessary.
BILIARY COMPLICATIONS
Biliary complications, including leakage, stenoses, obstructions caused by calculi or biliary mud, and sphincter of Oddi dysfunctions, are considered to be the Achilles heel of LT (33). They occur in up to 25% of patients. Stenoses are classified as early if they occur within the first month following LT, or late if they occur after the first month. They are classified as anastomotic stenoses (AS) or non-anastomotic stenoses (NAS) according to their locations (34). AS occur later and are related to technical factors, while NAS tend to happen earlier and their physiopathology is characterized by ischemic damage and immunological damage to the biliary epithelium. Reports of biliary complications in the early history of LTs were associated with important levels of morbidity and mortality. Although these have diminished over time, biliary complications still lead to death in up to 10% of patients (35). The main risk factors for biliary complications are T-tubes, Roux-en-Y anastomoses, ischemia-reperfusion lesions, hepatic artery injuries, CMV infection, mutual incompatibility of blood groups, the use of organs donated following cardiac arrest or antecedents of primary sclerosing cholangitis (PSC) or primary biliary cirrhosis (PBS) (36). Biliary complications can be characterized by asymptomatic elevation of biochemical liver tests, or by abdominal pain, fever and recurrent sepsis. Diagnosis requires evaluation of the biliary route with noninvasive methods and then confirmation with cholangiography. When clinical suspicion is high, performance of either endoscopic retrograde cholangiopancreatography (ERCP) or percutaneous transhepatic cholangiography (PTHC) is preferable, depending on the type of anastomosis that was performed and the experience of the center in accomplishment of therapeutic procedures such as dilatations and the use of biliary stents. When suspicion is low, cholangiography using MRI can be performed with a positive predictive value and 95% greater precision for diagnosing biliary route lesions (37). In a few cases surgical re-intervention is necessary, but in other cases, especially NAS, the damage can be irreparable and hepatic re-transplantation will become inevitable.
INFECTIONS
Infections continue to be the primary cause of death in the early period following LT, and more than 60% of these patients present some type of infection during the first year (38). Extended use of some prophylaxes has changed the epidemiology of post transplant infections by emphasizing the role of trimethoprim and sulfamethoxazole to control pneumocystis jiroveci. They also have the capacity to prevent other pathogens such as nocardia, legionella, listeria, toxoplasma, isospora, cyclospora and sensitive bacteria (39). In addition nystatin can be used to avoid upper gastrointestinal tract candidiasis. The risk for certain infections changes over time following LT and is closely associated with the intensity of immunosuppression and with patient location (Table 3) (40) Infections are not easy to recognize in LT patients due to immunosuppression and the presence of noninfectious causes of fever. Consequently the sharpest diagnostic skills must be brought to bear. This may include early performance x-rays, taking of cultures and invasive procedures depending on the particular case. Caution with antimicrobials is necessary due to their toxic effects and their interactions with immunosuppressants. CMV is very relevant for LT patients. This is not only because of its cytopathological eff ects within the organs that it attacks, but also because of its immune modulating capacity that can facilitate infections by bacteria and fungi and because of its relation to major rejection episodes. It can accelerate the reactivation of HCV resulting in chronic graft dysfunction and death (41, 42) Currently there are two strategies for avoiding CMV: universal prophylaxis including the use of anti-viral drugs for at least 3 months following LT (43), and preventive treatment with routine viral load tracking or testing with CMV pp65 antigenemia assay. When viral particles are detected treatment must begin immediately even if the patient is asymptomatic (44). Both strategies have been proven to diminish CMV infections, but only universal prophylaxis prevents indirect effects of the infection. Since it is associated with increased patient and graft survival rates, it has become the treatment of choice for high risk cases such as when the donor is CMV IgG positive but the recipient is CMV IgG Negative.
ACUTE AND CHRONIC REJECTION
In spite of advances in immunosuppression, rejection of the liver graft continues to be an important cause of morbidity and graft loss in some patients (45) Rejection can be hyperacute, acute or chronic. The first is very rare, is mediated by antibodies, and is related to mutual blood group incompatibility with complex immune deposits and hepatic sinusoid complements. Acute cellular rejection is most common. It occurs most frequently in the first month aft er LT. It is categorized as slight, moderate and severe according to hepatic biopsy findings for portal inflammatory infiltrate, compromised biliary epithelium and compromised vascular endothelium. Acute cellular rejection occurs in 40 - 60% of cases with 20% of patients experiencing a second episode. Although acute cellular rejection occurs in LT at the same frequency as in transplantation of other solid organs, the majority of authors consider that acute early rejection does not have an impact on delayed functioning of the liver graft or on the survival of the patients. The main risk factors associated with rejection are HLA incompatibility, young recipients, black race, prolonged cold ischemia time, creatinine under 2.0 mg/dL, donor age over 30 years, and transplants indicated by autoimmune hepatic disease, acute hepatic failure or hepatitis C infections (46). Acute rejection is characterized by hepatic biochemical alterations, fever, abdominal pain and ascites. The diagnosis is confirmed with a hepatic biopsy. Infectious, vascular and biliary complications must be ruled out. The treatment of choice, intravenous steroids, is effective in 90% of these patients. In refractory cases it is necessary to use lymphocyte eradication therapy. After rescue with steroids it is necessary to increase the immunosuppressant basis or to switch patients to treatment with tacrolimus and/or mycophenolate if they had not already been receiving them (47). Chronic rejection now occurs in less than 5% of patients. Although it can occur early, 85% of these cases happen 6 months or more after LT (48). Risk factors include PSC or CBP transplant, mutual HLA incompatibility, CMV infections, recurrent acute rejections and grafts which are refractory to steroids. Patients present cholestatic biochemical changes, jaundice, and pruritus. In some cases they may already have cirrhosis stigmata. Histological characteristics are ductopenia and obliterative vasculopathy of medium caliber arteries. Chronic rejection is treated with tacrolimus or increased dosages of steroids with the addition of mycophenolate and in many cases by hepatic re-transplant.
RELAPSES
Relapses of the basic disease are an important problem for long term follow-up after LT. In the case of cirrhosis due to hepatitis C virus recurrence is early and universal among patients with detectable viral loads at the time of transplant. Several relapse patterns occur. The most common is chronic hepatitis no different than in patients who have not undergone transplantation except that viremia is greater and progression to cirrhosis occurs in only 5 to10 years (49) Other patients (< 10%) present fibrosing cholestatic hepatitis, a severe condition related to high viral loads in patients with strong immunosuppression. Th e virus exerts a direct cytopathological effect in the absence of an immune response. This occurs during the first months and generally leads to graft failure in the first year. Independent of patterns of recurrence, cirrhosis occurs in 30% of LT patients, with decompensation occurring in the first year. Recurrence of cirrhosis is the main cause of graft failure and death in patients who receive transplants because of HCV. Treatment includes use of pegylated interferon and ribavirin for a year with maintenance of 30% viral response rates (50). Adverse effects, dose adjustments, suspension of medicines and acute rejection occur in less than 10% of patients. Retransplantation for this group of patients is very controversial due to the high rate of loss of new grafts. In the past HBV recurrence was so high and associated with such high mortality that transplantation was contraindicated for these patients. This changed with the introduction and use of a combination of immunoglobulin for hepatitis B and an antiviral. Recurrence rates have fallen to below 10% in this population (51). Currently we are working on defining early immunoglobulin withdrawal time and appropriate duration for administration of antiviral drugs. Th e recurrence of alcoholic liver disease is between 7% and 50%, but of these only 10% to 15% have severe enough alcohol consumption that it can lead to graft loss (52). Recurrence rates for other diseases and their respective risk factors are presented in Table 4.
LONG TERM COMPLICATIONS
Insofar as patients' post LT survival rates have increased it has become necessary for physicians to familiarize themselves with the long term complications that can occur and which can endanger graft functionality, patient quality of life and which can reduce patient life expectation. Many of these complications derive from the adverse eff ects of immune suppressants, sedentary life styles and failure to adhere to an appropriate diet. Arterial hypertension (AHT) develops in 65 - 70% of patients in the fi rst year following LT (53). It normally develops early and continues over time. Calcium antagonists and beta blockers are the preferred treatment. Angiotensin-converting-enzyme inhibitor and ALTAR II should not normally be administered during the first months following LT when high doses of calcineurin inhibitors are being administered. High blood pressure has been demonstrated to be a risk factor for death in long term follow-ups (54). Dyslipidemia, which occurs in 45 - 60% of cases following LT, is related to the use of calcineurin inhibitors and mammalian target of rapamycin (mTOR). Since it is commonly refractory to changes in diet, the physician should consider early introduction of normolipemic agents such as statins and fibrates which have only slight medicinal interactions with immunosuppressants. Pravastatin interacts the least. Diabetes mellitus occurs in 30 - 40% of post transplant patients (55) with 80% occurring within the first month. Diabetes can be transitory, it soon becomes well established. It occurs more frequently in obese individuals who have had transplants due to alcoholic cirrhosis or HCV and among those who are treated with tacrolimus. An attempt to clear steroids from the patient must made, but the principal treatment is administration of insulin. The presence of diabetes mellitus both prior to and following LT has been related to greater morbidity and mortality after the first year (56). Obesity occurs in up to 40% of patients after a transplant (57), but is most frequent among patients who receive transplants because of NAFLD. The greatest weight gain occurs during the first 6 months. The previous complications can be summarized within metabolic post-transplant syndrome which occurs in up to 50% of patients. It requires lifestyle changes, particularly adherence to healthy diet of fruit, polyunsaturated vegetables and fatty acids combined with weekly exercise.
These measures must be implemented as early as possible following transplantation (58). In the long term, advanced chronic renal disease occurs in 18% of LT recipients (59). It is associated with increased morbidity and mortality (60). The glomerular filtration rate and microalbuminuria of all patients at risk for renal failure must be checked every 6 months. The use of nephro-toxic substances must be prohibited and high blood pressure and diabetes mellitus must be treated appropriately.
LT recipients are at 2 to 4 times greater risk of suff ering neoplasias than patients of the same age and gender who do not receive transplants (61). It has been proposed that immunosuppressants diminish the immune system's capacity to monitor and control malignant cells and viruses with oncogenic properties. Indeed, the predominant neoplasias among these patients are related to viruses. Th ey include non-Hodgkin's lymphoma, Kaposi Sarcoma and uterine neck carcinoma (62). Skin cancer, which is the most frequent cancer encountered among these patients, sometimes develops more rapidly than usual with early metastases. Some groups of patients have specific risk factors for certain neoplasias. For example, patients with primary sclerosing cholangitis (PSC) must be monitored for development of cholangiocarcinoma, and patients PSC concomitant with ulcerative colitis must be monitored for colon cancer. Patients with alcoholic hepatic disease have a greater risk of neoplasias of the aerodigestive tract especially those who have concomitant tobacco addictions.
Mortality due to neoplasias is one of the main long term causes of death following LT, and occurs significantly more frequently than in the general population (63).
CONCLUSIONS
Liver transplantation is the treatment of choice for advanced liver disease and for some other entities. LT has very good long term results that depend on factors related to the patient, the donor, the surgery and the immune suppression therapy. Patients with cirrhosis should be referred to liver transplantation programs so that their conditions can be improved as much as possible prior to performance of surgery at the optimum moment. Post liver transplant care is complex because of the particular complications that occur in these patients, but it is possible to achieve total recovery and restore their social, family and work lives.
REFERENCES
1. National Institutes of Health Consensus Development Conference Statement: liver transplantation--June 20-23, 1983. Hepatology 1984; 4 (1 suppl): S107-10.
2. Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg 1968; 168(3): 392-415.
3. P Thuluvath, M Guidinger, et al. Liver Transplantation in the United States, 1999-2008. American Journal of Transplantation 2010; 10(Part 2): 1003-1019.
4. Hosein Shokouh-Amiri M, Osama Gaber A, Bagous WA, et al. Choice of surgical technique influences perioperative outcomes in liver transplantation. Ann Surg 2002; 231: 814.
5. Greif F, Bronsther OL, Van Thiel DH, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg 1994; 219: 40.
6. Hendriks HG, van der Meer J, de Wolf JT, et al. Intraoperative blood transfusion requirement is the main determinant of early surgical re-intervention after orthotopic liver transplantation. Transpl Int 2005; 17: 673-9.
7. Mandell MS, Lezotte D, Kam I, Zamudio S. Reduced use of intensive care after liver transplantation: influence of early extubation. Liver Transpl 2002; 8: 676-681.
8. Fan ST, Cheung ST, Lo CM. Indications for liver transplantation in patients with chronic hepatitis B and C virus infection and hepatocellular carcinoma. J Gastroenterol Hepatol 2000; 15(Suppl): E181-E186.
9. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693-699.
10. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33: 1394-403.
11. Julie Polson and William M Lee. AASLD Position Paper: The Management of Acute Liver Failure. Hepatology 2005.
12. Cross TJ, Antoniades CG, Muiesan P, Al-Chalabi T, et al. Liver transplantation in patients over 60 and 65 years: an evaluation of long-term outcomes and survival. Liver Transpl 2007; 13(10): 1382-8.
13. Eisenbach C, Merle U, Stremmel W, Encke J. Liver transplantation in HIV-positive patients. Clin Transplant 2009; 23(Suppl 21): 68-74.
14. Trotter JF. Impact of the model for endstage liver disease score on liver transplantation. Curr Opinion Organ Transplant 2007; 12: 294-297.
15. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000; 31(4): 864-871.
16. Habib S, Berk B, Chang CC, et al. MELD and prediction of postliver transplantation survival. Liver Transpl 2006; 12: 440-447.
17. Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant 2005; 5: 307-313.
18. Freeman RB, Mithoefer A, Ruthazer R, et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: A retrospective analysis of the UNOS/OPTN data-base. Liver Transpl 2006; 12: 1504-1511.
19. Machicao VI, Srinivas TR, Hemming AW, et al. Impact of implementation of the MELD scoring system on the prevalence and incidence of chronic renal disease following liver transplantation. Liver Transpl 2006; 12: 754-761.
20. Biggins SW, Rodriguez HJ, Bacchetti P, et al. Serum Sodium predicts mortality in patients listed for liver transplantation. Hepatology 2005; 41: 32-39.
21. Renz J, Kin C, Kinkhabwala M, et al. Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation. Ann Surg 2005; 242: 556-63.
22. Malag M, Rogiers X, Broelsch CE. Liver splitting and living donor techniques. Br Med Bull 1997;53: 860-7.
23. Giacomoni A, Lauterio A, Donadon M, et al. Should we still offer split-liver transplantation for two adult recipients? A retrospective study of our experience Liver Transpl 2008; 14: 999-1006.
24. Yamamoto S, Wilczek HE, Nowak G, et al. Liver transplantation for familial amyloidotic polyneuropathy (FAP): a single-center experience over 16 years. Am J Transplant 2007; 7: 2597.
25. Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979; 2(8151): 1033-1036.
26. Haddad E, McAlister V, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. The Cochrane Collaboration 2009.
27. Montalbano M, Neff GW, Yamashiki N, Meyer D, .Tzakis AG et al. A retrospective review of liver transplant patients treated with sirolimus from a single center: an analysis of sirolimus-related complications. Transplantation 2004; 78(2): 264-8.
28. Goralczyk AD, Hauke N. Interleukin 2 receptor antagonists for liver transplant recipients: a systematic review and metaanalysis of controlled studies. Hepatology 2011; 54(2): 541-54.
29. Stange BJ, Glanemann M, Nuessler NC, et al. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl 2003; 9: 612-20.
30. Orons PD, Sheng R, Zajko AB. Hepatic artery stenosis: prevalence and cholangiographic appearance of associated biliary complications. Am J Roentgenol 1995; 165: 1145-1149.
31. Settmacher U, Nussler NC, Glanemann M, et al. Venous complications after orthotopic liver transplantation. Clin Transplant 2000; 14: 235-241.
32. Michael D. Darcy. Management of Venous Outflow Complications After Liver Transplantation. Tech Vasc Interventional Rad 2007; 10: 240-245.
33. Hampe T, Dogan A, Encke J, et al. Biliary complications after liver transplantation. Clin Transplant 2006; 20: 93-96.
34. Koneru B, Sterling MJ, Bahramipour PF. Bile duct strictures after liver transplantation: a changing landscape of the Achilles heel. Liver Transpl 2006; 12: 702-704.
35. Welling TH, Heidt DG, Englesbe MJ, Magee JC, Sung RS, Campbell DA, Punch JD, Pelletier SJ. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl 2008; 14: 73-80.
36. Londoño MC, Balderramo D, Cárdenas A. Management of biliary complications after orthotopic liver transplantation: the role of endoscopy. World J Gastroenterol 2008; 14: 493-497.
37. Valls C, Alba E, Cruz M, Figueras J, Andia E, Sanchez A, Llado L, Serrano T. Biliary complications after liver transplantation: diagnosis with MR cholangiopancreatography. AJR Am J Roentgenol 2005; 184: 812-820.
38. Salizzoni M, Cerutti E, Romagnoli R, et al. The first one thousand liver transplants in Turin: a single-center experience in Italy. Transplant International 2005; 18: 1328-35.
39. Fishman JA. Pneumocystis carinii and parasitic infections in transplantation. Infect Dis Clin North Am 1995; 9(4): 1005-44.
40. Jay A. Fishman. Infection in Solid-Organ Transplant Recipients. N Engl J Med 2007; 357: 2601-14.
41. Rubin RH. The indirect effects of cytomegalovirus infection on the outcome of organ transplantation. JAMA 1989; 261: 3607-9.
42. Falagas ME, Snydman DR, Griffith J, Ruthazer R, Werner BG. Effect of cytomegalovirus infection status on first-year mortality rates among orthotopic liver transplant recipients. Ann Intern Med 1997; 126(4): 275-9.
43. Kalil AC, Levitsky J, Lyden E, et al. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med 2005; 143(12): 870-80.
44. Camille N Kotton, Deepali Kumar, Angela M Caliendo, et al. International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation. Transplantation 2010; 89: 779-795.
45. Klintmalm GB, Nery JR, Husberg BS, Gonwa TA, Tillery GW. Rejection in liver transplantation. Hepatology 1989; 10(6): 978-85.
46. Wiesner RH, Demetris AJ, Belle SH, et al. Acute hepatic allograft rejection: incidence, risk factors and impact on outcome. Hepatology 1998; 28: 638-45.
47. Anand A, Hubscher S, Gunson B, et al. Timing, significance and prognosis of late acute liver allograft rejection. Transplantation 1995; 60: 1098-103.
48. Neuberger J. Incidence, timing, and risk factors for acute and chronic rejection. Liver Transpl 1999; 5: S30.
49. Gane E. The natural history and outcome of liver transplantation in hepatitis C virus infected recipients. Liver Transpl 2003; 9: S28-34.
50. Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol 2008; 49(2): 274-87.
51. Yoshida EM, Erb SR, Partovi N, et al. Liver transplantation for chronic hepatitis B infection with the use of combined lamivudine and low-dose hepatitis B immune globulin. Liver Transpl 1999; 5: 520-5.
52. Cuadrado A, Fabrega E, Casafont F, Pons-Romero F. Alcohol recidivism impairs long-term patient survival after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl 2005; 11: 420-6.
53. Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L et al. Long-term medical complications in patients surviving > or = 5 years after liver transplant. Transplantation 2000; 69(5): 781-9.
54. Watt K, Pedersen R, Kremers W, Heimbach J, Charlton M. Evolution of causes and risk factors for mortality post liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant 2010; 10: 1-8.
55. Kuo HT, Sampaio MS, Ye X, Reddy P, Martin P, Bunnapradist S. Risk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing Database. Transplantation 2010; 89: 1134-1140.
56. John P, Thuluvath P. Outcome of patients with new onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl 2002; 8: 708-713.
57. Wawrzynowicz-Syczewska M, Karpin Ska E, Jurczyk K, Laurans L, Boron-Kaczmarska A. Risk factors and dynamics of weight gain in patients after liver transplantation. Ann Transplant 2009; 14: 45-50.
58. Kymberly D.S. Watt, Michael R. Charlton. Metabolic syndrome and liver transplantation: A review and guide to management. Journal of Hepatology 2010; 53: 199-206.
59. Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349(10): 931-40.
60. Brown RS Jr, Lombardero M, Lake JR. Outcome of patients with renal insufficiency undergoing liver or liver-kidney transplantation. Transplantation 1996; 62(12): 1788-93.
61. Haagsma EB, Hagens VE, Schaapveld M, van den Berg AP, de Vries EG, Klompmaker IJ, et al. Increased cancer risk after liver transplantation: a population-based study. J Hepatol 2001; 34: 84-91.
62. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a metaanalysis. Lancet 2007; 370: 59-67.
63. Herrero JI, Lorenzo M, Quiroga J, Sangro B, Pardo F, Rotellar F, et al. De novo neoplasia after liver transplantation: an analysis of risk factors and influence on survival. Liver Transpl 2005; 11: 89-97.
1. National Institutes of Health Consensus Development Conference Statement: liver transplantation--June 20-23, 1983. Hepatology 1984; 4 (1 suppl): S107-10. [ Links ]
2. Starzl TE, Groth CG, Brettschneider L, et al. Orthotopic homotransplantation of the human liver. Ann Surg 1968; 168(3): 392-415. [ Links ]
3. P Thuluvath, M Guidinger, et al. Liver Transplantation in the United States, 1999-2008. American Journal of Transplantation 2010; 10(Part 2): 1003-1019. [ Links ]
4. Hosein Shokouh-Amiri M, Osama Gaber A, Bagous WA, et al. Choice of surgical technique influences perioperative outcomes in liver transplantation. Ann Surg 2002; 231: 814. [ Links ]
5. Greif F, Bronsther OL, Van Thiel DH, et al. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg 1994; 219: 40. [ Links ]
6. Hendriks HG, Van der Meer J, De Wolf JT, et al. Intraoperative blood transfusion requirement is the main determinant of early surgical re-intervention after orthotopic liver transplantation. Transpl Int 2005; 17: 673-9. [ Links ]
7. Mandell MS, Lezotte D, Kam I, Zamudio S. Reduced use of intensive care after liver transplantation: influence of early extubation. Liver Transpl 2002; 8: 676-681. [ Links ]
8. Fan ST, Cheung ST, Lo CM. Indications for liver transplantation in patients with chronic hepatitis B and C virus infection and hepatocellular carcinoma. J Gastroenterol Hepatol 2000; 15(Suppl): E181-E186. [ Links ]
9. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996; 334: 693-699. [ Links ]
10. Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology 2001; 33: 1394-403. [ Links ]
11. Julie Polson and William M Lee. AASLD Position Paper: The Management of Acute Liver Failure. Hepatology 2005. [ Links ]
12. Cross TJ, Antoniades CG, Muiesan P, Al-Chalabi T, et al. Liver transplantation in patients over 60 and 65 years: an evaluation of long-term outcomes and survival. Liver Transpl 2007; 13(10): 1382-8. [ Links ]
13. Eisenbach C, Merle U, Stremmel W, Encke J. Liver transplantation in HIV-positive patients. Clin Transplant 2009; 23(Suppl 21): 68-74. [ Links ]
14. Trotter JF. Impact of the model for endstage liver disease score on liver transplantation. Curr Opinion Organ Transplant 2007; 12: 294-297. [ Links ]
15. Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology 2000; 31(4): 864-871. [ Links ]
16. Habib S, Berk B, Chang CC, et al. MELD and prediction of postliver transplantation survival. Liver Transpl 2006; 12: 440-447. [ Links ]
17. Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant 2005; 5: 307-313. [ Links ]
18. Freeman RB, Mithoefer A, Ruthazer R, et al. Optimizing staging for hepatocellular carcinoma before liver transplantation: A retrospective analysis of the UNOS/OPTN data-base. Liver Transpl 2006; 12: 1504-1511. [ Links ]
19. Machicao VI, Srinivas TR, Hemming AW, et al. Impact of implementation of the MELD scoring system on the prevalence and incidence of chronic renal disease following liver transplantation. Liver Transpl 2006; 12: 754-761. [ Links ]
20. Biggins SW, Rodriguez HJ, Bacchetti P, et al. Serum Sodium predicts mortality in patients listed for liver transplantation. Hepatology 2005; 41: 32-39. [ Links ]
21. Renz J, Kin C, Kinkhabwala M, et al. Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation. Ann Surg 2005; 242: 556-63. [ Links ]
22. Malag M, Rogiers X, Broelsch CE. Liver splitting and living donor techniques. Br Med Bull 1997;53: 860-7. [ Links ]
23. Giacomoni A, Lauterio A, Donadon M, et al. Should we still offer split-liver transplantation for two adult recipients? A retrospective study of our experience Liver Transpl 2008; 14: 999-1006. [ Links ]
24. Yamamoto S, Wilczek HE, Nowak G, et al. Liver transplantation for familial amyloidotic polyneuropathy (FAP): a single-center experience over 16 years. Am J Transplant 2007; 7: 2597. [ Links ]
25. Calne RY, Rolles K, White DJ, et al. Cyclosporin A initially as the only immunosuppressant in 34 recipients of cadaveric organs: 32 kidneys, 2 pancreases, and 2 livers. Lancet 1979; 2(8151): 1033-1036. [ Links ]
26. Haddad E, Mcalister V, Renouf E, Malthaner R, Kjaer MS, Gluud LL. Cyclosporin versus tacrolimus for liver transplanted patients. The Cochrane Collaboration 2009. [ Links ]
27. Montalbano M, Neff GW, Yamashiki N, Meyer D, .Tzakis AG et al. A retrospective review of liver transplant patients treated with sirolimus from a single center: an analysis of sirolimus-related complications. Transplantation 2004; 78(2): 264-8. [ Links ]
28. Goralczyk AD, Hauke N. Interleukin 2 receptor antagonists for liver transplant recipients: a systematic review and metaanalysis of controlled studies. Hepatology 2011; 54(2): 541-54. [ Links ]
29. Stange BJ, Glanemann M, Nuessler NC, et al. Hepatic artery thrombosis after adult liver transplantation. Liver Transpl 2003; 9: 612-20. [ Links ]
30. Orons PD, Sheng R, Zajko AB. Hepatic artery stenosis: prevalence and cholangiographic appearance of associated biliary complications. Am J Roentgenol 1995; 165: 1145-1149. [ Links ]
31. Settmacher U, Nussler NC, Glanemann M, et al. Venous complications after orthotopic liver transplantation. Clin Transplant 2000; 14: 235-241. [ Links ]
32. Michael D. Darcy. Management of Venous Outflow Complications After Liver Transplantation. Tech Vasc Interventional Rad 2007; 10: 240-245. [ Links ]
33. Hampe T, Dogan A, Encke J, et al. Biliary complications after liver transplantation. Clin Transplant 2006; 20: 93-96. [ Links ]
34. Koneru B, Sterling MJ, Bahramipour PF. Bile duct strictures after liver transplantation: a changing landscape of the Achilles heel. Liver Transpl 2006; 12: 702-704. [ Links ]
35. Welling TH, Heidt DG, Englesbe MJ, Magee JC, Sung RS, Campbell DA, Punch JD, Pelletier SJ. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl 2008; 14: 73-80. [ Links ]
36. Londoño MC, Balderramo D, Cárdenas A. Management of biliary complications after orthotopic liver transplantation: the role of endoscopy. World J Gastroenterol 2008; 14: 493-497. [ Links ]
37. Valls C, Alba E, Cruz M, Figueras J, Andia E, Sanchez A, Llado L, Serrano T. Biliary complications after liver transplantation: diagnosis with MR cholangiopancreatography. AJR Am J Roentgenol 2005; 184: 812-820. [ Links ]
38. Salizzoni M, Cerutti E, Romagnoli R, et al. The first one thousand liver transplants in Turin: a single-center experience in Italy. Transplant International 2005; 18: 1328-35. [ Links ]
39. Fishman JA. Pneumocystis carinii and parasitic infections in transplantation. Infect Dis Clin North Am 1995; 9(4): 1005-44. [ Links ]
40. Jay A. Fishman. Infection in Solid-Organ Transplant Recipients. N Engl J Med 2007; 357: 2601-14. [ Links ]
41. Rubin RH. The indirect effects of cytomegalovirus infection on the outcome of organ transplantation. JAMA 1989; 261: 3607-9. [ Links ]
42. Falagas ME, Snydman DR, Griffith J, Ruthazer R, Werner BG. Effect of cytomegalovirus infection status on first-year mortality rates among orthotopic liver transplant recipients. Ann Intern Med 1997; 126(4): 275-9. [ Links ]
43. Kalil AC, Levitsky J, Lyden E, et al. Meta-analysis: the efficacy of strategies to prevent organ disease by cytomegalovirus in solid organ transplant recipients. Ann Intern Med 2005; 143(12): 870-80. [ Links ]
44. Camille N Kotton, Deepali Kumar, Angela M Caliendo, et al. International Consensus Guidelines on the Management of Cytomegalovirus in Solid Organ Transplantation. Transplantation 2010; 89: 779-795. [ Links ]
45. Klintmalm GB, Nery JR, Husberg BS, Gonwa TA, Tillery GW. Rejection in liver transplantation. Hepatology 1989; 10(6): 978-85. [ Links ]
46. Wiesner RH, Demetris AJ, Belle SH, et al. Acute hepatic allograft rejection: incidence, risk factors and impact on outcome. Hepatology 1998; 28: 638-45. [ Links ]
47. Anand A, Hubscher S, Gunson B, et al. Timing, significance and prognosis of late acute liver allograft rejection. Transplantation 1995; 60: 1098-103. [ Links ]
48. Neuberger J. Incidence, timing, and risk factors for acute and chronic rejection. Liver Transpl 1999; 5: S30. [ Links ]
49. Gane E. The natural history and outcome of liver transplantation in hepatitis C virus infected recipients. Liver Transpl 2003; 9: S28-34. [ Links ]
50. Berenguer M. Systematic review of the treatment of established recurrent hepatitis C with pegylated interferon in combination with ribavirin. J Hepatol 2008; 49(2): 274-87. [ Links ]
51. Yoshida EM, Erb SR, Partovi N, et al. Liver transplantation for chronic hepatitis B infection with the use of combined lamivudine and low-dose hepatitis B immune globulin. Liver Transpl 1999; 5: 520-5. [ Links ]
52. Cuadrado A, Fabrega E, Casafont F, Pons-Romero F. Alcohol recidivism impairs long-term patient survival after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl 2005; 11: 420-6. [ Links ]
53. Sheiner PA, Magliocca JF, Bodian CA, Kim-Schluger L et al. Long-term medical complications in patients surviving > or = 5 years after liver transplant. Transplantation 2000; 69(5): 781-9. [ Links ]
54. Watt K, Pedersen R, Kremers W, Heimbach J, Charlton M. Evolution of causes and risk factors for mortality post liver transplant: results of the NIDDK long-term follow-up study. Am J Transplant 2010; 10: 1-8. [ Links ]
55. Kuo HT, Sampaio MS, Ye X, Reddy P, Martin P, Bunnapradist S. Risk factors for new-onset diabetes mellitus in adult liver transplant recipients, an analysis of the Organ Procurement and Transplant Network/United Network for Organ Sharing Database. Transplantation 2010; 89: 1134-1140. [ Links ]
56. John P, Thuluvath P. Outcome of patients with new onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl 2002; 8: 708-713. [ Links ]
57. Wawrzynowicz-Syczewska M, Karpin Ska E, Jurczyk K, Laurans L, Boron-Kaczmarska A. Risk factors and dynamics of weight gain in patients after liver transplantation. Ann Transplant 2009; 14: 45-50. [ Links ]
58. Kymberly D.S. Watt, Michael R. Charlton. Metabolic syndrome and liver transplantation: A review and guide to management. Journal of Hepatology 2010; 53: 199-206. [ Links ]
59. Ojo AO, Held PJ, Port FK, et al. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med 2003; 349(10): 931-40. [ Links ]
60. Brown RS Jr, Lombardero M, Lake JR. Outcome of patients with renal insufficiency undergoing liver or liver-kidney transplantation. Transplantation 1996; 62(12): 1788-93. [ Links ]
61. Haagsma EB, Hagens VE, Schaapveld M, van den Berg AP, de Vries EG, Klompmaker IJ, et al. Increased cancer risk after liver transplantation: a population-based study. J Hepatol 2001; 34: 84-91. [ Links ]
62. Grulich AE, van Leeuwen MT, Falster MO, Vajdic CM. Incidence of cancers in people with HIV/AIDS compared with immunosuppressed transplant recipients: a metaanalysis. Lancet 2007; 370: 59-67. [ Links ]
63. Herrero JI, Lorenzo M, Quiroga J, Sangro B, Pardo F, Rotellar F, et al. De novo neoplasia after liver transplantation: an analysis of risk factors and influence on survival. Liver Transpl 2005; 11: 89-97. [ Links ]











 text in
text in