Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957On-line version ISSN 2500-7440
Rev Col Gastroenterol vol.27 no.1 Bogotá Jan./Mar. 2012
Hepatosplenic peliosis: A case report and a literature review
Rafael Pila Pérez, MD, (1) Rafael Pila Peláez, MD, (1) Pedro Rosales Torres, MD, (1) Víctor Holguín Prieto, MD, (1) Etelivar Torres Vargas, MD. (1)
(1) Internal Medicine Service of Manuel Ascunce Domenech University Hospital in Camagüey, Cuba
Received: 09-06-11 Accepted: 21-02-12
Abstract
Peliosis is a very rare benign entity which is characterized by blood fi lled cavities filled within solid organs. They occur primarily in the mononuclear phagocyte system although peliosis can also affect organs such as the lungs, pleura, kidneys, adrenal glands and stomach. Only one lesion or multiple lesions may occur, and their size is also variable. The etiology is unknown but is related to ingestion of, or direct contact with, drugs and various toxic substances such as steroids and oral contraceptives. It is associated with hematologic cancers and infectious diseases, particularly tuberculosis. In some cases it is idiopathic. We present the case of a 60 years old woman with a history of hypertension. Physical examination and various imaging studies showed a giant nodular hepatomegaly without splenomegaly. Laboratory studies showed disorders due to liver failure. The patient finally died of hypovolemic shock and hemoperitoneum. An autopsy determined the presence of parenchymal hepatosplenic peliosis of other than idiopathic etiology. A literature review highlighting the history, pathogenesis, etiology, clinical aspects, diagnosis and treatment of hepatosplenic peliosis is included.
Key words
Peliosis, hepatomegaly, hemoperitoneum.
CLINICAL CASE
The patient was a 60 year old retired woman from a rural environment who had had no history of contact history with animals, drugs or toxic substances but had been treated for three years with angiotensin-converting-enzyme inhibitors and thiazides for primary hypertension. Four years prior she had undergone surgery for a mediastinal tumor which histopathologic examination had reported to be a benign pericardial. The patient remained asymptomatic for 20 days after which she began to present increasing abdominal volume, especially in the upper quadrant. Th is was combined with a feeling of early fullness and continuous mild epigastric pain in the right hypochondrium but without irradiation. Pain was exacerbated by movement.
The patient had also suffered from asthenia, anorexia, an unspecified weight loss and 7 days of constipation.
PHYSICAL EXAMINATION
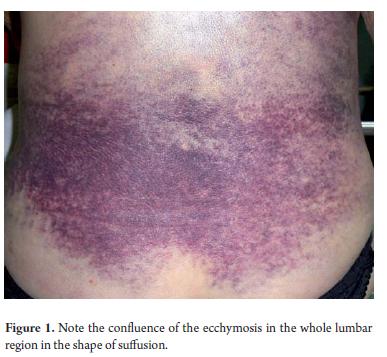
Respiratory rate: 17 breaths per minute, heart rate: 97 beats per minute, blood pressure: 130/80 mmHg. Th e patient's overall condition was poor. She was pale and weak and suffered from edema in her ankles (+++) but was not experiencing pain. Her respiratory system evidenced decreased vocal vibrations, dullness, and absence of vesicular murmur in the left hemithorax. Her abdomen was large, distended, and without contracture in the muscles. Th ere was approximately 24cm of hepatomegaly which was slightly painful. The area had an irregular surface, a blunt lower intercostal space and at the lower reaches the right iliac fossa. Th e left lobe extended to the ipsilateral iliac fossa. No ascites was found. Symptoms related to the hemolymphopoietic system included purple bruising throughout the lumbar region (Figure 1) and a spleen that was not palpable due to the enormous size of the left lobe of the liver. A rectal examination showed no anomalies or alterations. Th e patient was awake, well oriented in time, space and person, and had no motor defi cits. Th e rest of our examination showed no other alterations.
ANALYTICAL RESEARCH AND EVOLUTION
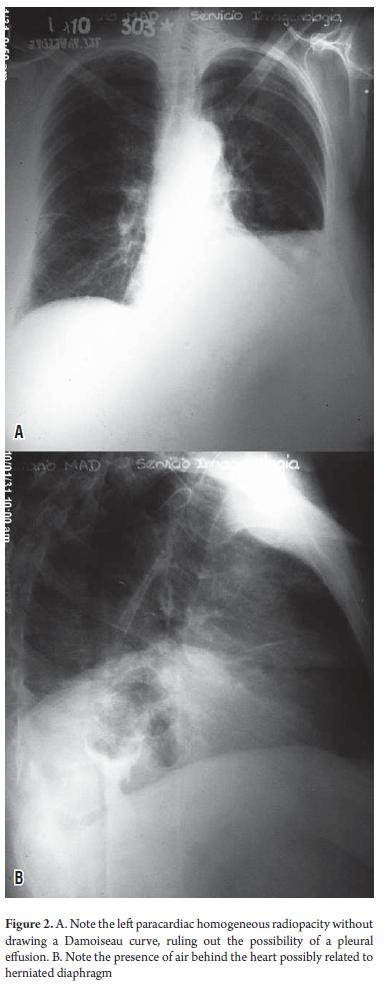
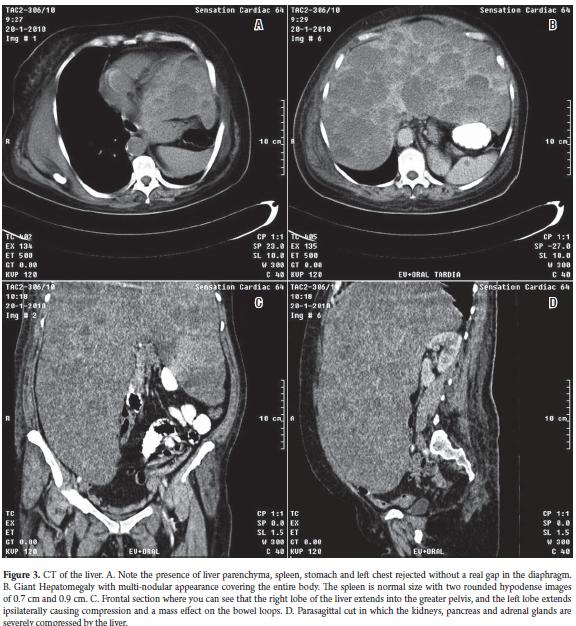
Table 1 shows some of the clinical findings from tests done when the patient was admitted to hospital. A complete blood count with blood smear showed hypochromia +++, and anemia ("anisopoiquilocitosis" a Cuban medical term meaning a type of severe anemia) +. Leukocytes, platelets, and urinary sediment were normal. An immunological study was normal. Blood cultures for HIV, HBsAg, HCV Ab and VDRL were normal. Urinalysis, serology, a culture for brucellosis, and a tuberculosis test were negative. An ECG showed sinus tachycardia. Anterior, posterior and lateral chest x-rays (Figure 2) showed a normal cardiac silhouett e and unaltered bony framework. At the upper horizontal edge of the left thorax homogenous paracardiac radiographic opacity with effacement of the ipsilateral costophrenic angle was observed. The lateral projection showed the presence of air in the center of the area of radiopacity which must have been related to the gastric cavity. Abdominal ultrasonography (USG) showed a multi-nodular hepatomegaly with giant lobes that extended to the corresponding iliac fossa. The quality of the sonic window for evaluation of other viscera was poor due to the size of the liver. Spleen size was normal in two echolucent rounded images with 0.7 cm and 0.9 diameters. No abdominal lymphadenopathy was observed. Simple computed tomography and a contrast x-ray of the abdomen (Figure 3) showed a giant multi-nodular hepatomegaly with a right lobe extending to the greater pelvis and a left lobe extending to the left a mass effect on the intestinal loops. It projected toward the left iliac fossa and the minor pelvis. The size of the spleen was in two rounded hypodense CT images 0.75 cm and 0.92 cm in diameter. Imaging showed elevation of the left hemidiaphragm, a pancreas compressed by the liver which appeared normal, kidneys and adrenal glands without alterations, unobservable abdominal lymph nodes, and a calcified fibroid uterus.
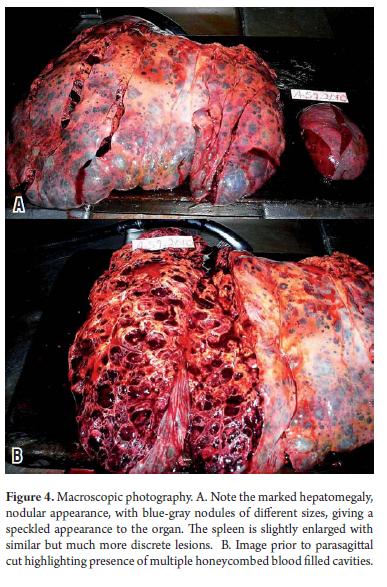
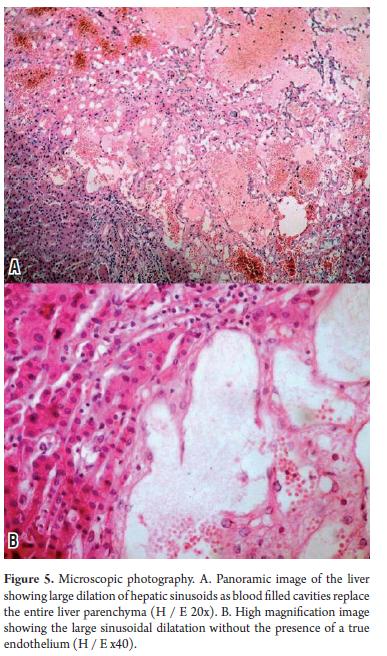
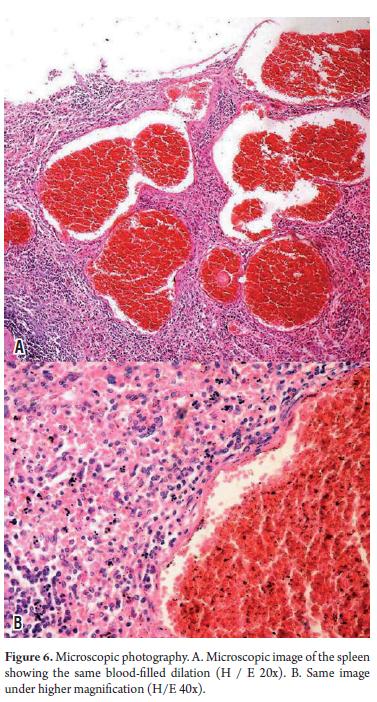
At admission, the patient needed multiple transfusions of red blood cells, fresh frozen plasma, and administration of vitamin K1. Analgesics were also administered because of daily pain intensification which even required the use of Opioid drugs. 15 days after admission, the patient showed signs of hypovolemic shock associated with intense abdominal pain radiating to the back with peritoneal reaction. She died several hours later. The autopsy (Figure 4) showed the nodular hepatomegaly with a slightly enlarged spleen with discreet nodular lesions. It also showed significant amounts of blood in the abdominal cavity resulting from cutting the two organs containing multiple blood-filled cavities. Microscopic examination (Figures 5 and 6) confirmed the diagnosis of hepatosplenic peliosis. Neither elements of malignancy nor infectious processes were found in the samples, so it was assumed to be an idiopathic etiology.
HEPATOSPLENIC PELIOSIS
Overview
Peliosis is a very rare benign entity characterized by blood filled cavities within solid organs (1). Traditionally, it was thought that peliosis was found in organs of the mononuclear-phagocyte system such as the liver, spleen, bone marrow and lymph nodes. Nevertheless, additional research (1) has shown that other organs such as lungs, parathyroid glands and kidneys can also be involved (2).
Hepatic peliosis is an infrequent benign condition. Cavities are distributed randomly, have irregular shapes, and range in size from 0.5 ml to several centimeters (1, 3, 4). Sometime there are multiples isolated lesions, but other times they gather in clusters or connect with each other laterally to create networks (5, 6).
History
Hepatic peliosis was first described by Wagner in 1861 (7), then in 1899 Schrone described several cases of peliosis (8). In 1916 Schoenlak et al. introduced the term peliosis which means "purple" or "maroon" because of the color observed in the hepatic tissue as a result of blood extravasation (9). In 1930 Hanser et al. described multiple hemorrhaging foci of spherical lesions as hepatic peliosis (10). These lesions consisted of well defined hepatocytes connected through centrilobular veins. In 1950, Zak described this entity for the first time in the English language literature and reviewed the entire hypothesis related to its etiology and pathogenesis (11). In 1964 Yanoff and Rawson differentiated between parenchymal and phlebectasial lesions (12) 103 years had passed by the time Caroli et al. first diagnosed peliosis in a live patient (13). In 1971 Drut and Pawlow described the first case of hepatosplenic peliosis in Argentina (14). In 1975 Fouquette and Lefebure showed this association for the first time in Canada while Chapoy et al. found it in France in 1976.15, 16 In 1992 Mayes et al. and Radin described the first cases in USA (17, 18).
Physiopathology
Peliosis fundamentally affects organs of the reticuloendothelial system (11, 21), Whether or not the liver is affected varies from case to case. In autopsies 10% to 50% of the livers were affected by peliosis (19). However, laparoscopic studies show a lower frequency (20). When the liver is severely affected, it looks like Swiss (Gruyere) cheese (20).
The blood contained in these cavities is usually liquid which indicates that is circulating rather than static blood. Fibrin thrombi such as those seen in disseminated intravascular coagulation have not been reported so far (21).
The peliotic cavities communicate with neighboring vascular structures, especially with sinusoids which are normally dilated (22). Cysts and dilated sinusoids have no preferred dispositions (21, 22).
Yanoff and Rawson described two types of disease: the parenchymal type which has blood-filled spaces lined with hepatocytes and which is usually associated with necrotic parenchymal hemorrhaging, and the phlebectasial type in which spaces are lined with endothelium and which are produced by dilatation of aneurysms of the central veins (12). In 1978, DeGott et al. described major peliosis hepatis and minor hepatic lesions with peliosis. The former consists of large confluent cavities compromising majority of the liver lobules within which the hepatic trabeculae are thinned or interrupted. In the minor form, the size of the cavities is small and lesions only affect one part of the liver lobules. Hepatocytes may be normal (23).
Since 1950 several theories have been postulated to explain the physiopathology of hepatosplenic peliosis. The most widely accepted include obstruction of the centrilobular and sinusoidal blood flow, hepatocellular necrosis and the effects of toxins on the sinusoidal barrier and the fibrous network.
Epidemiology
Since 1978 splenic peliosis (SP) has been reported on 34 occasions in the literature. There are 7 well documented cases of splenic rupture (3). Tada et al. examined 1,200 autopsies conducted over a 3 year period and found peliosis in 10 cases. Out of those cases, 8 had peliosis only in the spleens (4).
Hepatosplenic peliosis is very rare disease which has an incidence of 0.13%. In 1928 Gratzer showed a prevalence of 0.2% in autopsies of people who died as the result of tuberculosis (26). DeGott et al. showed a prevalence of 2-4% in laparoscopic studies of patients who had had renal transplants (23). Bruguera et al. reported a prevalence of 2.9% in liver biopsies (27). Cereceda et al. found a prevalence of 0.85% in laparoscopic studies (20).
Recently, as the result of increasing knowledge about this disease derived from the use of laparoscopy, hepatic biopsies and imaging studies, it has been found that peliosis is relatively frequent (1, 17, 18). Peliosis in the spleen is exceptional with less than 100 published cases in the global literature (28). It is almost always it is associated with hepatic peliosis and hepatosplenic peliosis is only slightly documented in the literature reviewed (14-18, 29).
In the majority of the cases peliosis is diagnosed in the fi fth decade of life, even though the literature notes that it can affect children and teenagers (24, 30). Both genders are affected although it seems to more common in the male gender (20).
Etiology
Hepatosplenic peliosis has been associated with a variety of other factors:
- As incidental findings with tumors including Wilms Tumor, hematological malignancies, peritoneal carcinomatosis, endocrine neoplasia, seminoma, and liver, adrenal, pancreatic, breast, and colon cancer (1, 3, 11, 28).
- Infectious diseases including retrovirus, mumps, parvovirus, herpes virus, rickettsia (mainly in AIDS patients with bacillary angiomatosis peliosis developing secondary to infection with Bartonella henselae), mycobacteria, hepatitis B and C, and staphylococcus aureus (20, 21, 24, 28).
- Systemic diseases including purpuric syndromes, Fanconi aplastic anemia, myelodysplastic syndrome, liver cirrhosis, portal hypertension, rheumatoid arthritis, leukemia, monoclonal gammopathy, systemic lupus erythematosus, heart failure, diabetes mellitus, mastocytosis, Castleman's disease, and histiocytosis (3, 11, 28).
- Congenital diseases including von Gierke disease, Osler-Weber-Rendu syndrome (21, 28, 29).
- Cobalt radiation therapy (28, 29, 31-34).
- Long term treatment with anabolic steroids, corticosteroids, oral contraceptives, cytostatic drugs, vitamin A, erythropoietin, allopurinol, paracetamol, aspirin, cimetidine, anti-choleretic drugs, ketoconazole, methyldopa, verapamil, nitrofurantoin, valproic acid, chlorpromazine, and oxazepam (1, 3, 20,28).
- Exposure to toxins including arsenic, vinyl chloride, thorium dioxide-Thorotrast, selenium, alcohol, phalloidin, and aflatoxin B1 (21, 23, 24, 28).
- Hormonal overload in pregnancy, childbirth and puerperal fever (21, 24, 28).
- Organ transplants (31)
- Splenic vein thrombosis (28, 35, 36).
Some authors have suggested that peliosis may be a congenital malformation (37). Although this theory is not gene-rally accepted, the fact that this entity is being observed in children must be taken into account (30, 31).
Duff ard-Thierman et al. indicate that in some cases no etiologic factors have been found to explain the presence of peliosis (38). In fact the medical literature reports that 25% to 50% of all cases have no identifiable causal factor (24, 25, 30, 39).
Clinical manifestations
Since patients with peliosis are usually asymptomatic, lesions are found incidental to biopsies, laparoscopic studies, imaging or surgical procedures (1, 20, 21). In some cases the patient presents hepatomegaly, splenomegaly, abdominal pain, a palpable abdominal mass, abdominal distension, or signs of the underlying etiology (2-4, 6). Infrequently peliosis presents through one of its complications such as portal hypertension, hemorrhaging due to rupture of the peritoneal cavity, liver failure, hepatorenal syndrome, peliotic cavity clotting, and hypersplenism (20, 31, 34). Nevertheless, it is sometimes hard to separate the eff ects of the drugs used in the treatment of an associated disease from peliosis itself (35).
Clinical evolution of peliosis varies. It may be asymptomatic unless a complication appears or focal liver lesions or splenic lesions are observed as casual findings in imaging (17, 31). Complications occur frequently in cases of large cavities (11, 23). Despite all of this, the natural evolution of peliosis is unknown. It may fluctuate from resolution of lesions after the agent causing them is suppressed to acute liver failure with fatal peritoneal hemorrhaging (40).
Diagnosis
Hepatosplenic peliosis should be suspected in patients undergoing prolonged treatment with drugs or known toxins capable of producing this disease and in patients who present persistent abdominal pain or whose imaging studies present are suggestive of its presence.
Because clinical manifestations of hepatosplenic peliosis are often absent or difficult to discern, diagnosis is oft en casual. Laparoscopy allows recognition of small lesions only millimeters in diameter. Larger lesions are found through angiography in which it is usual to observe numerous nodules of various sizes filled with contrast material during the late arterial and parenchymal phases which persist at the beginning of the venous phase. These nodules are easily distinguished from primary tumors of the liver, metastases and hemangiomas by their more uniform appearance (17, 18, 20, 34). Hepatic gammagraphy is recommended by some authors (13, 38).
Abdominal ultrasound generally shows homogeneous hypoechoic lesions in patients with hepatosplenic peliosis, but it can also demonstrate heterogeneous lesions in patients with hemorrhaging or in cases of hyperechoic lesions in apparently normal livers (41).
CT findings differ according to the size of the lesion, the extension of the communication with the sinusoid, the presence or absence of thrombi inside the cavity and the presence of blood. Lesions are regularly hypodense prior and after the administration of the contrast medium. If the peliosis cavities are smaller than 1 cm CT results may be normal. If the cavities are larger, and they communicate with the sinusoids, these cavities will have the same attenuation. In the case of thrombosed cavities, they will present the appearance of calcified nodules (40, 41). MRIs do not detect uncomplicated peliosis but does give suggestive findings for hyperintense lesions on T1 and T2. There may be small hyperintense foci due to the presence of methemoglobin (40-42).
Fine-needle aspiration produces histopathology through which a diagnosis can be confirmed. This can be performed without risk of accidents in cases of small lesions, but it cannot be performed when large lesions are present for fear of an iatrogenic result (20).
Histologically the process consists of dilation of the sinusoids with formation of cysts partially lined by endothelial cells. These constitute collections of blood cells surrounded by a membrane that perfectly lines the process of the surrounding structures which are usually unharmed (21, 43, 44).
Differential diagnosis
Differential diagnosis with CT scans requires the presence of adenomas which are hyperdense in the arterial phase but become isodense in relation to the rest of the parenchyma in the portal phase. Nodular hyperplasia is hypervascular in the arterial phase but become isodense in the late portal phase. Hypervascular metastases become hypodense in the late arterial phase due to rapid contrast washout which frequently produces distortion of the vascular network (3942). Nevertheless the fundamental diff erential diagnosis is among primary or metastatic neoplasms, hematological disorders of all kinds, liver abscesses and vascular tumors such as hemangiomas and hemangioendothelioma (11, 20, 24, 27, 40).
Treatment
Treatment of hepatosplenic peliosis depends on the underlying cause (45). When peliosis is bacterial, the administration of antimicrobials results in recovery and disappearance of lesions. When it is due to a medication such as anabolic steroids, immunosuppressants and vitamin A, discontinuation of medication allows the affected organ to recover. Similarly discontinuation of alcohol intake, contact with selenium and other toxics allows the affected organ to recover. In the majority of cases it is necessary to review and revise treatment because there are situations in which the responsible medication is overlooked (28).
For patients with intrahepatic hemorrhaging embolization through a catheter in the hepatic artery is used, but if hemorrhaging is severe a hepatectomy can be lifesaving. Endoscopic splenectomy has reduced mortality below those of conventional surgery (46). Nevertheless, currently endoscopic splenectomy is being prophylactically administered under conditions that can produce rupture of the spleen as happens in splenic sarcoidosis (47).
DISCUSSION
The case of hepatosplenic peliosis that we presented occurred in a 60 year old woman. Almost all of her liver was aff ected. 60% of her lesions were of very variable size and most of them were connected in a honeycombed visible to macroscopic observation. We concluded that this was a major parenchymal case of hepatosplenic peliosis.
The patient did not report consumption of any medication capable of producing this entity, although she did take thiazide-type drugs and ACE inhibitors. Her only surgical history consisted of excision of a benign pericardial cyst of unrelated etiology. Clinical studies did not show the existence of any other disease and histopathology of her biopsy showed nothing that could produce hepatosplenic peliosis. We concluded that this was an idiopathic etiology.
Clinical manifestations presented were related to the presence of giant hepatomegaly, progressively anemic blood trapped in the peliotic cavities, development of complications including extensive and large cavities, and liver failure and hemoperitoneum which were the cause of death.
Neither laparoscopy nor hepatic gammagraphy could be performed because of the patient´s poor condition. Abdominal ultrasound revealed hepatomegaly aff ecting not only its characteristic lesions and compression of neighboring organs, but also affecting the spleen through the presence of two not very echogenic nodules of 0.7 cm and 0.9 cm although there was no lymphadenopathy. Th e CT scan corroborated the gigantic multi-nodular hepatomegaly compromising both lobes and having hypodensities varying from 0.5 cm to several centimeters in diameter. There was no splenomegaly, but two hypodense lesions of 75mm and 92 mm were evident. All of these findings were confirmed at autopsy.
The patient died before we could arrive at a diagnostic conclusion. Hepatosplenic peliosis became evident during the postmortem.
CONCLUSION
Peliosis is such a rare benign pathological entity that this report is the first in our country. In hepatic peliosis irregularly shaped cavities of variable size are randomly distributed to varying extents. Hepatosplenic peliosis is poorly documented in the literature reviewed, although identification requires a high index of suspicion. It should be suspected in patients with prolonged treatment using drugs or toxins known to be capable of producing this disease and in patients who show persistent abdominal pain or whose CT scans, x-rays or sonography show suggestive images. Diagnosis is either incidental or through ruling out other diagnoses. Treatment depends on the underlying cause.
REFERENCES
1. Tsokos M, Erbersdobler A. Pathology of peliosis. Forensic Sci Int 2005; 149(1): 25-33.
2. Ichijima K, Kobashi Y, Yanabe H, Fujii Y, Inoue Y. Peliosis hepatis: an usual case involving multiple organs. Acta Pathol Jpn 1980; 30: 109-120.
3. Lashbrook D, James R, Phillips A, Holbrook A, Agomar A. Splenic peliosis with spontaneous rupture: report of two cases. BMC Surg 2006; 6: 9-14.
4. Tada T, Wakabayashi T, Kishimoto H. Peliosis of the spleen. Am J Clin Pathol 1983; 79(6): 708-13.
5. Fouquette B, Lefebure R. Péliose hépatosplénique. Signes angiographiques. Nouv Presse Med 1976; 5: 2455-2458.
6. Lacson A, Berman L, Neiman R. Peliosis of the spleen. Am J Clin Path. 1979; 71(5): 586-90.
7. Wagner E. Fall von bluteysten der leber. Arch D Heilk. 1961; 2: 369-70.
8. Schrohe T. Telangiektasien der leber. Virchows Arch F Pathol Anat 1899; 156: 37-61.
9. Schoenlank W. Ein fall won peliosis hepatis. Virchows Arch Pathol Anat 1916; 222: 358-364.
10. García RL, Khan MK, Berlin RB. Peliosis of the spleen with rupture. Hum Pathol 1982; 13(2): 177-9.
11. Zak FG. Peliosis hepatis. Am J Pathol 1950; 26: 1-15.
12. Yanoff M, Rawson A. Peliosis hepatis. An anatomic study with demonstration of two varieties. Arch Pathol 1964; 77: 159-165.
13. Caroli J, Julien C, Albano O. Péliose hépatique et plasmosarcomatose splénique. Première observation reconnue in vivo. Semaine des Hôpitaux de Paris 1964; 40: 1709-15.
14. Drut R, Pawlow D. Peliosis hepatoesplénica y peliosis hepática. Variedad parenquimatosa. Prensa Médica Argentina 1971; 58: 1739.
15. Fouquette B, Lefebure R. Péliose hépatique et splénique. L'union Médicale du Canada 1975; 104: 107.
16. Chapoy P, Sahel J, Burelle H, Bunneau H, Payan H, Clement J, et al. La péliose hépato-splénique. Signes angiographiques. Nouv Presse Med 1976; 5: 2455-2458.
17. Mayes C, Caron K, Bisset G. Splenic and hepatic peliosis. MR findings. A J R 1992; 158: 75-76.
18. Radin DR. Spontaneous resolution of the liver and spleen in a patient with HIV infection. A J R 1992; 158: 1409-11.
19. Solis Herugo J, Colina F, Muñoz-Yague M, Castellano G, Morillas J. Red purple areas of liver surface: the laparoscopic picture of peliosis hepatis. Endoscopy 1983; 15: 96-100.
20. Cereceda C, Solis J, Colina F, Muñoz-Yague M, Castellanos G, Castellanos G, et al. Peliosis hepática: estudio de 31 casos. An Med Intern 1986; 3(9): 371-6.
21. Zafrani E, Casier A, Baudelot A, Feldmann G. Ultrastructural lesions of the liver in human peliosis: a report of 12 cases. Am J Pathol 1984; 114: 349-359.
22. Zafrani E, Feldmann G. Primary lesions of the hepatic sinusoid. En: Bioulac-Sage P, Balabaud C, editors. Sinusoids in human liver: health and disease. Rijswijk, Th e Netherlands: Th e Kupffer Cell Foundation 1988. p. 207-222.
23. DeGott C, Rueff B, Kneis H, Duboust A, Potet F, Benhamou J. Peliosis hepatis in recipients of renal transplant. Gut 1978; 19: 748-750.
24. Samyn M, Hadzie N, Davenport M. Peliosis hepatis in childhood: case report and review of the literature. J Pediatr Gastroenterolol Nutr 2004; 39: 431-4.
25. Joseph F, Younis N, Haydon G. Peliosis of the spleen with massive recurrent haemorrhagic ascitis, despite splenectomy and associated with elevated levels of vascular endothelial growth factor. Eur J Gastroenterol Hepatol 2004; 16: 1401-6.
26. Gratzer g. Uber sogennate peliosis hepatis. Frankfurt Ztschr F Pathol 1928; 36: 134-145.
27. Bruguera M, Araguibel F, Ros E, Rodes J. Incidence and clinical significance of sinusoid dilatation in liver biopsies. Gastroenterology 1978; 75: 474-478.
28. Pérez-Holanda S, Tojo S, Calleja M, Fernández JA, Fernández P, Martínez MD, Fernández F, Rodríguez J, Valverde D. Peliosis esplénica, una entidad poco frecuente. Rev Esp Enferm Dig (Madrid) 2007; 99(6): 359-367.
29. Nesher G, Dollberg L, Zimran A, Henshko C. Hepatosplenic peliosis after danazol and glucocorticoids for ITP. N Engl J Med 1985; 312: 242-43.
30. Hiorns M, Ross U, Roebuck D. Peliosis hepatis causing inferior vena cava compression in a 3-year old child. Pediatr Radiol 2005; 35(2): 209-11.
31. Muñoz MM, Rodriguez ZN, Tordecilla C, Ureta E, Rizzardini C, Soto V, et al. Peliosis hepatis como complicación del uso de antoconceptivos orales en una paciente con mielodisplasia. Rev Chil Pediatr 2009; 80(4): 354-60.
32. González Bravo M, Hernández Borges A, Rodríguez J, López R, Lupiani P, Domenech E. Lesiones hepaticas en un paciente con anemia de Fanconi. BSCP Can Ped 2001; 25(3): 213-6.
33. Corti M, Villafaña M, Castello T, Mendez N, Gancedo E, Polmieri O. Angiomatosis bacilar con peliosis hepática en un paciente con SIDA. Medicina (Buenos Aires) 2006; 66: 153.
34. Abbot RM, Levy AD, Aguilera NS, Gorospe L, Th ompson WM. Primary vascular neoplasms of the spleen: Radiologicpathologic correlation. Radiographic 2004; 24: 1137-63.
35. Zafrani E, Pinaudeau Y, Dhumeaux D. Drug-induced vascular lesions of the liver. Arch Intern Med 1983; 143: 405-502.
36. Gushiken FC. Peliosis hepatis after treatment with 2-Chloro3'-deoxyadenosine. South Med J 2000; 93(6): 625-6.
37. Weir M, Decherd J, Beathard G. A unique case of peliosis hepatis. Texas report of biology and Medicine 1969; 11(5): 27.
38. Duff aurd-Thierman D, Hecht Y, Ragasol A, Ferrier J, Gallard P, Goldlust D. Péliose criptogenetique régressive: un cas. Nouvelle Presse Medicale 1977; 6: 163-6.
39. Resnick I, Salvin S, Or R. Peliosis hepatis following treatment with androgen-steroids in patients with bone marrow failure syndromes. Hematologica 2007; 92: 106-10.
40. Vignaux O, Leghmann P, De Pinieux G. Hemorrhagic necrosis due to peliosis hepatis: imaging findings and pathological correlation. Eur Radiol 1999; 9: 454-456.
41. Ferrozzi F, Tognini G, Zuccoli G, Cademartiri F, Pavone P. Peliosis hepatis with pseudotumoral and hemorrhagic evolution: CT and MR findings. Abdominal Imagin 2001; 26: 197-199.
42. Jamadar D, D'Souza S, Thomas E. Case report: Radiological appearances in peliosis hepatis. Br j Radiol 1994; 67: 102-104.
43. Rosai J. Spleen. En: Rosai J. Ackerman's surgical pathology. 8th ed. St-Louis, Missouri: Mosby Year Book Inc; 1996. p. 1782.
44. Crawford JM. Hígado y vías biliares. En: Schoen FJ, Cotran RS, Kumar V, Robbins SL, editores. Robbins. Patología Estructural y Funcional. 5ª edición. Madrid: McGraw-Hill-Interamericana de España; 1996, p. 919-991.
45. Navarro Ibánez V, Sabater Marco V. Peliosis hepática asociada a meningitis por N. meningitidis. Patología 1996; 29: 141-2.
46. Katkhouda N, Mayor E. Laparoscopic splenectomy. Surg Clin North Am 2000; 80(4): 1285-97.
47. Zia H, Zemon H, Brody F. Laparoscopic splenectomy for isolated sarcoidosis of the spleen. J Laparoendoscop Adv Surg Tech 2005; 15(2): 160-2.
1. Tsokos M, Erbersdobler A. Pathology of peliosis. Forensic Sci Int 2005; 149(1): 25-33. [ Links ]
2. Ichijima K, Kobashi Y, Yanabe H, Fujii Y, Inoue Y. Peliosis hepatis: an usual case involving multiple organs. Acta Pathol Jpn 1980; 30: 109-120. [ Links ]
3. Lashbrook D, James R, Phillips A, Holbrook A, Agomar A. Splenic peliosis with spontaneous rupture: report of two cases. BMC Surg 2006; 6: 9-14. [ Links ]
4. Tada T, Wakabayashi T, Kishimoto H. Peliosis of the spleen. Am J Clin Pathol 1983; 79(6): 708-13. [ Links ]
5. Fouquette B, Lefebure R. Péliose hépatosplénique. Signes angiographiques. Nouv Presse Med 1976; 5: 2455-2458. [ Links ]
6. Lacson A, Berman L, Neiman R. Peliosis of the spleen. Am J Clin Path. 1979; 71(5): 586-90. [ Links ]
7. Wagner E. Fall von bluteysten der leber. Arch D Heilk. 1961; 2: 369-70. [ Links ]
8. Schrohe T. Telangiektasien der leber. Virchows Arch F Pathol Anat 1899; 156: 37-61. [ Links ]
9. Schoenlank W. Ein fall won peliosis hepatis. Virchows Arch Pathol Anat 1916; 222: 358-364. [ Links ]
10. García RL, Khan MK, Berlin RB. Peliosis of the spleen with rupture. Hum Pathol 1982; 13(2): 177-9. [ Links ]
11. Zak FG. Peliosis hepatis. Am J Pathol 1950; 26: 1-15. [ Links ]
12. Yanoff M, Rawson A. Peliosis hepatis. An anatomic study with demonstration of two varieties. Arch Pathol 1964; 77: 159-165. [ Links ]
13. Caroli J, Julien C, Albano O. Péliose hépatique et plasmosarcomatose splénique. Première observation reconnue in vivo. Semaine des Hôpitaux de Paris 1964; 40: 1709-15. [ Links ]
14. Drut R, Pawlow D. Peliosis hepatoesplénica y peliosis hepática. Variedad parenquimatosa. Prensa Médica Argentina 1971; 58: 1739. [ Links ]
15. Fouquette B, Lefebure R. Péliose hépatique et splénique. L'union Médicale du Canada 1975; 104: 107. [ Links ]
16. Chapoy P, Sahel J, Burelle H, Bunneau H, Payan H, Clement J, et al. La péliose hépato-splénique. Signes angiographiques. Nouv Presse Med 1976; 5: 2455-2458. [ Links ]
17. Mayes C, Caron K, Bisset G. Splenic and hepatic peliosis. MR findings. A J R 1992; 158: 75-76. [ Links ]
18. Radin DR. Spontaneous resolution of the liver and spleen in a patient with HIV infection. A J R 1992; 158: 1409-11. [ Links ]
19. Solis Herugo J, Colina F, Muñoz-Yague M, Castellano G, Morillas J. Red purple areas of liver surface: the laparoscopic picture of peliosis hepatis. Endoscopy 1983; 15: 96-100. [ Links ]
20. Cereceda C, Solis J, Colina F, Muñoz-Yague M, Castellanos G, Castellanos G, et al. Peliosis hepática: estudio de 31 casos. An Med Intern 1986; 3(9): 371-6. [ Links ]
21. Zafrani E, Casier A, Baudelot A, Feldmann G. Ultrastructural lesions of the liver in human peliosis: a report of 12 cases. Am J Pathol 1984; 114: 349-359. [ Links ]
22. Zafrani E, Feldmann G. Primary lesions of the hepatic sinusoid. En: Bioulac-Sage P, Balabaud C, editors. Sinusoids in human liver: health and disease. Rijswijk, Th e Netherlands: Th e Kupffer Cell Foundation 1988. p. 207-222. [ Links ]
23. DeGott C, Rueff B, Kneis H, Duboust A, Potet F, Benhamou J. Peliosis hepatis in recipients of renal transplant. Gut 1978; 19: 748-750. [ Links ]
24. Samyn M, Hadzie N, Davenport M. Peliosis hepatis in childhood: case report and review of the literature. J Pediatr Gastroenterolol Nutr 2004; 39: 431-4. [ Links ]
25. Joseph F, Younis N, Haydon G. Peliosis of the spleen with massive recurrent haemorrhagic ascitis, despite splenectomy and associated with elevated levels of vascular endothelial growth factor. Eur J Gastroenterol Hepatol 2004; 16: 1401-6. [ Links ]
26. Gratzer g. Uber sogennate peliosis hepatis. Frankfurt Ztschr F Pathol 1928; 36: 134-145. [ Links ]
27. Bruguera M, Araguibel F, Ros E, Rodes J. Incidence and clinical significance of sinusoid dilatation in liver biopsies. Gastroenterology 1978; 75: 474-478. [ Links ]
28. Pérez-Holanda S, Tojo S, Calleja M, Fernández JA, Fernández P, Martínez MD, Fernández F, Rodríguez J, Valverde D. Peliosis esplénica, una entidad poco frecuente. Rev Esp Enferm Dig (Madrid) 2007; 99(6): 359-367. [ Links ]
29. Nesher G, Dollberg L, Zimran A, Henshko C. Hepatosplenic peliosis after danazol and glucocorticoids for ITP. N Engl J Med 1985; 312: 242-43. [ Links ]
30. Hiorns M, Ross U, Roebuck D. Peliosis hepatis causing inferior vena cava compression in a 3-year old child. Pediatr Radiol 2005; 35(2): 209-11. [ Links ]
31. Muñoz MM, Rodriguez ZN, Tordecilla C, Ureta E, Rizzardini C, Soto V, et al. Peliosis hepatis como complicación del uso de antoconceptivos orales en una paciente con mielodisplasia. Rev Chil Pediatr 2009; 80(4): 354-60. [ Links ]
32. González Bravo M, Hernández Borges A, Rodríguez J, López R, Lupiani P, Domenech E. Lesiones hepaticas en un paciente con anemia de Fanconi. BSCP Can Ped 2001; 25(3): 213-6. [ Links ]
33. Corti M, Villafaña M, Castello T, Mendez N, Gancedo E, Polmieri O. Angiomatosis bacilar con peliosis hepática en un paciente con SIDA. Medicina (Buenos Aires) 2006; 66: 153. [ Links ]
34. Abbot RM, Levy AD, Aguilera NS, Gorospe L, Th ompson WM. Primary vascular neoplasms of the spleen: Radiologicpathologic correlation. Radiographic 2004; 24: 1137-63. [ Links ]
35. Zafrani E, Pinaudeau Y, Dhumeaux D. Drug-induced vascular lesions of the liver. Arch Intern Med 1983; 143: 405-502. [ Links ]
36. Gushiken FC. Peliosis hepatis after treatment with 2-Chloro3'-deoxyadenosine. South Med J 2000; 93(6): 625-6. [ Links ]
37. Weir M, Decherd J, Beathard G. A unique case of peliosis hepatis. Texas report of biology and Medicine 1969; 11(5): 27. [ Links ]
38. Duff aurd-Thierman D, Hecht Y, Ragasol A, Ferrier J, Gallard P, Goldlust D. Péliose criptogenetique régressive: un cas. Nouvelle Presse Medicale 1977; 6: 163-6. [ Links ]
39. Resnick I, Salvin S, Or R. Peliosis hepatis following treatment with androgen-steroids in patients with bone marrow failure syndromes. Hematologica 2007; 92: 106-10. [ Links ]
40. Vignaux O, Leghmann P, De Pinieux G. Hemorrhagic necrosis due to peliosis hepatis: imaging findings and pathological correlation. Eur Radiol 1999; 9: 454-456. [ Links ]
41. Ferrozzi F, Tognini G, Zuccoli G, Cademartiri F, Pavone P. Peliosis hepatis with pseudotumoral and hemorrhagic evolution: CT and MR findings. Abdominal Imagin 2001; 26: 197-199. [ Links ]
42. Jamadar D, D'Souza S, Thomas E. Case report: Radiological appearances in peliosis hepatis. Br j Radiol 1994; 67: 102-104. [ Links ]
43. Rosai J. Spleen. En: Rosai J. Ackerman's surgical pathology. 8th ed. St-Louis, Missouri: Mosby Year Book Inc; 1996. p. 1782. [ Links ]
44. Crawford JM. Hígado y vías biliares. En: Schoen FJ, Cotran RS, Kumar V, Robbins SL, editores. Robbins. Patología Estructural y Funcional. 5ª edición. Madrid: McGraw-Hill-Interamericana de España; 1996, p. 919-991. [ Links ]
45. Navarro Ibánez V, Sabater Marco V. Peliosis hepática asociada a meningitis por N. meningitidis. Patología 1996; 29: 141-2. [ Links ]
46. Katkhouda N, Mayor E. Laparoscopic splenectomy. Surg Clin North Am 2000; 80(4): 1285-97. [ Links ]
47. Zia H, Zemon H, Brody F. Laparoscopic splenectomy for isolated sarcoidosis of the spleen. J Laparoendoscop Adv Surg Tech 2005; 15(2): 160-2. [ Links ]











 text in
text in