Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.27 no.3 Bogotá jul./set. 2012
Current approaches to the study and treatment of cystic tumors of the pancreas: A brief analysis of currently available tools
Diego Aponte Martín, MD. (1)
(1) Internist at the Universidad Javeriana, Gastroenterologist at the Universidad National, Academic Coordinator of the Gastroenterology Masters Degree program at the Fundación Universitaria Sanitas in Bogotá, Colombia.
Received: 01-08-12 Accepted: 17-08-12
Because of their increasing frequency, cystic tumors of the pancreas have clearly become a topic of great interest. This is related to the development, improvement and common use of radiological images such as CT scans and magnetic resonance imaging (MRI). In most cases these images are ordered for diagnosis of various gastrointestinal symptoms and, as a consequence, these cysts are often found as incidental findings.
Although the exact prevalence of cystic lesions of the pancreas is not known, different studies report prevalences between 2% and 16% in radiological studies. This is corroborated by autopsy findings of 25% with approximately half being cystic neoplasms of the pancreas (CNP) (1-3).
Although, proportions of the different types of (CNP) vary from one region of the world to another, it is clear that some (CNP) are more common than others. Serous cystic neoplasms (SCN) account for 32% to 39%, mucinous cystic neoplasms (MCN) account for 10% to 45%, intraductal papillary mucinous neoplasms (IPMN) account for 21% to 33%, and solid pseudopapillary neoplasms (SPN) account for less than 10% of all cystic neoplasms of the pancreas (4).
Types of (CNP) must be differentiated from each other since the prognosis, treatment and monitoring for each is clearly different from the others. Nevertheless, distinguishing them apart is not easy. For this reason different opinions appear in the literature about the best approach for diagnosis and treatment of these lesions. One group has even proposed surgical resection for all CNP in patients with suitable states of health (5, 6). In my medical opinion and those of other authors, this treatment might be defined as aggressive and as a course of action which minimizes the risks of failure to diagnose and treat lesions with malignant components. In addition this treatment could subject many healthy patients with benign lesions to unnecessary surgical risks of morbidity which on some occasions can be very high despite the undeniable progress in this surgical specialty. Moreover, we should take into account the fact that in recent years there has been significant progress in developing tools that generate greater diagnostic certainty and accuracy which helps to establish which patients should be referred to surgery (5, 7, 10-12).
To respond in detail to the question of, "What should the current focus of study and management of cystic tumors be? We propose to evaluate the following tools in this important section of the magazine:
1. Clinical diagnosis
2. Radiological imaging
3. Endoscopic ultrasound
4. Analysis of cyst fluid. At the end we will give a recommendation and suggestions regarding treatment.
CLINICAL DIAGNOSIS
Epidemiology is useful since there are significant differences in ages and genders of patients who present different types of CNP. A mnemonic that can be useful in the study of CNPs, and which is being employed in various groups around the world, uses family relationships to help remember each neoplasia:
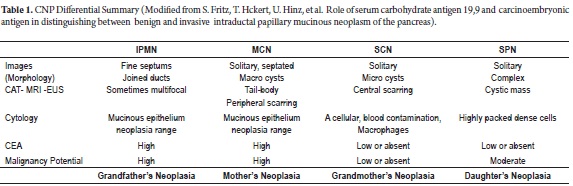
Grandmother's Tumors are SCNs because they primarily occur in 60-70 year old women as benign lesions. There have only been about 25 cases of malignancy described anywhere in the world. These tumors are solitary and can be big (2).
Mother's Tumors are MCNs which occur mainly in women 50 years old and younger as pre-malignant or malignant lesions. They are encapsulated, do not communicate with the pancreatic duct, and are mostly located in the tail or body (2).
Daughters' Tumors are SPNs which occur most often in women between the ages of 20 and 30 years old (2).
Grandfather's Tumors are IPMNs because they have slightly higher frequencies among men, with a peak presentation at approximately 70 years old. They clearly communicate with the main or secondary pancreatic duct and can range from benign to frankly malignant. Malignant IPMNs are frequently associated with main ducts which are more than 1 cm or with a secondary duct that is more than 3 cm (2).
40% to 75% of pancreatic cystic tumors are asymptomatic and found incidental to searches for other gastrointestinal disorders. When there are symptoms, they are often vague nonspecific symptoms including abdominal pain, nausea and dyspepsia (5). Patients with large tumors or with associated malignant components may present weight loss, jaundice, associated pancreatitis or diabetes (6).
Routine blood tests to evaluate liver, pancreatic, renal and hematological functions are usually normal or have unclear results. They may show nonspecific changes or CA 19.9 and carcinoembryonic antigen (CEA) levels with no diagnostic value. Some reports have shown 80% elevations of these two antigens in patients with invasive IPMN compared with only 18% in patients with noninvasive cases. (8)
RADIOLOGICAL IMAGES
Great advances have been made in perfecting studies of the morphology of cystic neoplasms of the pancreas. These are used to describe their anatomical locations, their relationship with other organs and blood vessels, to determine presence or absence of metastases, and to determine whether or not the lesion communicate with the pancreatic ducts. Each neoplastic lesion has specific characteristics as seen in Table 1 (8).
ENDOSCOPIC STUDIES AND USEFULNESS OF STUDYING MATERIAL FROM THE CYST
Even though increasing amounts of evidence indicate that endoscopic ultrasound (EUS) can be very useful for refining morphological details of CNP, its greatest diagnostic contribution is its use for performance of fine needle aspiration (FNA) to obtain cyst fluid for cytological studies. When an expert cytopathologist is present within the study room we can obtain a good approximation of whether or not cells suggestive of malignancy are present and also determine what type of cyst is suggested by cellular differentiation. Cyst material for use in antigenic studies, primarily CEA, can also be extracted with the same method. Dr. Brugge's classic studies show that a 192 ng/ml point can differentiate mucinous from non-mucinous tumors (10). DNA analysis in very recent studies shows that the RAS K mutation is highly specific (96%) to mucinous cysts. High mutation values with large amplitudes and specific sequences are indicators of malignancies (11, 14).
Several current articles demonstrate the usefulness of high resolution optical images which are not yet available in Colombia. These include optical coherence tomography which explores the morphology of pancreatic tissue and can differentiate mucinous neoplasms from non-mucinous neoplasms with 95% sensitivity and 95% specificity. Confocal laser endomicroscopy studies in vivo histology with micro-probes that reach pancreatic tissue with the assistance of EUS. These new techniques will soon become key tools for diagnosing different types of CNP (12, 13).
SUGGESTED APPROACH FOLLOWING DISCOVERY OF A PANCREATIC CYST
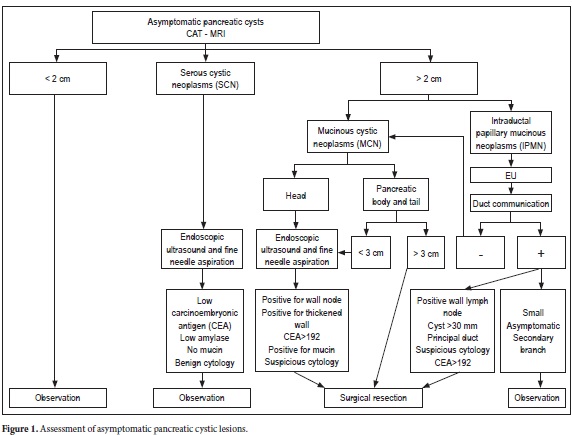
The various algorithms suggested for the study and management of pancreatic cysts place great emphasis on the size and morphology of the lesion.
The following recommendations are modified versions of those found in the various published articles, and are also based on my own point of view regarding management current management of cystic tumors of the pancreas in our country.
The intention of most of the algorithms is to differentiate benign from malignant or potentially malignant tumors. Once pseudocysts are ruled out, we probably face a CNP. The first differentiation which must be made is whether this is a benign neoplasm or a potentially malignant serous cyst such as an MCN or IPMN variation. Then we should evaluate its characteristics to define the risk of malignancy and decide on whether to simply monitor the patient or to refer the patient for surgery. In order to do this we must use the tools previously discussed which obviously vary depending on availability, training, experience in each particular institution in our country.
In addition, this should always be analyzed and evaluated in a multidisciplinary environment where clinicians, endoscopists and surgeons jointly evaluate and determine which course of action has the most benefits and the least risks for the patient (2, 9).
Symptomatic pancreatic lesions
Bigger than 3 cm: Surgical Resection
Asymptomatic pancreatic lesions (Figure 1)
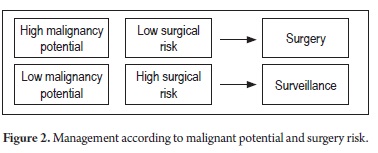
It is also important to define malignant potential and surgical risk in general (Figure 2).
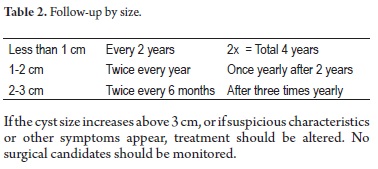
Follow-up of indeterminate cystic tumors
A recent recommendation by Dr. Koenraad Mortele, Associate Radiology Professor and Director of the Division of Resonance at Harvard Medical School is viewed in Table 2.
If the cyst size increases above 3 cm, or if suspicious characteristics or other symptoms appear, treatment should be altered. No surgical candidates should be monitored.
Some new therapeutical strategies have evolved thanks to the possibility of injecting substances such as cytotoxic agents guided by EUS. At first, various concentrations (5% to 80%) of ethanol were used in 25 patients without any reported complications. Cysts resolved completely in 8 patients. Another clinical trial using saline solution report that cyst sizes decreased by 33%. Recently, other agents such as paclitaxel have been applied. Since but since these studies are still early, and although they seem promising, additional scientific data is needed before a precise recommendation can be made about their use (15, 16).
REFERENCES
1. Scheiman JM. Cystic Lesion of the pancreas. Gastroenterology 2005; 128: 263-9.
2. Dewhurst CE, Mortekele KJ. Cystic tumors of the pancreas: imaging and management. Radiol clin North Americ 2012; 50(3): 467-86. Epub 2012 Mar 28.
3. Basturk O, Coban I, Adsay NV. Pancreatics cysts: Pathological classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med 2009; 133: 423-38.
4. Brugge WR, Lawers GY, Sahani D, et al. Cystic neoplasms of the pancreas. Nengl J Med 2004; 351: 1218-26.
5. Aponte Diego. Paciente con quiste pancreático incidental. Rev Col Gastroenterolog 2010; 25(3): 274-280.
6. Horvath KD, Chaboth JA, et al. An aggressive resectional approach to cystic neoplasms of the pancreas. Am J surg 1999; 178(4): 269-74.
7. Sohn TA, Yeo CJ, Cameron JL, et al. Intraductal Papillary mucinous neoplasms of the pancreas: an updated experience, Ann Surg 2004; 239: 788-97.
8. Fritz S, Hckert T, Hinz U, et al. Role of serum carbohydrate antigen 19,9 and carcinoembryonic antigen in distinguishing between benign and invasive intraductal papillary mucinous neoplasm of the pancreas. Br J Surg 2011; 98: 104-10.
9. Won Jae, Brugge William. Pancreatic Cystic neoplasms: Diagnosis and Management. Gastroenterol Clin N Ame 2012; 41: 103-118.
10. Brugge WR, Lewandroski K, Lee L, et al. Diagnosis of pancreatic cyst neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 2004; 126: 1330-6.
11. Khalid A, Zahid M, Finkelstein SD, et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA Study. Gastrointest Endosc 2009; 69: 1095-102.
12. Iftimia N, Won J, Brugge W. Cystic Lesions of the Pancreas: more reliable differentiation with in situ high resolution optical imaging? Expert Rev Gastroenterol Hepatol 2012; 6(2): 125-127.
13. Konda VJ, Aslanian HR, Wallace MB, et al. First assessment of needle-based confocal laser endomicroscopy during EUS-FNA procedures of the pancreas (with videos). Gastrointest Endosc 2011; 74: 1049-60.
14. Kwon RS. Advances in the diagnosis of cystic neoplasms of the pancreas. Curr Opin Gastroenterol 2012; 28(5): 494-500.
15. De Witt J, Dimaio CJ, Brugge WR. Long Term follow-up of pancreatic cysts that resolve radiologically after EUS-guided ethanol ablation. Gastrointest Endosc 2010; 72: 862-6.
16. Oh HC, Seo DW, Song TJ, et al. Endoscopic ultrasonography-guided ethanol lavage with paclitaxel injection treats patients with pancreatic cysts. Gastroenterology 2011; 140: 172-9.











 texto em
texto em