Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.27 no.3 Bogotá jul./set. 2012
Gastric cancer in young patients in Colombia
Martín Gómez, MD. (1), William Otero, MD. (2), Jorge Eduardo Caminos, MD, PhD. (3)
(1) Assistant Professor in the Gastroenterology Unit of the Department of Internal Medicine at the Universidad Nacional de Colombia and Hospital El Tunal in Bogotá, Colombia.
(2) Professor in the Gastroenterology Unit of the Department of Internal Medicine at the Universidad Nacional de Colombia, Hospital El Tunal, Clínica Fundadores, and Clínica de la Mujer in Bogotá, Colombia.
(3) Associate Professor in the Biochemistry Unit of the Physiology Department of the Universidad Nacional de Colombia in Bogotá, Colombia.
Received: 12-01-12 Accepted: 17-08-12
Abstract
Gastric cancer is the leading cause of death among cancers in Colombia. Although its incidence has fallen among older people throughout the world, in recent years it has been increasing among patients under 40 years old. Poor prognoses for these patients is due in part to late diagnoses and in part to the aggresivity of the disease. For these reasons the objectives of this study were to evaluate clinical, endoscopic and histological factors in gastric cancer patients under 40 years of age and compare those factors with findings from older patients in order to establish differences which might allow characterization of this group of patients.
Materials and Methods. This is a retrospective, descriptive study of patients diagnosed with adenocarcinoma in the Hospital el Tunal between December 2005 and December 2010. During this period cancer patients under 40 years of age were compared with those over 40 years of age. Information collected on forms included patients ages and sex and tumor, classification, type of and location. Of the 15,550 upper endoscopies performed during the study period, 226 patients were diagnosed with gastric adenocarcinoma and had their diagnoses confirmed histologically. 20 patients (8.8%) were under 40 years old and 206 were over 40 years old.
Results. The 20 patients under 40 years old were labeled Group 1 and the 206 patients who were over 40 years old were labeled Group II. Average patient age in Group I was 33.5 years while average patient age in Group II was 63 years old. Patient age range in Group I was 17 to 40 years old while in Group 2 it was 41 to 95 years old. 45% of the patients in Group 1 were men while 61.7% of the patients in Group 2 were men (p:<0.05). 90% of the patients in Group I had diffuse adenocarcinomas while only 23.8% (49) of the patients in Group II had diffuse adenocarcinomas (p:<0.05). Only one case was diagnosed early in Group I (5%) while 42 cases (20.4%) were diagnosed early in Group II (5%) (p:<0.05). Of the 19 advanced cases in Group I there were 5 cases of linitis plastica (26.3%) while there were 30 cases (14.5%) found in Group II (p:<0.05).
Conclusion. Gastric cancer is slightly more prevalent among young patients in our study than the prevalence that has been reported elsewhere. These patients presented more advanced stages of cancer than did older patients, and the majority of their cancers were diffuse types (90%) which resulted in a high mortality rate. Early performance of endoscopy is mandatory for young patients. In addition preventative measures such as genetic studies for CDH1 carriers to protect family members from this terrible disease should also be mandatory.
Key words
Gastric cancer, young patients, diffuse.
INTRODUCTION
Worldwide, gastric cancer (GC) is one of the most frequent gastrointestinal tract cancers. It is the fourth most common cancer. It causes 700,000 deaths annually making it the second leading cause of death among cancers (1). In our country GC accounts for the second largest number of cancer cases among males, and accounts for the fourth largest number of cancer cases among females. It is the leading cause of cancer deaths. The estimated incidence of GC was of 20.7 per 100,000 females and 36 per 100,000 males during 1995 and 1999 (2). In 2008, GC occupied sixth place as a cause of death, accounting for 4,549 deaths out of a total of 196,324 deaths. It was preceded by acute myocardial infarcts, murders, chronic pulmonary disease, acute respiratory infections and complications of diabetes mellitus, but GC remains the deadliest cancer (3). Due to these characteristics, GC should be a primary objective for prevention in our health care system. It is very plausible that we should target early stage detection to change the reality that over 90% of the cases diagnosed late currently face: poor prognoses with a survival rate of less than 10% at 5 years (4). If detected at an early stage the prognosis would be radically different: a survival rate greater than 90% at 5 years (4). Hence, the importance of studies that provide insight into the epidemiological, clinical and pathological characteristics of patients with GC to design a strategy that will lead to early detection.
Adenocarcinoma is the primary type of GC. There are two histological types of adenocarcinomas: (5) the most frequent type is intestinal gastric cancer (IGC) while diffuse gastric cancer (DGC) occurs less frequently. IGC is Bly associated with Helicobacter pylori (H. Pylori) and environmental factors such as tobacco consumption and diet (especially salty foods) (6). In addition, according to Pelayo Correa, IGC follows a sequence from chronic inflammation, through gastric atrophy, incomplete intestinal metaplasia and dysplasia, concluding with cancer (7). DGC is less frequent and has origins more Bly related to genetic factors of the host (8). In the case of IGC, the long period of chronic inflammation that precedes the appearance of the tumor makes it more likely that early detection through upper endoscopy will allow the physician to opportunely offer the patient endoscopic or surgical treatment (9). Unfortunately, diffuse cancer is not the same. It generally appears "abruptly" in younger individuals with no history of chronic or specific symptoms and without the preceding chain of inflammatory events that characterizes IGC (10).
Classically, GC is considered to be an adult disease since its incidence increases with age with as primary peak of incidence between the ages of 50 and 70 years (11). However, it is clearly recognized that it can occur in younger individuals. Various series have found that from 2.4% to 8% of all GC cases occur in patients under the age of 40 (12). Studies which have looked at patients younger than 30 years have found that the percentage of total cases accounted for by this groups ranges from 0.5% to 5.2% (13). In Latin America it has been found that the percentage of cases accounted for by young adults is 6.1% in Brazil (14), 5% in Chile (15) and 3.1% in Peru (15).
Recently, a decrease in both the incidence and mortality of IGC has been found in developed countries. This has been attributed to the control of some dietary risk factors, a decrease in the prevalence of H. Pylori, and better conservation of food (16). In contrast, in recent years there has been an increase in DGC especially among younger people. As already noted, the prognosis for DGC is poor primarily because of late diagnosis and the more aggressive course characteristic of this type of tumor (17). In Colombia, there have been no studies which have specifically targeted his group of patients. For this reason we found it appropriate to conduct this research. Given all of this, one objective of this work is to evaluate clinical, endoscopic and histological factors in GC patients under 40 years and compare them with GC patients over 40 years of age. With this comparison we can establish differences and make recommendations that will enable early detection of this tumor which should lead to more appropriate treatment and more favorable prognoses.
MATERIALS AND METHODS
This is a cross-sectional and analytical study of patients diagnosed with GC. The study population consisted of patients diagnosed with gastric cancer by histological examination of endoscopic gastric biopsies or surgical specimens at a university hospital in Colombia during the period between January 2006 and December 2010. The study population was limited to those patients for whom we obtained complete information on variables considered necessary for this study. This information was recorded on a form designed specifically for this purpose. The study population was divided into two groups: those under 40 years old, and those over 40 years.
DEFINITION OF VARIABLES
Clinic variables
- Dyspepsia: pain or discomfort in the mid upper abdomen as per the Rome III criteria.
- Upper GI bleeding: hematemesis or presence of melena.
- Dysphagia: difficulty swallowing.
Morphological variables
- Macroscopic: According to the classic classification adopted by Bormann for the World Endoscopy Organization. (18)
- Type I Polypoid cancer: proliferating exophytic polypoid tumors which are not ulcerated and have generally irregular surfaces.
- Type II Fungating ulcerated cancer: disk-shaped circumscribed lesion with sharply defined margins and ulceration.
- Type III Ulcerated and infiltrating cancer: usually ulcerated tumors with extended irregular and indefinite margins. Ulcerations are usually variegated and the base is usually infiltrated by tumor.
- Type IV Diffuse infiltrating cancer or plastic linitis: diffuse, deep infiltrating lesion in which deep mucosal folds may remain intact or may be distorted.
Histologic Type: According to the classification of Lauren. (19)
- Intestinal: characterized by the formation of tubular structures resembling intestinal glands.
- Diffuse: not characterized by tubular structures but rather by the formation of signet ring patterns of nests of malignant cells.
EVOLUTION TIME OF THE DISEASE AND BACKGROUND
Methodology
We reviewed all endoscopy and pathology reports of all patients with gastric adenocarcinoma at Hospital El Tunal during the study period. Subsequently, we searched the medical records of these patients in the general archives of the institution.
Statistical analysis
A data base created with the study variables was used for statistical analysis which was performed with EpiInfo 6.04.
The student statistical test was used to analyze variables with normal distributions. Non-parametric statistical tests were used for variables with abnormal distributions.
Qualitative variables were expressed as percentages, and quantitative variables as means with standard deviations. Univariate analysis using the chi-square test and Fishers exact test were used to compare qualitative variables. Results were considered significant at p <0.05.
OUTCOMES
During the study period, 15,550 endoscopies where performed in the Department of Gastroenterology, and 226 patients with histologically confirmed diagnoses of gastric adenocarcinoma were found. Out of this patients 20 (8.8%) were 40 years old or younger (Group I) while the other 206 patients were older than 40 years of age (Group II). The study compared the two groups.
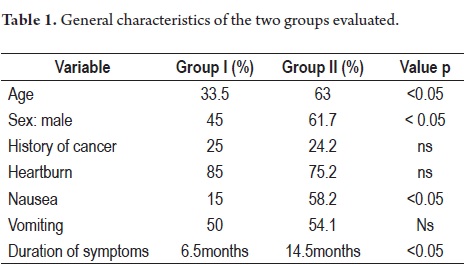
The average age of patients in Group I was 33.5 years old with a range of 17 to 40 years while the average of group II was 63 years old with a range of 41 to 95 years. 45% of the patients in Group I were male while in group II 61.7% were male (p: <0.05). 20% of group I (five patients) had first degree family histories of cancer while 24.2% of Group II had first degree family histories of cancer (p:ns).
The primary symptom reported by 85% of the cases in Group I and by 75.2% of Group II was heartburn (p:ns) (Table 1). The average duration of symptoms was 6.4 months for Group I and 10.5 months for Group II (p:<0.05). The primary warning symptom was weight loss which was present in 55% of patients in Group I and 56.4% of the patients in group II (p:ns). Other symptoms included hematemesis (20% in Group I, 20.2% in Group II), melena (40% in Group I, 36% in Group II), and anemia (10% in Group I, 12.5% in Group II). 70% of the patients in Group I presented some type of warning symptom.
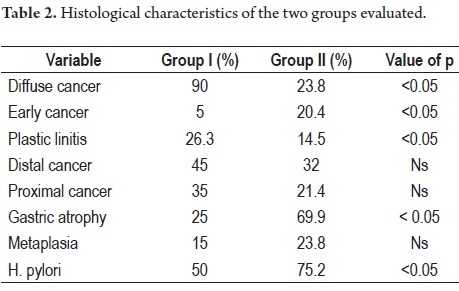
90% of patients in group I had DGC while only 23.8% (49 patients) in group II had DGC (p <0.05).
Only one case (5%) in Group I had a tumor confined to the submucosa (early or T1) in contrast to 20.4% (42 cases) of the patients in group II (p:<0.05). Out of the 19 advanced cases of Group I, five (26.3%) had plastic linitis while only 30 patients (14.5%) in group II had plastic linitis (p = 0.05).
Nine patients (45%) in Group I had distally located lesions while 66 patients (32%) in Group II had distally located lesions (p = ns). Seven patients (35%) in Group I had proximal lesions vs. 44 patients (21.4%) in group II. Five patients (25%) in Group I had associated pathological atrophy vs. 144 patients (69.9%) in group II (p <0.05). Three patients in Group 1 (15%) had associated intestinal metaplasia vs. 49 (23.8%) in group II. H. pylori was present in 50% of group I and in 75.2% of group II (p:<0.05) (Table 2).
At time of this writing, 16 patients in group I (80%) had died. Average survival time was 11 months.
DISCUSSION
Although GC is one of the most common cancers in our country, and it is the leading cause of death among cancers, the medical community still lacks awareness of its prevalence. Most likely this is one of the reasons why diagnoses are made late which in turn has resulted in a 5-year survival rate of less than 10% for young patients (2, 3). This finding highlights the importance of research to establish the epidemiological, clinical, endoscopic, and pathological characteristics of these patients so that we can apply this knowledge in daily clinical practice. In this study, 8.8% of GCs occurred in people 40 years old or younger. This low prevalence is generally similar to that found in other countries. In an extensive study published this year of with more than 33 000 patients with gastric cancer in the United States, 8.3% were under 45 years of age. Although in general the tumors were in more advanced stages, in contrast to our study, the prognosis was better for young people than for older patients (20). Although there is controversy about clinical outcomes for young GC patients, with some research reporting better prognoses (21), others reporting similar survival rates (22, 23) and still others reporting poor prognoses (24), the results of our study are very disappointing in terms of survival. Probably this dismal picture is due to the fact that most tumors in young patients in our study were diffuse cancers which have worse prognoses than do intestinal tumors (25). In our study, only one patient (5%) of the group under 40 years was diagnosed at an early stage, but 20.4% of the group of patients over 40 were diagnosed early. Furthermore, 26% of young patients had plastic linitis, a condition in which the tumor spreads aggressively and involves the whole thickness of the gastric wall.
One case highlights the aggressiveness of this tumor: we found a 39 year old woman who had a bilateral ovarian tumor. When an upper endoscopy was performed to find the primary tumor, a 10 mm depression in the gastric corpus was found. Echoendoscopy demonstrated that the entire thickness of the gastric wall had been compromised, a finding which is compatible with gastric cancer which has metastasized to the ovaries (Bilateral Krukenberg tumor). In this case the diagnosis of DGC was demonstrated histologically with an endoscopic biopsy and pathology from the ovaries which had been resected.
In this study, 90% of younger patients had DGC, a prevalence which is higher than those in other series. Japanese authors found DGC in 26% of young patients while a recent study done in China, a country with a high incidence of GC, found 64% of 210 patients with under 40 years of age had diffuse GC (27). In the Chinese study, 19% of young patients with GC had first degree family histories of the disease. This is the almost the same as the 20% found in our study and highlights the importance of investigating all first-degree relatives of patients with this condition. Different series studying young patients have shown that at the moment of diagnosis there is a higher proportion of patients with advanced GC meaning that less patients are diagnosed in early stages. This correlates well with findings in Peru where GC is the leading cause of death among malignant neoplasias in males between 30 and 39 years old (28). Often the literature explains aggressiveness of tumor in young patients by delayed diagnoses. This is most probably due to the fact that there are few symptoms early in the disease. Physicians are often unwilling to perform diagnostic tests in this population since the disease is not usually considered in the diagnosis differential for young patients with upper gastrointestinal symptoms (29).
It is possible that this is due to the fact that many doctors often begin treatment of symptoms before having an etiological diagnosis of the origin of symptoms. This is even more so in many health centers in our country where there is no possibility of endoscopic examination. In our opinion, the aggressiveness of these tumors is mostly due to the diffuse type tumor usually found in these patients. Symptoms from these tumors have only short durations. In our series average duration was 6.7 months compared to average duration of 10 months for patients who were over 40. In other words, older patients did not seek attention late or they were not treated late. 80% of young patients had diffuse tumors while only 23.8% of older patients had DGC. Moreover, in 95% of the cases in young patients the tumor was already advanced when it was diagnosed.
The literature shows a predominance of men among GC patients (30-32). Our study is striking because 55% of the group of young patients were women while only 38.3% of the older patients were women. This could be a risk factor to be considered in future studies. Given the high portion of GC patients under 40 years of age in our study (8.8%), we consider that it is necessary for physicians to perform upper digestive endoscopies for Colombian patients with warning signs for GC regardless of the patients age. The youngest of our patients was a 17 year old who had an adenocarcinoma in the esophagogastric junction. Symptoms lasted for 12 months, but given his young age the patient was never referred for endoscopy despite his constant complaints of dysphagia and weight loss. This patient survived only 5 months after diagnosis. An earlier prospective study conducted in Colombia demonstrated that the 50 years old and up rule for performing upper endoscopies when there is suspicion of dyspepsia that is recommended in the United States should be lowered to 30 years old and up (33). A corollary to this is that they should be performed at younger ages if the patient exhibits warning signs. In this study it is clear that clinical manifestations of gastric cancer are not specific for either young or old patients. This is consistent with reports elsewhere in the literature (34). The most frequent symptoms in our study population were weight loss and dyspepsia, as has been reported in other countries (35).
We observed distal tumor locations were the most frequent locations among our younger patients, similar to locations found among older patients and in agreement with findings in other studies (36, 37).
Helicobacter pylori are associated with chronic diffuse surface gastritis in the corpus and antrum (38-40). Various findings suggest that this microorganism is the critical agent in gastric carcinogenesis of 70% to 94% of patients with GC (41). The diffuse-type gastric cancer prevalent in young adults is less frequently associated with chronic H. pylori infection. This correlates well with our findings in which 50% of young patients were infected while 75% of the older patients were (p <0.05).
Biological behavior of GC depends not only on the degree of invasion at diagnosis but also on histology (42, 43). DGC exhibits a pattern of early metastasis to the lymphatic system and extensive peritoneal seeding. This is unlike differentiated intestinal cancer which spreads primarily through the blood and most frequently involves metastasis to the liver. In our series and in other studies, young patients most frequently are diagnosed histologically with undifferentiated diffuse intestinal cancer (44). Presumably, younger patients have fewer years available for the development of chronic atrophic gastritis and intestinal metaplasia which are precursors of intestinal cancer which may be why they exhibit a higher frequency of diffuse cancer than found in the older population (45).
In our series, only 25% of younger patients had atrophy while 69.9% of older patients did. Only 15% of younger patients had intestinal metaplasia while 23.8% of older patients did. This might be explained by the fact that older patients mostly have intestinal cancer, followed in frequency by the cascade of Pelayo Correa. Young people probably do not because they most often have DGC.
CGD may be sporadic or hereditary. Hereditary CGD is called hereditary diffuse gastric cancer (HDGC) (46). Unlike sporadic forms, the hereditary form can be predicted when genetic studies demonstrate the CDH1 mutation. When this genetic abnormality is identified in first degree relatives, current alternatives are prophylactic gastrectomy or endoscopic surveillance since mutations are found in about 40% of the families studied with HDGC (47-49). Male carriers of a mutation in the CDH1 gene have an 83% risk of developing gastric cancer while women who carry the mutation have a 67% DGC risk. These women also have a 39% risk of lobular breast carcinoma which is far greater than that of the general population (50-51).
Among new criteria for suspecting HDGC is if a patient with diffuse cancer is less than 40 years old. This raises an interesting discussion because of the 20 patients under 40 years of age in our study who had gastric cancer. 18 had diffuse cancer which means that they meet the criteria for consideration of having HDGC. We are conducting an ongoing study to check whether they have CHD1 mutations. If they test positive, we will extend the study to their families in order to identify carriers of the mutation who are asymptomatic. In these cases early detection of this terrible disease that causes high mortality may still be possible.
CONCLUSIONS
1. This study has found that GC in people 40 years and under represents 8.8% of the GC cases.
2. DGC was present in 90% of younger patients. Only one case among the younger patients was early GC. Most of these cases were plastic linitis.
3. Most cases of GC in people under 40 years of age is diffuse. This type of cancer has very poor prognosis, especially since its biological behavior is to spread early metastasis throughout the lymph system. This requires that physicians think of performing upper endoscopy on young people with dyspepsia who do not improve quickly or who exhibit any warning symptoms.
REFERENCES
1. Kelley J, Dugan J. Gastric cancer epidemiology and risk factor. J Clin Epidemiol 2003; 56: 1-9.
2. Piñeros M, Ferlay J, Murillo R. Cancer incidence estimates at the nation and district levels in Colombia. Salud Pública Mex 2006; 48: 455-465. 443.
3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90.
4. Shah MA, Kelsen DP. Gastric Cancer: A Primer on the Epidemiology and Biology of the Disease and an Overview of Medical Management of Advanced Disease. J Nat Comprehensive Cancer Network 2010; 8: 438-447.
5. Muñoz N, Correa P, Cuello C, Duque E. Histologic types of gastric cancer in high and low areas. Int J Cancer 1968; 3: 809-18.
6. Cochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best Pract Res Clin Gastroenterol 2007; 21: 281-97.
7. Correa P, Chen VW. Gastric Cancer. Cancer Surv 1994; 19: 55-76.
8. Correa P. Schneider BG. Etiology of gastric cancer: what is new? Cancer Epidemiol Biomarkers Prev 2005; 14: 1180-6.
9. Mueller A, Falkow S, Amieva MR. Helicobacter pylori and gastric cancer: what can learn by studying the response of gastric epithelial cells to the infection? Cancer Epidemiol Biomarkers Prev 2005; 14: 1859-64.
10. Crowe SE. Helicobacter pylori infection, chronic inflammation and the development of malignancy. Curr Opin Gastroenterol 2005; 21: 32-8.
11. Ikossi-OConnor W, Douglass H. Gastric Cancer in Young Patients. Journal of Surgical Oncology 1984; 26: 219-224.
12. Boles RS, Ipsen J, Ruedy J. A Comparative study of the frequency of carcinoma of the stomach. Cancer.1963; 16: 1584-1585.
13. Kubo T. Gastric carcinoma in New Zealand: some epidemiologic-pathologic aspects. Cancer 1973; 31: 1498-1501.
14. Ganan M, Massayuki C. Neoplasia gástrica maligna en jóvenes: a propósito de 22 casos. An Paul Med Cir 1988; 115: 15-21.
15. Pisano R, León J, Hernández H, Aguirre R. Cáncer gástrico en la provincia de Valdivia (Chile) 1977-1987. Rev Med Chile 1990; 118: 158-163.
16. Ecstrom AM, Hansson LE, Signorello LB, et al. Decreasing incidence of both major histological subtypes of gastric adenocarcinoma. Br J Cancer 2000; 83: 391-396.
17. Lochhead P, El-Omar EM. Gastric cancer. Br Med Bull 2008; 85: 87-100.
18. Palmer WL. Carcinoma of the Stomach: clinical considerations. En HL Bockus. Gastroentrology I. Saunders: Philadelphia; 1974. p. 949.
19. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal type carcinoma. Acta Pathol Microbiol inmunol Scand 1965; 64: 31-49.
20. Al-Refaie WB, Hu CY, Pisters PWT, Chang GJ. Gastric adenocarcinoma in young patients: a population-based appraisal. Ann Surg Oncol 2011; 18: 2800-7.
21. Katai H, Sasako M, Sano T, et al. Gastric adenocarcinoma in Young adults. Jpn J Clin Oncol 1996; 26: 139-43.
22. Santoro R, Carboni F, Lepiane P, et al. Clinicopathological features and prognosis of gastric cancer in Young European adults. Br J Surg 2007; 94: 737-42, 23.
23. Matley PJ, Dent DM, Madden MV, et al. Gastric carcinoma in Young adults. Ann Surg 1988; 208: 593-6.
24. Theuer CP, de Virgilio C, Keese G, et al. Gastric adenocarcinoma in patients 40 years of age or younger. Am J Surg 1996; 172: 473-6.
25. Lauren P. The two histological main types of gastric carcinomas: Diffuse and so-called intestinal-type carcinoma. Acta Path Microbiol Scand 1965; 64: 31-49.
26. Nakamura R, Saikawa Y, Takahashi T, Takeuchi H, Asanuma H, Yamada Y, Kitagawa Y. Retrospective analysis of prognostic outcome of gastric cancer in Young patients. Int J Clin Oncol 2011; 16: 328-34.
27. Bai Y, Li ZS. Endoscopic, clinicopathological features and prognosis of very Young patients with gastric cancer. J Gastroenterol Hepatol 2011, early release 2011 acceso octubre 8, 2011.
28. García R, Bernui F, Machado A. Cáncer gástrico en adultos menores de 40 años. Bol Soc Per Med Int 1993; 5(2): 33-36.
29. Piñeros M, Hernández G, Bray F. Increasig mortality rates of comon malignancies in Colombia. Cancer 2004; 101: 2285-92.
30. International Agency for Research on Cancer (IARC), Schistosomes liver flukes and Helicobacter pylori. IARC monograph on the evaluation on carcinogenic risks to humans 1994; 61.
31. IARC 1994, Helicobacter and Cancer Colaborative Group. Gastric Cancer and Helicobacter pylori: a combined analyses of 12 case-control Studies nested within prospective cohorts. Gut 2001; 49: 347-53.
32. Rocco A, Nardone G. Diet H. pylori infection and gastric cancer: evidence and controversies. World J Gastroenterol 2007; 13: 2901-12.
33. Pineda LF, Otero W, Gómez M, Arbeláez V, Otero E. Enfermedad estructural y valor predictivo de la historia clínica en pacientes con dispepsia no investigada. Rev colomb gastroenterol 2004; 19(1): 13-25.
34. Medina-Franco H, Heslin MJ, Cortes-González R. Clinicopathological characteristics of gastric carcinoma in young and elderly patients: a comparative study. Ann Surg Oncol 2000; 7: 515-519.
35. Greene FL, Page DL, Fleming ID, et al, editors. AJCC (American Committee on Cancer) Cancer Staging Manual. 6th edition. New York; Springer- Verlag. 2002.
36. McNeer G. Cancer of the stomach in the young. AJR 1941; 45: 537-50.
37. Matley PJ, Dent DM, Madden MV, et al. Gastric carcinoma in young adults. Ann Surg 1988; 208: 593-6.
38. Woodward M, Tunstall-Pedoe H, McColl K. Helicobacter pylori infection reduce systemic availability of dietary vitamina C. Eur J Gastroenterol 2001; 13: 2559-66.
39. Meining A, Morgner A, Mielhke S, et al. Atrophy-metaplasia-dysplasia-carcinoma sequence in the stomach: a reality or merely an hypothesis?. Best Pract Res Gastroenterol 2001; 15: 983-8.
40. El Zimaity HM, Ramchatesingh J, Sabed MA, et al. Gastric Intestinal metaplasia: subtypes and natural history. J Clin Pathol 2001; 54: 679-83.
41. Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow derived cells. Science 2004; 306: 1568-71.
42. Breaux J, et al. Adenocarcinoma de estómago: Una revisión de 35 años, 71.710 casos. World Journal of Surgery. 1990; 14: 580-586.
43. ArmB C, Dent D. Factors influencing prognosis in gastric carcinoma. Surg Gynecol Obstet 1986; 162: 343-348.
44. Fujimoto S, Takahasi M, Ohkubo H, et al. Comparative clinicopathologic features of early gastric cancer in young and older patients. Surgery 1994; 516-520.
45. Keller G, Vogelsang H, Becker I, et al. Diffuse type gastric and lobular breast carcinoma in a familial gastric cancer patient with an E-cadherin germline mutation. Am J Pathol 1999; 155: 337-342.
46. Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998; 392: 402-405.
47. Newman EA, Mulholland MW. Prophylactic gastrectomy for hereditary diffuse gastric cancer syndrome. J Am Coll Surg 2006; 202: 612-617.
48. Huntsman DG, Carneiro F, Lewis FR, et al. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N Engl J Med 2001; 344: 1904-1909.
49. Lewis FR, Mellinger JD, Hayashi A, et al. Prophylactic total gastrectomy for familial gastric cancer. Surgery 2001; 130: 612-619.
50. Chun YS, Lindor NM, Smyrk TC, et al. Germline E-cadherin gene mutations: is prophylactic total gastrectomy indicated? Cancer 2001; 92: 181-187.
51. Blair V, Martin I, Shaw D, et al. Hereditary diffuse gastric cancer: diagnosis and management. Clin Gastroenterol Hepatol 2006; 4: 262-275.
1. Kelley J, Dugan J. Gastric cancer epidemiology and risk factor. J Clin Epidemiol 2003; 56: 1-9. [ Links ]
2. Piñeros M, Ferlay J, Murillo R. Cancer incidence estimates at the nation and district levels in Colombia. Salud Pública Mex 2006; 48: 455-465. 443. [ Links ]
3. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011; 61: 69-90. [ Links ]
4. Shah MA, Kelsen DP. Gastric Cancer: A Primer on the Epidemiology and Biology of the Disease and an Overview of Medical Management of Advanced Disease. J Nat Comprehensive Cancer Network 2010; 8: 438-447. [ Links ]
5. Muñoz N, Correa P, Cuello C, Duque E. Histologic types of gastric cancer in high and low areas. Int J Cancer 1968; 3: 809-18. [ Links ]
6. Cochhead P, El-Omar EM. Helicobacter pylori infection and gastric cancer. Best Pract Res Clin Gastroenterol 2007; 21: 281-97. [ Links ]
7. Correa P, Chen VW. Gastric Cancer. Cancer Surv 1994; 19: 55-76. [ Links ]
8. Correa P. Schneider BG. Etiology of gastric cancer: what is new? Cancer Epidemiol Biomarkers Prev 2005; 14: 1180-6. [ Links ]
9. Mueller A, Falkow S, Amieva MR. Helicobacter pylori and gastric cancer: what can learn by studying the response of gastric epithelial cells to the infection? Cancer Epidemiol Biomarkers Prev 2005; 14: 1859-64. [ Links ]
10. Crowe SE. Helicobacter pylori infection, chronic inflammation and the development of malignancy. Curr Opin Gastroenterol 2005; 21: 32-8. [ Links ]
11. Ikossi-O'Connor W, Douglass H. Gastric Cancer in Young Patients. Journal of Surgical Oncology 1984; 26: 219-224. [ Links ]
12. Boles RS, Ipsen J, Ruedy J. A Comparative study of the frequency of carcinoma of the stomach. Cancer.1963; 16: 1584-1585. [ Links ]
13. Kubo T. Gastric carcinoma in New Zealand: some epidemiologic-pathologic aspects. Cancer 1973; 31: 1498-1501. [ Links ]
14. Ganan M, Massayuki C. Neoplasia gástrica maligna en jóvenes: a propósito de 22 casos. An Paul Med Cir 1988; 115: 15-21. [ Links ]
15. Pisano R, León J, Hernández H, Aguirre R. Cáncer gástrico en la provincia de Valdivia (Chile) 1977-1987. Rev Med Chile 1990; 118: 158-163. [ Links ]
16. Ecstrom AM, Hansson LE, Signorello LB, et al. Decreasing incidence of both major histological subtypes of gastric adenocarcinoma. Br J Cancer 2000; 83: 391-396. [ Links ]
17. Lochhead P, El-Omar EM. Gastric cancer. Br Med Bull 2008; 85: 87-100. [ Links ]
18. Palmer WL. Carcinoma of the Stomach: clinical considerations. En HL Bockus. Gastroentrology I. Saunders: Philadelphia; 1974. p. 949. [ Links ]
19. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal type carcinoma. Acta Pathol Microbiol inmunol Scand 1965; 64: 31-49. [ Links ]
20. Al-Refaie WB, Hu CY, Pisters PWT, Chang GJ. Gastric adenocarcinoma in young patients: a population-based appraisal. Ann Surg Oncol 2011; 18: 2800-7. [ Links ]
21. Katai H, Sasako M, Sano T, et al. Gastric adenocarcinoma in Young adults. Jpn J Clin Oncol 1996; 26: 139-43. [ Links ]
22. Santoro R, Carboni F, Lepiane P, et al. Clinicopathological features and prognosis of gastric cancer in Young European adults. Br J Surg 2007; 94: 737-42, 23. [ Links ]
23. Matley PJ, Dent DM, Madden MV, et al. Gastric carcinoma in Young adults. Ann Surg 1988; 208: 593-6. [ Links ]
24. Theuer CP, de Virgilio C, Keese G, et al. Gastric adenocarcinoma in patients 40 years of age or younger. Am J Surg 1996; 172: 473-6. [ Links ]
25. Lauren P. The two histological main types of gastric carcinomas: Diffuse and so-called intestinal-type carcinoma. Acta Path Microbiol Scand 1965; 64: 31-49. [ Links ]
26. Nakamura R, Saikawa Y, Takahashi T, Takeuchi H, Asanuma H, Yamada Y, Kitagawa Y. Retrospective analysis of prognostic outcome of gastric cancer in Young patients. Int J Clin Oncol 2011; 16: 328-34. [ Links ]
27. Bai Y, Li ZS. Endoscopic, clinicopathological features and prognosis of very Young patients with gastric cancer. J Gastroenterol Hepatol 2011, early release 2011 acceso octubre 8, 2011. [ Links ]
28. García R, Bernui F, Machado A. Cáncer gástrico en adultos menores de 40 años. Bol Soc Per Med Int 1993; 5(2): 33-36. [ Links ]
29. Piñeros M, Hernández G, Bray F. Increasig mortality rates of comon malignancies in Colombia. Cancer 2004; 101: 2285-92. [ Links ]
30. International Agency for Research on Cancer (IARC), Schistosomes liver flukes and Helicobacter pylori. IARC monograph on the evaluation on carcinogenic risks to humans 1994; 61. [ Links ]
31. IARC 1994, Helicobacter and Cancer Colaborative Group. Gastric Cancer and Helicobacter pylori: a combined analyses of 12 case-control Studies nested within prospective cohorts. Gut 2001; 49: 347-53. [ Links ]
32. Rocco A, Nardone G. Diet H. pylori infection and gastric cancer: evidence and controversies. World J Gastroenterol 2007; 13: 2901-12. [ Links ]
33. Pineda LF, Otero W, Gómez M, Arbeláez V, Otero E. Enfermedad estructural y valor predictivo de la historia clínica en pacientes con dispepsia no investigada. Rev colomb gastroenterol 2004; 19(1): 13-25. [ Links ]
34. Medina-Franco H, Heslin MJ, Cortes-González R. Clinicopathological characteristics of gastric carcinoma in young and elderly patients: a comparative study. Ann Surg Oncol 2000; 7: 515-519. [ Links ]
35. Greene FL, Page DL, Fleming ID, et al, editors. AJCC (American Committee on Cancer) Cancer Staging Manual. 6th edition. New York; Springer- Verlag. 2002. [ Links ]
36. McNeer G. Cancer of the stomach in the young. AJR 1941; 45: 537-50. [ Links ]
37. Matley PJ, Dent DM, Madden MV, et al. Gastric carcinoma in young adults. Ann Surg 1988; 208: 593-6. [ Links ]
38. Woodward M, Tunstall-Pedoe H, McColl K. Helicobacter pylori infection reduce systemic availability of dietary vitamina C. Eur J Gastroenterol 2001; 13: 2559-66. [ Links ]
39. Meining A, Morgner A, Mielhke S, et al. Atrophy-metaplasia-dysplasia-carcinoma sequence in the stomach: a reality or merely an hypothesis?. Best Pract Res Gastroenterol 2001; 15: 983-8. [ Links ]
40. El Zimaity HM, Ramchatesingh J, Sabed MA, et al. Gastric Intestinal metaplasia: subtypes and natural history. J Clin Pathol 2001; 54: 679-83. [ Links ]
41. Houghton J, Stoicov C, Nomura S, et al. Gastric cancer originating from bone marrow derived cells. Science 2004; 306: 1568-71. [ Links ]
42. Breaux J, et al. Adenocarcinoma de estómago: Una revisión de 35 años, 71.710 casos. World Journal of Surgery. 1990; 14: 580-586. [ Links ]
43. Armstrong C, Dent D. Factors influencing prognosis in gastric carcinoma. Surg Gynecol Obstet 1986; 162: 343-348. [ Links ]
44. Fujimoto S, Takahasi M, Ohkubo H, et al. Comparative clinicopathologic features of early gastric cancer in young and older patients. Surgery 1994; 516-520. [ Links ]
45. Keller G, Vogelsang H, Becker I, et al. Diffuse type gastric and lobular breast carcinoma in a familial gastric cancer patient with an E-cadherin germline mutation. Am J Pathol 1999; 155: 337-342. [ Links ]
46. Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998; 392: 402-405. [ Links ]
47. Newman EA, Mulholland MW. Prophylactic gastrectomy for hereditary diffuse gastric cancer syndrome. J Am Coll Surg 2006; 202: 612-617. [ Links ]
48. Huntsman DG, Carneiro F, Lewis FR, et al. Early gastric cancer in young, asymptomatic carriers of germ-line E-cadherin mutations. N Engl J Med 2001; 344: 1904-1909. [ Links ]
49. Lewis FR, Mellinger JD, Hayashi A, et al. Prophylactic total gastrectomy for familial gastric cancer. Surgery 2001; 130: 612-619. [ Links ]
50. Chun YS, Lindor NM, Smyrk TC, et al. Germline E-cadherin gene mutations: is prophylactic total gastrectomy indicated? Cancer 2001; 92: 181-187. [ Links ]
51. Blair V, Martin I, Shaw D, et al. Hereditary diffuse gastric cancer: diagnosis and management. Clin Gastroenterol Hepatol 2006; 4: 262-275. [ Links ]











 texto em
texto em