Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.27 no.4 Bogotá Oct./Dec. 2012
Original articles
(1) Centro de Investigaciones de Enfermedades Digestivas (CIED - Center for the Investigation of Digestive Diseases) at the Centro Hospital la Rosa of Pasto Salud ESE
Max Meyer Award. Colombian congress of digestive diseases. Medellín, December 10th, 2011.
Received: 19-09-12 Accepted: 23-10-12
Abstract
Atrophic chronic gastritis (ACG), intestinal metaplasia (IM) and mild dysplasia (MD)) are all precursor lesions which have been clearly demonstrated by many studies to be related to risks for development of gastric cancer (GC). This is especially true in our region which is considered to be a high risk area for this disease.
We conducted a prospective study of 212 patients between the ages of 11 and 89 years who were from the yellow zone of Nariño. Patients were cared for in the Centro de Investigaciones de Enfermedades Digestivas (CIED - Center for the Investigation of Digestive Diseases) at the Centro Hospital la Rosa which is part of the public health care system of Pasto. Seven gastric mucosa biopsies were taken from each subject and stained with specially modified Giemsa stain to detect precursor lesions and the presence of Helicobacter pylori.
The prevalence of ACG was 38.6%, the prevalence of IM was 24.4%, and the prevalence of MD was 1.5%. Prevalence of H. pylori infections among patients with ACG was 73.5% while among patients with IM it was 52%, but prevalence rose to 100% among patients with MD. When severity of precursor lesions on the OLGA-staging (Operative Link for Gastritis Assessment) scale was 11.5%, lesions were classified as stage III and IV. With one exception, all of these patients were H. pylori positive. We would like to ask the medical community to consider CIED's Follow-up and Monitoring Protocol for precursor lesions in order to demonstrate that the best strategy continues to be GC prevention in high risk regions.
Key words
Precursor lesions, gastric cancer (GC), atrophic chronic gastritis (ACG), intestinal metaplasia (IM), mild dysplasia (MD).
Gastritis is a very frequent pathological entity in the Andes Region which is treated by general practitioners, surgeons, internists and gastroenterologists in Colombia. A large number of these professionals do not have clear or profound knowledge of the risks, natural history, possible interventions and monitoring required when faced with a pathological report of chronic atrophic gastritis with or without intestinal metaplasia.
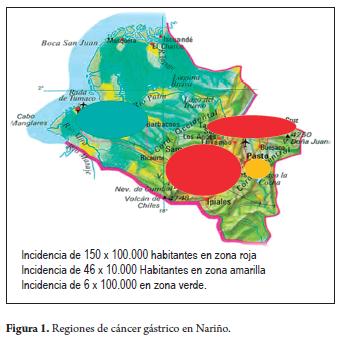
The department of Nariño, located in the southwest of Colombia has a very special social, ethnic and geographic situation. It consists of a high mountainous Andean region which has a high incidence of gastric cancer and a low coastal region which has a low incidence of gastric cancer. This makes it special, perhaps maybe unique in the world, since this small territory of 33.265 km2 has two clearly defined regions of gastric cancer. Two red zones in the north and southwest have one of the highest incidences of gastric cancer worldwide (150 for every 100.000 inhabitants). This region also has a high prevalence of premalignant lesions including chronic atrophic gastritis, intestinal metaplasia and mild dysplasia as well as high prevalence of Helicobacter pylori infections. The capital, San Juan de Pasto, and the surrounding municipalities located on the right bank of the Guaitara River are considered to be a yellow zone. This area has a gastric cancer incidence of 46 per 100,000 inhabitants, a lower prevalence of precursor lesions, but a high prevalence of Helicobacter pylori infections. The Pacifc Coast is considered to be a green zone with an incidence of gastric cancer of only with 6 per 100,000 inhabitants, a lower prevalence of precursor lesions, and a high prevalence of Helicobacter pylori infections. This is currently known as the Tumaqueño, Nariñense or Colombian enigma (Figure 1) (1,2,3).
MATERIALS AND METHODS
The design chosen for this study was descriptive and prospective. The study population consisted of patients between the ages of 11 and 89 years who were treated at the CIED at the Hospital Center La Rosa in Pasto, Colombia from January to December of 2010. Examinations were performed by a single endoscopist with training in early cancer diagnosis.
The sample included 212 patients from the yellow zone. Material used came from 7 biopsies of the gastric mucosa taken from each patient using Olympus Actera 150 video endoscopy. Two (2) biopsies were taken from the antrum fve centimeters from the pylorus; one (1) from the angular incision, two (2) from the corpus ten centimeters from the cardia and two (2) from the subcardia. They were placed in 10% bufered formalin, dehydrated and placed in parafn for 24 hours. At the moment of incision the tissues were oriented so that the edge of the mucosa was perpendicular to the cuting surface. Four (4) micron thick histological sections were cut from each biopsy fragment, placed on glass slides in groups of three (3) histological sections. Each slice was prepared with hematoxylin eosin stain for routine histological examination of with an especially modified Giemsa stain for detection of Helicobacter pylori.
The analog visual scale of the updated version of the Sydney system was used to stage gastritis in histological evaluations. Gastritis was graded as negative (0), mild (1), moderate (2), severe (3) and undifferentiated (9). The antral region was labeled region A, the fundo-corporal region was labeled region C, and the subcardial region was labeled region S. The following parameters were evaluated: polymorphic nuclear neutrophils, mononuclear leuko-cytes, intraepithelial lymphocytes, regenerative activity, mucus depletion and Helicobacter pylori density.
Twenty (20) patients were excluded from the study population. Six (6) were excluded because they presented adenocarcinomas. Five of the six were in advanced stages while one (1) was in an early stage, classified as type I. Fourteen (14) patients were excluded because they were diagnosed with erythematous gastritis that could not be classified because of one or several low quality biopsies.
RESULTS
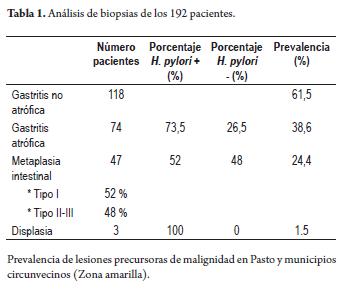
In the study population of 192 patients, 118 histological studies showed active non-atrophic chronic gastritis, 74 patients had chronic atrophic gastritis with a 73% prevalence of Helicobacter pylori infections, 47 patients had intestinal metaplasias. Of these 52% had complete, or type I, intestinal metaplasia and 48% had incomplete or mixed typed II and III intestinal metaplasias. Three patients had mild dysplasia and were positive for Helicobacter pylori. The prevalence of chronic atrophic gastritis was 38.6%, the prevalence of intestinal metaplasia was 24.4%, and that of mild dysplasia 1.5% (Table 1).
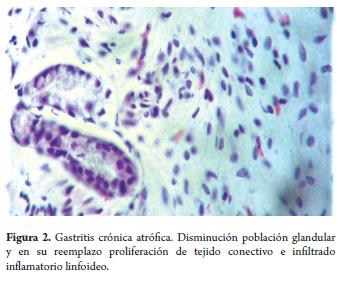
Microscopic examination of the biopsies of patients with chronic atrophic gastritis showed decreased numbers or size, or complete disappearance, of the normal gastric glands and replacement through fibrosis of the lamina propria (Figure 2).
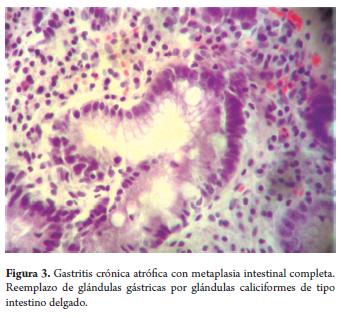
Cases of complete intestinal metaplasia (type I) showed loss of the gastric glands due to the replacement of normal glands by metaplastic glandular structures coated with incipient small intestine epithelium (Figure 3).
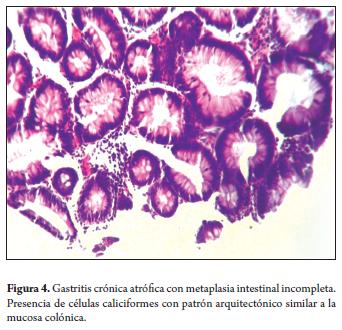
Incomplete intestinal metaplasia (type II) showed replacement of epithelium by caliciform glands with an architectonic patern similar to colonic mucosa (Figure 4).
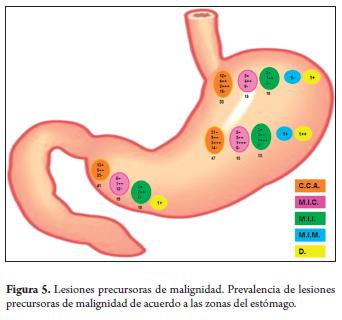
The prevalence of premalignant lesions related to the antrum was observed in 41 patients with chronic atrophic gastritis, in 19 patients with complete intestinal metaplasia, in 10 patients with incomplete intestinal metaplasia and in 1 patient with dysplasia. In the oxyntic or fundo-corporal region, 47 patients had chronic atrophic gastritis, 15 had complete intestinal metaplasia, 13 had incomplete intestinal metaplasia, 1 had mixed intestinal metaplasia and 1 had mild dysplasia. The significant prevalence of premalignant lesions and their association with Helicobacter pylori infection in the subcardial biopsies deserves atention (Figure 5).
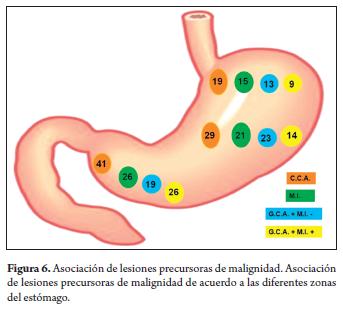
Out of 41 patients with chronic atrophic gastritis in the antrum, 29 also had atrophy in the corpus of the stomach. Out of 26 patients with antral intestinal metaplasia, 21 also had intestinal metaplasia in the corpus of the stomach (Figure 6).
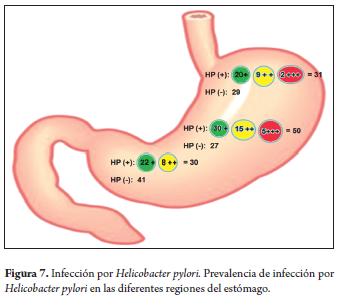
The presence of the Helicobacter pylori bacteria in one or several biopsies was denominated a positive case of infection. 50 cases in the corpus of the stomach were positive for Helicobacter pylori while just 30 cases in the antral region were positive. A large number of these had moderate and severe bacterial loads (Figure 7).
The study was also demonstrated that the higher the bacterial load the greater the patient's compromise due to histological damage (Figure 8).
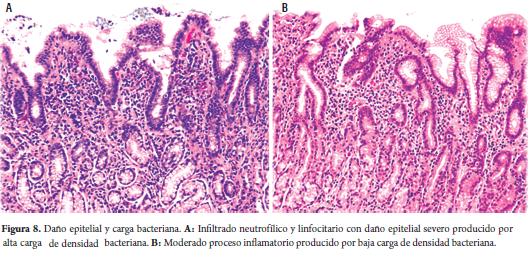
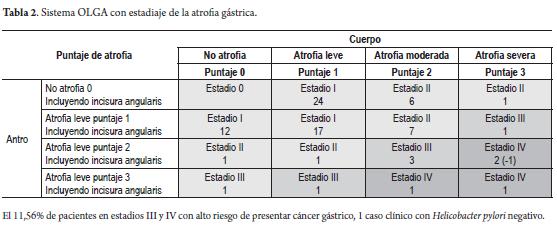
The risk evaluation from our histological findings using the visual analog scale of the updated version of the Sydney system taken to the OLGA scale (operative link for gastritis assessment) found 20.2% in stage II, 6.3% in stage III and 5.4% in stage IV. One stage IV patient was negative for Helicobacter pylori infection (Table 2).
DISCUSSION
The Andes region, especially the department of Nariño, is characterized by elevated prevalence of gastrointestinal diseases in which gastritis accounts for the largest number of cases. It begins in childhood as demonstrated by a study of a population of children in Pasto in which 88% of asymptomatic children under 10 years of age showed some inflammatory change in the gastric mucosa (4-5).
The problem for the atending physician begins when she or he faces pathology report indicating chronic atrophic gastritis, whether or not intestinal metaplasia is present. This entity is classified as a precursor lesion of malignancy that is characterized by slow progression towards more advanced lesions that may end in neoplasia (6,7,8,9,10).
The prevalence of premalignant lesions varies greatly according to geographic zones and age which accounts for the importance of their prevalence in the region in question. If the department of Nariño is analyzed, the study of Correa P. (11) reports that red regions have a prevalence of chronic atrophic gastritis of 57%, 38% for intestinal metaplasia and 10% for mild dysplasia in those older than 40. A study of and 86,243 biopsies reports in 16 Colombian cities by Bravo L. (12) shows prevalence varying greatly in the cities studied. 40.5% of the chronic atrophic gastritis cases were in Pasto, a number similar to the 38.6% chronic atrophic gastritis reported in our own study. Maybe the small diference is Bravo's study included patients from red and green regions. A study of asymptomatic children under 10 years old from Pasto published by Bedoya A. (4) showed active multifocal chronic atrophic gastritis in 5% of the population studied. The author highlights the great caution that must be taken by this population considering that gastric gland atrophy that quite possibly could progress to intestinal metaplasia, dysplasia and gastric carcinoma with time and other associated factors occurs at such young ages (13). This is especially true when one takes into account the investigation done by the National Cancer Institute of the USA (3) in which they clearly enunciate that gastric cancer is an infectious disease that starts during childhood and includes precursor lesions of malignancy in its early stages.
Intervention to treat these lesions is of great importance because of their slow progressive onset and dynamic processes that might last for several years. A considerable percentage of them, together with other risk factors, result in the appearance of gastric carcinoma as has been confirmed in many studies (14,15,16).
Intervention by health promotion and disease prevention medical groups, using the medical advice available in each region, should include eradication treatment for Helicobacter pylori infection (13) since it has been widely demonstrated that the eradication of this bacterial infection decreases the risk of gastric cancer (17-18-19-20). In our study we found large colonies of Helicobacter pylori in the gastric corpus with the moderate to severe bacterial loads associated with chronic atrophic gastritis and intestinal metaplasia. This phenomenon indirectly indicates an advanced development of premalignant lesions in the antrum, which is the normal habitat of these bacteria. The atrophic and metaplastic changes associated with these lesions create an inhospitable and hostile microenvironment for the bacteria in the antrum causing them to migrate to the gastric corpus. This migratory process can be interpreted as an indirect measure of the extent of the damage to the gastric mucosa, and as such, as a phenomena that we may consider to be an indicator for risk of carcinoma (21,22,23).
The use of antioxidants and carotenes and intervention against risk factors by health promotion and disease prevention medical groups, are strategies with which we aim to reduce the impact of the premalignant lesions that have been demonstrated in studies performed in Nariño (24,25,26,27,28).
Although we understand that premalignant lesions are histological alterations that by themselves are strong predictors of risk for intestinal type gastric cancer (6,27,29,30,31,32), in our environment the study of chronic atrophic gastritis with or without intestinal metaplasia has not achieved its full impact for all of its clinical implications in relation to severity and risk.
For this reason professionals responsible for managing this pathology who are worried about progression to catastrophes such as gastric cancer have proposed a system for calibrating chronic atrophic gastritis, including intestinal metaplasia (33-34-35-36). Called the OLGA system (Operative Link for Gastritis Assessment), it grades lesions from stage I to IV. Stages III and IV have up to 84% of risk of gastric cancer (36). In our study we found 11.5% of the patients were in stages III and IV with a very high risk of developing gastric cancer. Seven patients were positive for Helicobacter pylori while only one was negative for the bacteria. This population is under strict vigilance by health promotion and disease prevention medical groups under the premise that the best strategy for disease control is prevention.
While the OLGA system is of great importance for the risk that these entities represent, especially for many research groups and for referral centers for these pathologies, the daily work of professionals who care for patients with to these types of pathologies cannot easily, practically or routinely use OLGA Classification. Instead, what we consider to be indispensably useful is use of the analog scale of the updated version of the Sydney system for the pathology report (37).
To date, vigilance and monitoring of chronic atrophic gastritis with or without intestinal metaplasia has not been defined, and there is no uniform national or international consensus. Consequently, we invite professionals who work with this type of pathology to work on the protocol that the CIED has proposed for regions with a high risk of gastric cancer.
For patients with mild or moderate antral chronic atrophic gastritis with mild atrophy of the corpus an endoscopic check up after 5 years is recommended. If the patient is under 40 years old, endoscopic checkups every 3 years are recommended.
For patients with extensive complete intestinal metaplasia of the corpus with or without atrophy in the antrum endoscopic checkups every 2 years are recommended. If the patient is under 40 years old and endoscopic checkups every year are recommended.
For patients with extensive incomplete or mixed intestinal metaplasia of the corpus with or without atrophy of the antrum endoscopic checkups every year are recommended. If the patient is under 40 years old and endoscopic checkups every 6 months are recommended.
We emphasize more frequent checkups for patients under 40 years old because we are aware, as mentioned in our studies (4,5,27), that the lower the age of onset of the dynamic process of development of premalignant lesions, the faster is the progression towards more advanced lesions and the faster the intervention must be for primary prevention. The point becomes even more important when we take into account the study of the pO1 ca028842 project report of the National Cancer Institute of the USA (3) which says that invasive gastric cancer is preceded by a prolonged process that starts during childhood as a result of the Helicobacter pylori infection. Stages following infection include multifocal atrophy of the glands, intestinal metaplasia and dysplasia as we related in our research 10 years ago (4,5,6).
All of the above emphasis on more frequent checkups, especially for younger patients, aims at making early detection of gastric cancer a priority and at observing regression of premalignant lesions resulting from intervention by health promotion and disease prevention medical groups. We believe that in the long term following our recommendations might decrease both the mortality rate and incidence of gastric cancer in our region.
We present this investigation convinced of the great impact on public health it can have. We base this conviction on the fact that the majority of intestinal gastric cancer takes roots in premalignant lesions, the belief that by contributing to the knowledge of all the professionals involved with this pathology we can bring clarity to the treatment of risk factors. By eradicating Helicobacter pylori infections, monitoring and intervening to treat premalignant lesions, controlling salt intake, and by introducing nutritional policies to increase intake of micronutrients and antioxidants, we may conquer the scourge known as gastric cancer.
REFERENCES
1. Holcombe C. Helicobacter pylori: The African enigma. Gut 1992; 33: 429-431. [ Links ]
2. Correa P, Piazuelo B. Cáncer gástrico: El enigma colombiano. Rev Col Gastroenterol 2010; 25: 334-337. [ Links ]
3. Correa P. Cáncer gástrico: Una enfermedad infecciosa. Rev Colombiana de Cirugía 2011; 26: 111-117. [ Links ]
4. Bedoya A, Arcos M, Sanson F, Del Castillo G. Helicobacter pylori y cambios histológicos de la mucosa gástrica en menores de 10 años Pasto 1999. Rev Col Gastroenterol 2002; 17: 36-42. [ Links ]
5. Bedoya A, Garay J, Sanson F, Bravo L, Bravo J, Correa H, et al. Histopathology of gastritis in Helicobacter pylori infected children form population at high and low gastric cancer risk. Human Pathol 2003; 34: 206-213. [ Links ]
6. Bedoya A. Infección por Helicobacter pylori y su asociación con el cáncer gástrico. Temas escogidos de gastroenterología 2003; 32: 142-146. [ Links ]
7. Correa P, Haenszel W. Cuello C, Tannenbaum S, Archer M, et al. A Model for gastric cancer epidemiology. Lancet 1975; 2: 58-60. [ Links ]
8. Correa P, Haenszel W, Cuello C, Zabala D, Fontaham E, Zarama G, et al. The gastric precancerous in a high risk Population: Cohort follow-up. Cancer Res 1990; 50: 4737-4740. [ Links ]
9. Correa P, Bravo L. Investigaciones sobre cáncer gástrico en Nariño. Rev Med Hospital Universitario Departamental de Nariño 2007; 3: 6-12. [ Links ]
10. Correa P, Pazuelo M, Wilson K. Pathology of gastric intestinal metaplasia: Clinical implications. Am J Gastroenterology 2010; 105: 493-498. [ Links ]
11. Correa P, Haenszel W, Cuello C, Zabala D, Fontham E, Zarama G. et al. The gastric precursors process in a high risk population: Cross- sectional studies. Cancer Res 1990; 50: 1731-1736. [ Links ]
12. Bravo L, Cortez A, Carrascal E, Jaramillo R, García L, Bravo P. et al. Patología y prevalencia en biopsias gástricas en Colombia. Colombia Médica 2003; 34: 124-131. [ Links ]
13. Bedoya A. Infección por Helicobacter pylori y cáncer gástrico. Rev Debates 2010; 38: 22-23. [ Links ]
14. Correa P. Piazuelo M, Camargo M. Etiopathogenesis of gastric cancer. Scand J Surg 2006; 95: 218-224. [ Links ]
15. Cassaro M, Rugge M, Gutiérrez O, Leandro G, Graham D, Genta R. Topographic patterns of intestinal metaplasia and gastric cancer. Am. J. Gastroenterology 2000; 95: 1431-1438. [ Links ]
16. Correa P. Is gastric Cancer preventable? Gut 2005; 53: 1217-1221. [ Links ]
17. Montes S. Standpoint for the management of Helicobacter pylori infections in endemic zones for gastric cancer. Red Med Extension Portuguesa 2009; 3: 38-40. [ Links ]
18. Serrano A, Hernández M, De la Garza J, Herrera L. Helicobacter pylori y cáncer gástrico. Cancerología 2009; 4: 193-204. [ Links ]
19. Ramírez A, Sánchez R. Helicobacter pylori y cáncer gástrico. Rev Gastroenterol Perú 2008; 28: 258-266. [ Links ]
20. Ricaurte O. Asociación entre cáncer gástrico y la infección por Helicobacter pylori Rev Col Gastroenterol 1997; 12: 25-31. [ Links ]
21. Meining A, Kowfisch A, Stolte M. Comparative classification and grading of Helicobacter pylori gastritis in patients with gastric cancer and patients with functional dyspepsia. Scand J Gastroenterology 2003; 38: 707-711. [ Links ]
22. Bravo L, Bravo J, Realpe J, Zarama G, Piazuelo M, Correa P. Fuentes de variabilidad en el diagnóstico de gastritis crónica atrófica multifocal asociada con la infección de Helicobacter pylori. Colombia Médica 2008; 39: 58-65. [ Links ]
23. Correa P, Fontham E, Bravo J, Bravo L, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: Randomized trial of antioxidant supplement and anti- Helicobacter pylori therapy. J Nat Cancer Insat 2000; 92: 1881-1888. [ Links ]
24. Fontham E, Correa P, Mera R, Bravo L, Bravo J, Piazuelo M, et al. Duration of exposure a neglected factor in chemoprevention trials. Cancer Epidemiol Bromarkers Prev 2005; 14: 2465-2466. [ Links ]
25. Mera R, Fontham E, Bravo L, Bravo J, Piazuelo M, Camargo M, et al. Long term follow-up of patients treated for Helicobacter pylori infections. Gut 2005; 54: 1536- 1540. [ Links ]
26. Bedoya A. Gastritis crónica atrófica: Lesión precursora de malignidad. Rev Debates 2008; 26: 36-37. [ Links ]
27. Bedoya A, Yépez Y. Hábitos alimentarios y gastritis crónica atrófica en el departamento de Nariño. Rev Col Gastroenterol 2000; 15: 165-169. [ Links ]
28. Bedoya A. Cáncer gástrico en Nariño: Un problema de salud pública. Rev Debates 2006; 14: 32-33. [ Links ]
29. Bedoya A. Cáncer gástrico: Nariño está perdiendo la guerra. Rev Debates 2007; 20: 21-22. [ Links ]
30. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007; 133: 679-672. [ Links ]
31. Ohata M, Kitauchi S., Yoshimura N, Mugitani K, Iwane M, Nakamura H, et al. Progression of chronic atrophic gastritis associated with Helicobacter pylori infection increases risk of gastric cancer. Int J Cancer 2004; 109: 138-143. [ Links ]
32. Rugge M, Genta R, OLGA group. Staging gastritis: an international proposal. Gastroenterology 2005; 129: 1807-1808. [ Links ]
33. Rugge M, Genta R. Staging and grading of chronic gastritis. Human Pathol 2005; 36: 228-233. [ Links ]
34. Rugge M, Meggio A, Pannelli G, Piscioli F, Giacomelli L, Depretis G, et al. Gastritis staging clinical practice: the OLGA system. Gut 2007; 56: 631-636. [ Links ]
35. Satoh K, Osawa H, Yoshizawa M, Nakano H, Hirasawa T, Kihira K, et al. Assessment of atrophic gastritis using the OLGA system. Helicobacter 2008; 13: 225-229. [ Links ]
36. Dixon M, Genta R, Jardley H, Correa P. And the participants in the international work. Classification and grading of gastritis- The up dated Sydney system. Am. J. Sug Pathol 1996; 20: 1161-1181. [ Links ]











 text in
text in 

