Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev Col Gastroenterol vol.27 no.1 Bogotá Jan./Mar. 2012
Endoscopic techniques for gastrointestinal stenting: When and how to stent, how to manage complications, stent selection and costs
Rodrigo Castaño Llano, MD. (1)
(1) Gastrointestinal Surgeon and Endoscopist, Chief of Postgraduate General Surgery at the Universidad Pontificia Bolivariana. Professor in the Gastrohepatology Group at the Universidad de Antioquia and at the Hospital Pablo Tobón Uribe, Instituto de Cancerología, Clínica Las Américas in Medellín, Colombia
Received: 18-01-12 Accepted: 21-02-12
Abstract
Over the past century the use of stents has evolved to the point where they are no used throughout the gastrointestinal tract. The evolution of endoscopic ultrasound and signifi cant improvements in stent design have allowed endoscopists to drive the use of gastroenterological stents in new directions. Endoscopic creativity remains crucial for the evolution of new endoscopic technology. Finally, the use of multidisciplinary teams which include endoscopists, radiologists, and surgeons allows for the exchange of ideas and procedural planning necessary for successful innovation. This is a review of indications for stenting, technical aspects of implementation, appropriate stent selection for different locations and management of complications.
Key words
Self-expanding metallic stent, malignant gastrointestinal obstruction, endoscopy, complications.
INTRODUCTION
A physician does not need to be a specialist to place a stent in the gastrointestinal tract. Interventional radiologists, gastroenterologists or surgeons with appropriate training and experience can routinely perform these procedures. Until now, there has been no standard training or certification in any country for performance of these procedures. Development of such standards, which should include a minimum number of stents per year to maintain expertise, would be ideal. The physician should keep a log or, ideally, a database to monitor results.
At the minimum, an endoscopist who wants to work with stents must have knowledge and experience in radiological and endoscopic techniques. Ideally, these physicians should become tutors for the development of these skills.
PROCEDURES
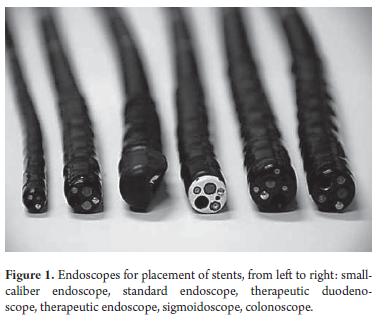
For through-the-scope stent placement (TTS) in the gastrointestinal tract a therapeutic endoscope with a wide channel (3.2 mm) should be used so that the stent can pass through the scope. A standard caliber (2.8 mm) endoscope can be used for over-the-wire stenting (OTW) whether it is peroral or per-anal (Figure 1).
Endoscopic procedures are indicated for placement of stents in the esophagus, stomach, duodenum and large intestine. Stenoses are in generally malignant, but benign stenoses, particularly in the esophagus, can also be treated with stents. In addition, stents are indicated for esophageal respiratory fistulas, for esophageal benign fistulas and for perforations.
SKILLS AND EXPERIENCE
Basic prerequisites for stent placement include skills in upper endoscopy and colonoscopy, fluoroscopic image interpretation, and experience in stenosis dilation. It is very useful to have training in endoscopic retrograde cholangiopancreatography (ERCP).
There are many degrees of difficulty in stent placement. Esophageal stents are generally easier to place, though the difficulty varies. For example, stents for medium esophageal obstruction are easier to place than those for proximal esophageal stenoses which are closer to the inferior pharyngeal constrictor muscle.
Gastroduodenal or colorectal stents are more difficult to place due to the severity of these obstructions and to the local anatomical situation. Endoscopists who perform ERCP are often capable of penetrating complex duodenal or colon stenoses. Also, they are capable of interpreting complex fluoroscopic images and properly placing stents in malignant obstructions. In general, it is diffi cult to place a colon stent in the scenario of a severe colonic obstruction. Th e difficulty can be caused by the patient's situation, failure to prepare the colon, inadequate colon preparation, or distorted anatomy, oft en with displaced lumina.
Another not less important issue is the wide variety of stents and stent introduction systems. It is impossible to master every new product or to know the specific features of all of the stents and delivery systems available. All digestive tract stents have some ability to contract and lengthen.
SPECIAL CONSIDERATIONS
The expertise required for placing gastrointestinal stents is gained soonest in tertiary referral centers. Patients who require stents are found both within these hospitals and in gastrointestinal oncology services. Training is based on development of skills for dilation of stenoses. Th e physician must know the indications and contraindications for stent placement, and must anticipate and know how to handle potential complications (1).
SPECIFIC TECHNIQUES AND COGNITIVE SKILLS
The placement of stents requires the following types of skills:
- Knowing indications for stenting of malignant and benign digestive tract stenoses, benign esophageal stenoses, anastomotic fistula and esophageal-respiratory fi stulas.
- Interpreting non-invasive images such as computed axial tomography (CAT) and radiological studies using contrast media.
- Evaluating and interpreting signs and symptoms aft er stent release.
- Managing endoscopic and non endoscopic complications and recurrent obstructions.
- Removing stents in cases of benign pathologies.
- Placing a second stent in case of the failure of the first placement.
Required technical skills include upper and lower therapeutic endoscopy, use of guide wires, and placement of stents. Because stents are occasionally required for critical stenosis, the physician must be able to dilate these stenoses with a balloon or a Savary-Gilliard dilator. Furthermore, because stents are used with increasing frequency for benign pathologies, the physician must have the skills to remove different types of stents.
It is essential to have skills in the management of intraoperative complications such as bleeding and perforation and for follow-up procedures to treat problems such as fistulas, re-obstruction, and migration.
It is important to note the stenosis location and its anatomical relation to its neighborhood before stent deployment. This is particularly useful for proximal esophageal obstruction, spontaneous ruptures and obstructions in the gastric tract exit.
Finally, proper interaction with other team members such as surgeons, oncologists, broncoscopists, and radiologists is very important. This is essential in the manipulation of proximal esophageal obstructions that compromise the air passages and require group intervention.
EQUIPMENT
- Esophagus
- Endoscope with normal or therapeutic work channel
- Material to mark the top and bottom of the tumor:
- Injection needle with soluble contrast material (Lipiodol)
- External metallic label with adhesive tape for fixation
- Rigid guide wire, preferably calibers 0.018" to 0.038"
- Plug or dilation balloon (maximum 10 to 12 mm)
- Metallic stent partially or fully covered for OGW or TT S placement
- Distal stomach/duodenum
- Therapeutic endoscope (11.5 to 14 mm) or duodenoscope (11 to 12.5 mm)
- Cannulation catheter
- Water-soluble contrast medium
- Rigid guide wire, preferably calibers 0.018" to 0.038"
- Plug or dilation balloon (maximum 10 to 12 mm)
- Metallic stent partially or fully covered for OGW or TT S placement
- Colon and rectum
- Sigmoidoscope or colonoscope
- Material for marking the proximal and distal ends of the tumor:
- Injection needle with soluble contrast material (Lipiodol)
- Cannulation catheter
- Water-soluble contrast medium
- Flexible guide wire, preferably calibers 0.018" to 0.038"
- Plug or dilation balloon (maximum 10 to 12 mm)
- Metallic stent partially or fully covered for OGW or TT S placement
- Back-up Safety Equipment
- Accessories for hemostasia: injection needle, epinephrine, endoclips, bipolar cautery unit, argon plasma
- Foreign body forceps
- Polypectomy handle
- A second stent
KEY STEPS FOR PROPER TECHNIQUE
Esophagus
Placing a stent to relieve dysphagia or to close esophageal respiratory fistulas is a therapeutic alternative for patients not eligible for surgery. Stents have also been used for dilation of benign stenoses and to seal ruptures or esophageal fistulas (2, 3).
Evaluation prior to stent placement
Before placing stent the endoscopist should evaluate the following:
Patient conditions
In cases of malignant esophageal stenoses it is important to note the general conditions of the patient in order to have an advantage with stent placement. In patients with an overall WHO index activity score of 4 (100% lying in bed), placement should be carefully evaluated.
Tumor location
Esophageal tumors can have upper, middle or distal locations. Tumors in the proximal or medium esophagus risk have risks of invasion to the trachea or bronchial tree and risks of airway compression. Placing a tracheal stent should be considered before considering placing the stent in the esophagus. The stents that cross the gastroesophageal junction present high risks of migration and gastro esophageal reflux. In addition the distal end of the stent can damage the stomach's minor curve when the stent is located too distally.
Stent selection
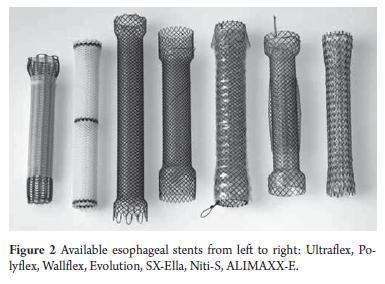
Partially or fully covered stents are used most oft en because it has been shown that uncovered stents have a high tumor colonization rate and light occlusion (Table 1 and Figure 2) (4).
The main causes of stent dysfunction are recurrent dysphagia caused by stent migration, tumor growth inside or over the stent, and food impact. Stent design can prevent migration (Figure 2). The selection of the appropriate for each clinical scenario is of critical importance. The use of partially covered stents may be a valid option for malignant esophageal stenosis.
Fully covered stents or plastic stents are preferred for easy removal from benign stenoses since they prevent or reduce colonization by non cancerous hyperplastic tissue which makes stent removal more diffi cult (2).
For ruptures of benign esophageal fistulas without stenosis, large caliber covered stents are used. If a conventional caliber stent is used, migration is very possible. Th ough fully covered stents may be more easily removed than partially covered, many experts prefer partially covered stents in these situations because they allow for bett er sealing in the open portion. However, this recommendation is not widespread or widely accepted.
Although it is widely accepted that stents used to treat extrinsic esophageal compression a high migration rate, this notion is not based on comparative studies. Nevertheless, experts only use partially covered stents to treat extrinsic esophageal compression.
Stents are increasingly used to treat pathologies of the proximal esophagus close to the upper esophageal sphincter. The location of the stent should avoid proximity to, or crossing into, the upper sphincter to avoid the sensation of a foreign body. Another complication is pain induced by stents and extrinsic compression, particularly in the air passage. In these cases a tracheal stent should be placed first. Place a flexible prosthesis in the proximal esophagus is recommended in order to minimize complications. Given the great variability in stent features, routine use of only two (e.g. one covered stent and one uncovered stent) or three prostheses is recommended so that the physician will achieve familiarity with the stents he or she uses (Table 2).
Coagulation tests
Although bleeding is not a common complication of stenting, it can have a poor prognosis. Although coagulation tests are not routinely recommended, prothrombin time (PT) and partial thromboplastin time (PTT) are recommended for patients using warfarin or heparin or who have hepatic dysfunction.
ESOPHAGEAL STENT PLACEMENT
The antegrade technique for location of prosthesis is described in the following (5):
The stent is placed with the patient conscious but under sedation.
Top and bottom margins must be identified. If the tumor does not allow passage of the endoscope, it is permissible to dilate the passage to 10 or 12 mm in order to measure the tumor and to leave an advanced guide wire. However, since dilation involves risk of perforation, it is preferable to use small caliber endoscopes.
When stents are placed using fluoroscopic vision, the proximal and distal margin of the stenosis is marked with clips on the skin or by intramucosal injection of a radiopaque agent.
The next step is to advance a rigid guide wire. 0.038" Savary guide wires or 0.035" Amplatz guide wires are the best choices when the guides will be left in the duodenum. Rigidity is important to prevent bending of the guide, but it is not important when using TT S placement.
The stent is advanced over the guide. Most stent diameters reduce during expansion. This must be taken into account when positioning the introduction system. The stent should be about 4 inches longer than the stenosis and should be released when it extends one or two centimeters from both ends of the stenosis. For stents crossing the gastroesophageal junction, the stent length should be determined by the rule that says that the stent should not be more than 2-3 cm distal to the tumor. This is to avoid laceration of the posterior wall of the stomach and to prevent stent angulations.
Stent release can be monitored fl uoroscopically and/or endoscopically. Introduction of the endoscope into the released stent should be avoided because there is a risk of proximal or distal migration of the partially expanded stent after removal of the endoscope. Th e expansion of the stent should be evaluated with a contrast study, although this is most relevant for gastroduodenal stents.
Stenting is an outpatient procedure that takes 15 to 20 minutes. It should be insisted to patients that the stent does not contract peristaltically with the gastrointestinal tract, so food should be eaten in small bites accompanied by plenty of fluids. Also, patients should be advised not to lie down within the 3 or 4 hours following eating. This is especially important for stents crossing the gastroesophageal junction.
For stents that cross the gastroesophageal junction prescription of a double dose of proton pump blocker is recommended to reduce acid reflux. Th ere are stents with antireflux mechanisms, but the results have been erratic.
HANDLING AFTER ESOPHAGEAL STENTING
Stent removal in benign cases
It is uncertain how long stents should be left in place in benign cases. The cause that motivated placement and stenosis length are factors that influence duration of stent time. The following considerations should be taken into account for cases of benign esophageal stenosis (2):
- In stenoses following ischemia which are not larger than 5 cms, stents should remain 12 to 16 weeks.
- For anastomosis stenoses and stenoses caused by caustics, stents should be placed for short periods of 4 to 8 weeks.
- When symptoms occur after stent removal and there is reappearance of stenosis, a second stent can be considered.
- When using partially covered stents, an endoscopy can be done every 4 weeks to evaluate the uncovered end for colonization by hyperplastic tissue. If this happens, the stent should be removed and replaced with a fully covered one.
- Fully covered stents also have a risk of hyperplastic growth. For this reason follow-up endoscopy is recommended every six weeks. If stenosis persists aft er removing the stent, another stent should be considered.
HANDLING OF COMPLICATIONS
Procedure-related complications
These complications appear within one week of stent placement. Fortunately, they are rare and occur in less than 5% of the cases (6).
Perforation is caused by dilation, by forced passage of the endoscope, or by the expansion of the stent itself. Th ese cases are treated with stenting. However, one should be prepared for infectious complications.
Bleeding results from friable tumor manipulation, from dilation of the endoscope passage, and from the stent. Bleeding is difficult to treat. Sometimes, endoscopic therapies are effective, but other times 5 sections of 4 Gy (Gray) radiation therapy are needed.
Thoracic pain, more intense and frequent in patients previously treated with chemotherapy and/or radiation therapy, is treated with analgesics. In severe cases the stents should be removed.
Aspiration (pneumonia) appears early and is associated with stent placement in sedated patients in supine positions. In particular, critical esophageal stenosis and stents crossing the gastroesophageal junction predispose patients to this complication.
Long-term complications
Long term complications become evident a week or more after stent placement. They are more common than procedure-related complications and occur in 20% to 30% of all cases (7).
Bleeding is the most severe long-term complication. It is not related to placement but to tumor growth when large caliber vessels compromise the area around the esophagus. External radiation is usually not effective. Successive transfusions should be done. Mortality is very high for this complication.
Fistulas are more frequent in the proximal extreme and are related to pressure and ulceration of the prosthesis ends. They can be treated with passage of another coaxial stent over the first stent.
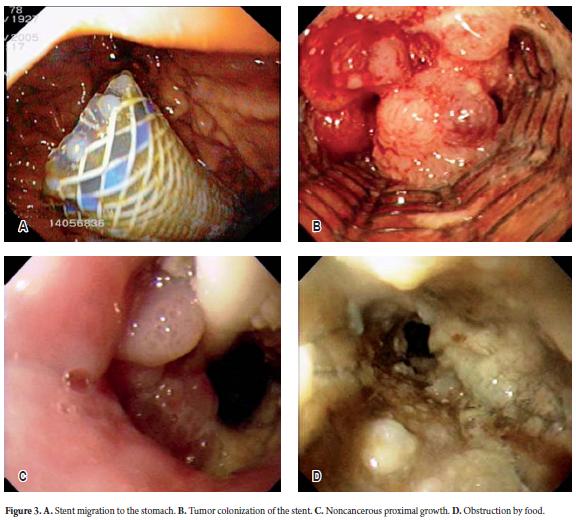
Recurrent dysphagia is usually caused by stent migration (Figure 3A), by neoplastic colonization (malignant cases) (Figure 3B), by proximal overgrowth (benign or malignant cases) (Figure 3C) or by food impact (Figure 3D).
In cases of stent migration to the stomach, try to reposition the stent using foreign body forceps or a polypectomy snare or to remove the stent. Some doctors prefer to use an overtube to remove the stent.
Remove the stent by taking an end of the stent with a forceps and pulling it along its long axis through the esophagus. Leaving the stent in the esophagus can cause complications, especially pain. When it is not possible to return a stent which has migrated to its placement, another stent should be placed. It is better to use a stent with a diff erent design to reduce the risk of a second migration.
Placement of a new stent is the chosen treatment in patients with tumor colonization or with benign overgrowth at the stent ends.
Cleaning the stent is the preferred option for cases of food impact. It should be emphasized that the occluded stent must be manipulated carefully because of the high risk of migration while stirring the food inside the stent.
DISTAL STOMACH AND DUODENUM
Traditionally, a gastroenteric bypass (gastrojejunostomy) was the only option for palliation of malignant obstruction of the stomach exit or duodenum. However, gastrojejunostomy requires general anesthesia and is often associated with high morbidity and mortality rates due to the generally poor condition of patients. An attractive alternative treatment is stenting that restore permeability of the passageway for patients with malignant gastroduodenal obstructions who are not candidates for surgery (8).
EVALUATION PRIOR TO GASTRODUODENAL STENTING
The following aspects should be evaluated before placing a gastroduodenal stent.
Patient condition: More than 50% of obstructions in our country are caused by gastric cancer. In other countries they are caused by pancreatic cancer. Patients with peritoneal or extensive hepatic compromise are in poor condition, scoring 4 on the World Health Organization scale of activity. These patients will not benefit from stent placement. In addition, since obstructions caused by peritoneal metastases occur more distally in the jejunum or ileum, gastroduodenal stents will not resolve their symptoms (9).
Location of the tumor. An important issue to consider before placing the gastroduodenal stent is to check if there is biliary compromise.
- When a stent has been placed in the bile duct, its characteristics are very important (plastic or metal). A plastic stent must be removed and changed to a metallic stent before the gastroduodenal prosthesis.
- In the case of biliary compromise and gastroduodenal simultaneous engagement a biliary stent should be placed first and then a gastroduodenal stent.
- If there is no biliary obstruction when placing gastroduodenal stent, but aft erwards, this determines the ideal position of the stent. It is preferred to leave the papilla not covered for future intervention.
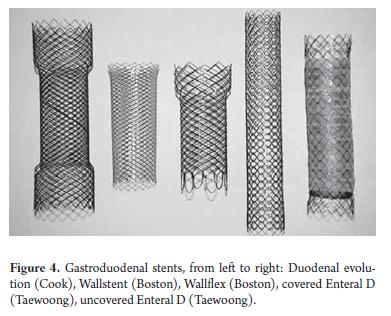
- Stent election from available covered and uncovered stents to be released through the endoscope (Figure 4).
For many years uncovered prostheses were preferred because the diameters of early types of covered stents and their introducers were much larger to allow passage through the endoscope's working channel. Covered stents suitable for TTS have only recently become available (Table 3).
- It has been suggested that covered stents have a higher risk of migration, although the latest stent generation has features to prevent migration.
- It has been reported that covered stents in the duodenum may predispose to cholangitis or pancreatitis when released in front of the papilla. Initial assessments show favorable results. One disadvantage of uncovered stents is the risk of tumor or hyperplastic colonization. Particularly, in patients with prolonged survival a second stent may need to be placed.
GASTRODUODENAL STENT PLACEMENT
The following steps are recommended for placing stents in malignant gastroduodenal obstructions (10, 11):
- Place stent when patient is conscious but sedated.
- Identify the distal and proximal ends to determine the extent of the tumor. If the severity of the obstruction does not allow passage of the endoscope, an ERCP cannulation catheter and the guide wire should be passed through the stenosis. An injection of contrast medium through the catheter will allow determination of tumor length and characteristics and will allow the physician to rule out more distal obstructions. It is often necessary to dilate stenoses in the pylorus and duodenum. They must be dilated to a diameter up to 12 mm.
- These stents can be placed with a therapeutic endoscope (11mm to 14.5 mm) or a duodenoscope (11mm to 12.5 mm). Using a therapeutic gastroscope can be difficult when a loop forms in a distended stomach, and also because this equipment is too flexible for this procedure. In these circumstances a colonoscope can be used, which is longer and less flexible than a gastroscope and does not produce loops in the stomach.
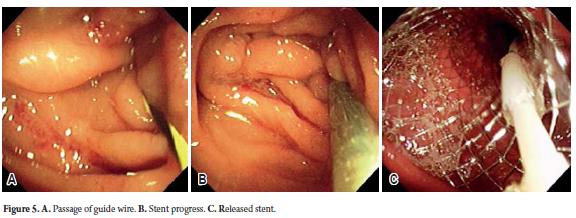
- Next, a 0.038" guide wire with a flexible tip is advanced through the stenosis as distally as possible. Fluoroscopy permits assessment of the distal passage of the guide wire (Figure 5A).
- The enteral stent is carefully advanced through the endoscopes work channel, through the stenosis, and as distally as possible (Figure 5B). Most stents shorten during release. During this process the introducer should be removed to correct stent shortening (Figure 5C). If this is not done, the stent could be positioned too distally and fail to alleviate the stenosis. Prevention of distal migration is another reason for not placing the stent too distally.
- Enteral stents should be guided endoscopically and fl uoroscopically. The stent should be chosen so that it extends by least 2 centimeters beyond each end of the stenosis.
- This is an outpatient procedure that takes 20 to 25 minutes. When a biliary stent is simultaneously placed the procedure takes longer. Because of the large storage capacity of the stomach, it is difficult to determine stent dysfunction based solely on the patient's symptoms. When there is a suspicion of stent dysfunction, an endoscopy is recommended.
Placement of a gastroduodenal stent with respect to the papilla is an important topic to consider. It is important to leave the papilla free when the stent is deployed to allow for the possibility of ERCP in the future in case of biliary obstruction. If the stent crosses above the papilla, bile duct cannulation through the uncovered stent framework is possible. Some authors report the use of argon plasma on the stent to create a window after which the papilla can be addressed and a stent placed into the bile duct. If this is not possible, a percutaneous approach is recommended.
ASSESSMENT AFTER GASTRODUODENAL STENT
Biliary obstruction
Patients should be instructed regarding the appearance (or recurrence) of biliary obstruction after placing the gastroduodenal stent. Biliary obstruction in the case of a previous stent is due to tumor growth inside the stent or to benign hyperplasia. In either case, a primary or secondary stent should be placed.
Management of complications
Complications related to the procedure
Complications related to the procedure are rare, occurring in 5% to 7% of cases (9).
- The most critical complications are bleeding and perforation. Endoscopic treatment is very diffi cult, if not impossible.
- Other complications are early stent migration and stent dysfunction. Often, they can be managed endoscopically by removing the stent and placing another enteral stent.
- Sedation may have adverse effects. Because the possibility of bronco-aspiration increases due to some obstructions, some doctors recommend placing a nasogastric tube the night before to guarantee that the airway remains open during the procedure.
Late complications (12)
Late complications, more common than procedure related complications, occur in less than 20% of cases (13). Th e most frequent late complications are stent migrations and neoplastic origin or food occlusion. Th ese complications can be managed endoscopically by placing a second stent into the occluded one. Stents occluded by food can be managed endoscopically, but the physician must be careful not to move the stent during the procedure.
Colorectal stents
Approximately 10% to 30% of patients with colorectal cancer (CRC) present obstructions. The instructions for stent use have expanded as a first step for patients with acute obstruction and for those who have potentially operable colon cancer. The stent allows decompression, complete colon preparation and resection all in one step (14).
EVALUATION PRIOR TO COLONIC STENTING
Before placing the colorectal stent it is important to consider the following (15).
Patient's condition. It is not clear whether placing a colonic stent in a healthy 50 year old person with total and acute colonic obstruction prior to surgery presents a greater or lower risk than does placing a colonic stent in an 80 year old diabetic person with partial obstruction and metastatic disease whose treatment is palliative. The most probable is that the decision to place a stent should be determined by the risk of complications in an acute intervention for malignant obstruction and by life expectancy aft er stent placement in the case of palliative treatment (15).
The use of agents such as bevacizumab has recently been recognized as an important complicating factor in patients who have colonic stents placed for palliative reasons. Recently, it was reported that the use of bevacizumab increases the risk of perforation about three times to almost 20%. It has also been established that average perforation time is reduced to 22 days (16).
Tumor location. Although it is more diffi cult to place a stent on the right side of the colon than on the left side, some studies report similar efficacy and safety for right and left side colon stents. As a general rule, the more distal the tumor, the easier it is to place a stent (17).
Stents placed in the last 10 cm of the rectum should be handled very carefully since pain and rectal tenesmus are associated with this distal location. Another complication is that stents in this location are frequently expelled in defecation.
Stents in angled sections places have higher risks of pressure and ulceration in the gastric wall which can result in bleeding and/or perforation. These sections include the sigmoid, splenic and hepatic angles.
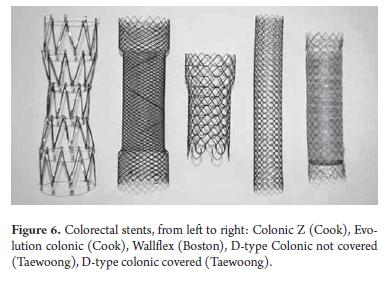
Stent selection. As mentioned above, covered and uncovered endoscopic enteral stents are available for the colon and rectum. In addition, they are also available for OTW placement (Table 4).
OTW stents may be placed in left side colonic tumors as high as the proximal sigmoid and in the descending colon. An advantage of the stents with metallic guide wire is that they are covered and have greater diameters than colorectal TT S stents. This feature can reduce the risk of migration (Figure 6).
PLACEMENT OF COLORECTAL STENTS
The reason for placement of colorectal stents is an important factor. In general, stent placement prior to surgery is likely to involve critical stenoses, making these procedures more diffi cult than palliative stenting.
Whenever possible, dilation of the colon and rectum must be avoided since dilation increases the risks of perforation. The use of an ERCP cannulation guide wire can give sufficient information for stent placement.
There is an increased risk of perforation of in the angled sigmoid section when it is obstructed to the point that there is no clear opening. In this situation pass the metallic guide wire through the stenosis can be a challenge. Using a small size gastroscope (4mm to 6 mm) is recommended in order to pass through the obstruction. Alternatively, a highly flexible hydrophilic covered guide wire (Terumo, Somerset, NJ, USA) can be considered.
It is important to emphasize that other potential locations of malignant obstructions should be ruled out through MRI, sonography, or radiography. When another malignant obstruction is found, placement of an additional stent should be considered, but if the cancer has metastasized it is better to use another treatment option.
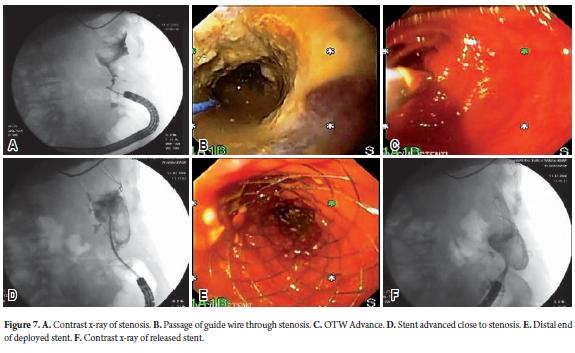
TTS for colorectal stents is not very diff erent from the TTS technique for esophageal and gastroduodenal stents (Figure 7), but there are additional maneuvers and technical problems which will be discussed. The technical problems in placing stents in stenosing colorectal lesions will also be discussed.
COLONIC STENT FOLLOW-UP EVALUATION
Management of complications
Early complications
Most early complications of colonic stenting are no diff erent from those previously discussed although tenesmus, pain, and fecal incontinence can occur aft er rectal stenting. These complications should be treated with antispasmodics and laxatives, although occasionally the stent must be removed. To prevent stent obstruction a soft diet with feces softeners or laxatives is recommended.
Late complications
Late complications resulting from colonic stenting are no different from those previously discussed in relation to esophageal and gastroduodenal stents.
TRAINING
Developing skills for placing stents is left for the second and third years of training in gastroenterology during the interventional endoscopy rotation. How much training is required to obtain sufficiency in the placement of the stents is unknown. Training including no less than 10 esophageal stents, and at least 15 gastroduodenal or colonic stents has been suggested. Continued practice placing these prostheses is recommended. This is most easily achieved in third level centers. To maintain skills it is suggested that the physician place at least one esophageal or enteral stent every month. Placement of practice prostheses can be done in animal or plastic models. However, the benefit of such training has yet not been validated.
DEFINITION OF RESPONSIBILITIES
The definition of responsibilities can be problematic and depends upon the volume of cases available for developing skill in placing stent. Currently, there are no tools available to measure these skills, nor are there established parameters of competency. Different authors' opinions establish that the first thing to look at in evaluating these skills is competence in accurately placing guide wires through different kinds of stenoses. Second, the technical skills for safely and efficiently dilating difficult stenoses should be evaluated. Finally, the ability to place different kinds of stents should be examined. There is no point of reference in expert practices for achievement of these skills.
COST-BENEFIT RELATIONSHIP
Various studies that evaluate cost benefit relationships in the use of stents in different locations show favorable outcomes. These studies are more comprehensive for gastroduodenal stenting (18) and colonic stenting (19) to prevent and close colostomies than for other types of stenting.
Conflicts of interests
The author has no connection with any stent manufacturer.
REFERENCES
1. Castaño R, Álvarez O, Lopera J. Endoprótesis metálicas autoexpandibles en la obstrucción maligna esofágica y gastroduodenal. Rev Col Cirugía 2005; 20: 33-48.
2. Castaño R, Alvarez O, Lopera J, Sanín E, Nuñez E, García L. Expandable Nitinol Stent for Treatment of Malignant and Benign Esophagorespiratory Fistulas. Gastrointestinal Endoscopy 2008; 67: AB 151-152.
3. Castaño R, Ruiz MH, Sanín E. Stent esofágico de nitinol en el manejo de las fístulas esofagorrespiratorias malignas, Revista Col de Gastroenterol 2003; 18: 78-82.
4. Vakil N, Morris AI, Marcon N, Segalin A, Peracchia A, Bethge N, Zuccaro G, Bosco JJ, Jones WF. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. The American journal of gastroenterology 2001; 96: 1791-1796.
5. Castaño R, Ruiz M, Juliao F, Sanín E, et al. Eficacia de un nuevo stent de nitinol fabricado localmente, en el tratamiento de la obstrucción maligna esofágica, Revista Col de Gastroenterol 2003; 18: 211-221.
6. Castaño R. Stents en el tracto gastrointestinal y biliar. Edited by Feris J. Bogotá, Sociedad Colombiana de Gastroenterología 2007. p. 309-329.
7. Castaño R. Prótesis metálicas autoexpansibles gastroduodenales, intestinales y colónicas. Landazábal G. Bogotá, Ediciones Médicas Latinoamericanas S.A. 2011. p. 389-408.
8. Lopera JE, Alvarez O, Castaño R, Castaneda-Zuniga W. Initial experience with Song’s covered duodenal stent in the treatment of malignant gastroduodenal obstruction, Journal of vascular and interventional radiology : JVIR 2001; 12: 1297-1303.
9. Castaño R, Álvarez O, Ruiz MH. Nitinol autoexpandable stent in malignant gastric outlet obstruction., Endoscopy 2004; 36(Suppl 1): A242.
10. Baron T, Hani A. Manejo endoscópico de la obstrucción gastroduodenal maligna. Castaño R, Artifon EL. Medellín, Editorial Universidad de Antioquia, 2011. p. 115-132.
11. Castaño R, Álvarez O, Ruiz MH, Cárdenas A, Sanín E, Erebrie F. Comparación entre la gastroyeyunostomía y el stent metálico autoexpandible para la paliación de la obstrucción maligna gastroduodenal. Rev Col Gastroenterol 2006; 21: 62-67.
12. Castaño R, Álvarez O, Lopera J, Sanín E, Erebrie F, Nuñez E, García L. Endoscopic Stenting Versus Surgical Gastrojejunostomy for Palliation of Malignat Gastroduodenal Obstruction. Gastrointestinal Endoscopy 2008; 67: AB 151.
13. Castaño R, Alvarez O, Lopera J, Ruiz MH, Sanín E, Erebrie F. El uso de prótesis de nitinol parcialmente cubiertas en la obstrucción gastroduodenal maligna. Rev Gastroenterol Perú 2006; 26: 233-241.
14. Castaño R, Puerta JD, Restrepo JI, Alvarez O, Sanín E, Erebrie F, Nuñez E, García L. Manejo actual de la obstrucción maligna colorrectal: grandes incisiones, pequeñas incisiones o sin incisiones. Rev Col Gastroenterol 2007; 23: 57-66.
15. Castaño R, Puerta JD, Álvarez O, Lopera J, Sanín E, Erebrie F, Nuñez E, García L. Self-Expanding Metal Stents in Malignant and Benign Colonic Obstructions. Gastrointestinal Endoscopy 2008; 67: AB 151.
16. Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc 2010; 71: 560-572.
17. Kang SG, Ruiz MH, Castaño R. Stents colónicos en la obstrucción maligna del colon. Castaño R, Artifon EL. Medellin, Editorial Universidad de Antioquia 2011. p. 133 -148.
18. Jeurnink SM, Polinder S, Steyerberg EW, Kuipers EJ, Siersema PD. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. Journal of gastroenterology 2010; 45: 537-543.
19. Varadarajulu S, Roy A, Lopes T, Drelichman ER, Kim M. Endoscopic stenting versus surgical colostomy for the management of malignant colonic obstruction: comparison of hospital costs and clinical outcomes. Surg Endosc 2011; 25: 2203-2209.
1. Castaño R, Álvarez O, Lopera J. Endoprótesis metálicas autoexpandibles en la obstrucción maligna esofágica y gastroduodenal. Rev Col Cirugía 2005; 20: 33-48. [ Links ]
2. Castaño R, Alvarez O, Lopera J, Sanín E, Nuñez E, García L. Expandable Nitinol Stent for Treatment of Malignant and Benign Esophagorespiratory Fistulas. Gastrointestinal Endoscopy 2008; 67: AB 151-152. [ Links ]
3. Castaño R, Ruiz MH, Sanín E. Stent esofágico de nitinol en el manejo de las fístulas esofagorrespiratorias malignas, Revista Col de Gastroenterol 2003; 18: 78-82. [ Links ]
4. Vakil N, Morris AI, Marcon N, Segalin A, Peracchia A, Bethge N, Zuccaro G, Bosco JJ, Jones WF. A prospective, randomized, controlled trial of covered expandable metal stents in the palliation of malignant esophageal obstruction at the gastroesophageal junction. The American journal of gastroenterology 2001; 96: 1791-1796. [ Links ]
5. Castaño R, Ruiz M, Juliao F, Sanín E, et al. Eficacia de un nuevo stent de nitinol fabricado localmente, en el tratamiento de la obstrucción maligna esofágica, Revista Col de Gastroenterol 2003; 18: 211-221. [ Links ]
6. Castaño R. Stents en el tracto gastrointestinal y biliar. Edited by Feris J. Bogotá, Sociedad Colombiana de Gastroenterología 2007. p. 309-329. [ Links ]
7. Castaño R. Prótesis metálicas autoexpansibles gastroduodenales, intestinales y colónicas. Landazábal G. Bogotá, Ediciones Médicas Latinoamericanas S.A. 2011. p. 389-408. [ Links ]
8. Lopera JE, Alvarez O, Castaño R, Castaneda-Zuniga W. Initial experience with Song's covered duodenal stent in the treatment of malignant gastroduodenal obstruction, Journal of vascular and interventional radiology : JVIR 2001; 12: 1297-1303. [ Links ]
9. Castaño R, Álvarez O, Ruiz MH. Nitinol autoexpandable stent in malignant gastric outlet obstruction., Endoscopy 2004; 36(Suppl 1): A242. [ Links ]
10. Baron T, Hani A. Manejo endoscópico de la obstrucción gastroduodenal maligna. Castaño R, Artifon EL. Medellín, Editorial Universidad de Antioquia, 2011. p. 115-132. [ Links ]
11. Castaño R, Álvarez O, Ruiz MH, Cárdenas A, Sanín E, Erebrie F. Comparación entre la gastroyeyunostomía y el stent metálico autoexpandible para la paliación de la obstrucción maligna gastroduodenal. Rev Col Gastroenterol 2006; 21: 62-67. [ Links ]
12. Castaño R, Álvarez O, Lopera J, Sanín E, Erebrie F, Nuñez E, García L. Endoscopic Stenting Versus Surgical Gastrojejunostomy for Palliation of Malignat Gastroduodenal Obstruction. Gastrointestinal Endoscopy 2008; 67: AB 151. [ Links ]
13. Castaño R, Alvarez O, Lopera J, Ruiz MH, Sanín E, Erebrie F. El uso de prótesis de nitinol parcialmente cubiertas en la obstrucción gastroduodenal maligna. Rev Gastroenterol Perú 2006; 26: 233-241. [ Links ]
14. Castaño R, Puerta JD, Restrepo JI, Alvarez O, Sanín E, Erebrie F, Nuñez E, García L. Manejo actual de la obstrucción maligna colorrectal: grandes incisiones, pequeñas incisiones o sin incisiones. Rev Col Gastroenterol 2007; 23: 57-66. [ Links ]
15. Castaño R, Puerta JD, Álvarez O, Lopera J, Sanín E, Erebrie F, Nuñez E, García L. Self-Expanding Metal Stents in Malignant and Benign Colonic Obstructions. Gastrointestinal Endoscopy 2008; 67: AB 151. [ Links ]
16. Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc 2010; 71: 560-572. [ Links ]
17. Kang SG, Ruiz MH, Castaño R. Stents colónicos en la obstrucción maligna del colon. Castaño R, Artifon EL. Medellin, Editorial Universidad de Antioquia 2011. p. 133 -148. [ Links ]
18. Jeurnink SM, Polinder S, Steyerberg EW, Kuipers EJ, Siersema PD. Cost comparison of gastrojejunostomy versus duodenal stent placement for malignant gastric outlet obstruction. Journal of gastroenterology 2010; 45: 537-543. [ Links ]
19. Varadarajulu S, Roy A, Lopes T, Drelichman ER, Kim M. Endoscopic stenting versus surgical colostomy for the management of malignant colonic obstruction: comparison of hospital costs and clinical outcomes. Surg Endosc 2011; 25: 2203-2209. [ Links ]











 text in
text in