Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.27 no.4 Bogotá out./dez. 2012
Review articles
(1) Internist, Gastroenterologist and Hepatologist at the Clínica Colombia (Organización Sanitas). and Clínica del Country in Bogotá, Colombia
(2) Resident II in Gastroenterology at Organización Sanitas
(3) Medical Pathologist and Epidemiologist at Clínica Colombia and Reina Sofía (Organización Sanitas), Professor at the Universidad Nacional de Colombia in Bogotá, Colombia
Received: 17-04-12 Accepted: 23-10-12
Abstract
Autoimmune hepatitis is a condition which can be asymptomatic or can present as acute hepatitis or liver cirrhosis. Diagnosis is based on clinical criteria and laboratory criteria. Laboratory criteria include elevated levels of immunoglobulin G and/or autoantibodies and histological criteria such as hepatitis interface, the presence of plasma cells and lymphocytic infiltrate. In diffcult to diagnose cases original or modified scoring systems can be used. Treatment is based on the use of immunosuppressants such as corticosteroids and azathioprine that have changed the natural history of disease.
Key words
Autoimmune hepatitis, acute hepatitis, cirrhosis, hepatitis interface, scoring systems, steroids.
DEFINITION
Autoimmune hepatitis (AIH) was first described in 1950, but has been known since by different names including active chronic hepatitis, aggressive chronic hepatitis, lupoid hepatitis, plasmatic cell hepatitis and more comonly, autoimmune chronic active hepatitis. In 1992, the International Autoimmune Hepatitis Group recommended that autoimmune hepatitis was the most appropriate term for this disease (1). AIH is defined as a generally persistent or unresolved chronic hepatitis of unknown origin (2).
EPIDEMIOLOGY
This disease is present in all races and in all geographic areas of the world (2, 3). As with other autoimmune diseases, the average initial age of appearance is around forty years of age but can vary and may appear from the first year of life until eighty years of age. Among children, the mean age for appearance of type 1 autoimmune hepatitis is between 10 and 11 years of age while it is between 6 and 7 years of age for type 2 AIH (3-6). Women are afected more frequently than men with a female to male ratio of 3.6:1 (7).
In the USA there are no clear epidemiological data, but, in Norway and Sweden the mean incidence is 1 to 2 for every 100,000 people per year and its prevalence is from 11 to 17 per 100,000 people per year. Similar incidence and prevalence are assumed for North America's Caucasian population. However, due to the subclinical nature of the disease in an important proportion of patients, it is possible that these numbers are greater (8, 9).
NATURAL HISTORY
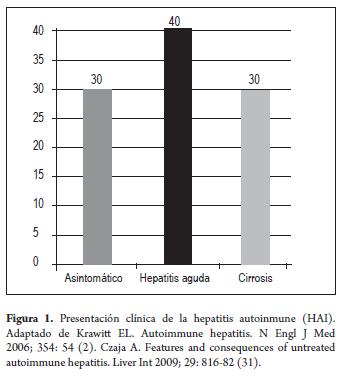
The natural evolution of the untreated disease is known as the result of experiences published before the use of immunosuppressive drugs for AIH became generalized and before the detection of hepatitis C (HCV). These studies showed that 40% of the patients with severe untreated disease died within 6 months of diagnosis and that the survivors frequently developed cirrhosis with esophageal varicose veins and subsequent hemorrhaging (10-15). The acute presentation of the disease was common (40%) and sometimes present together with severe acute hepatic insuficiency with hepatic encephalopathy developing within 8 weeks of the clinical symptoms (16-19). Approximately 30% of cases were completely asymptomatic and insidious form while another 30% began as cirrhotic. The possibility of cirrhosis could be predicted by the histological findings. 17% of the patients had developed interface hepatitis at 5 years, 49% developed mild to moderate alterations within 15 years, 82% developed bridging (or multilobular) cirrhosis. Of these the 5 year mortality rate was 44% (10, 12).
Three randomized treatment controlled clinical trials have established that prednisone alone or in combination with azathioprine improves symptoms, laboratory test results, histological results and immediate survival rates (11-13). These studies led to the acceptation of immuno-suppressive regimes as standard treatment and supported an autoimmune pathogenesis of the disease.
Liver transplantation has also evolved as efficient treatment for patients with decompensated cirrhosis, and the 5 year graf and patient survival rates now exceed 80% (20-23).
PATHOGENESIS AND GENETICS
The exact pathogenesis of autoimmune hepatitis is unknown although molecular mimicry is considered to be the generator of autoimmunity. One theory postulates that environmental triggers cause the loss of mechanisms for immune tolerance in genetically predisposed patients which induces an immunological atack mediated by T-cells on liver antigens which leads to progressive necroin-fammation and fibrosis (24).
While the exact relation between genes and the autoimmune process has not been defined, it is believed that the antigen, the major histocompatibility complex (MHC) and the T-cell receptor (TCR) are involved at the molecular level in which small segments called complementary determinant regions (CDR) identify and contact the MHC complex.
Viruses, medications, herbs and vaccines have been suggested as triggering agents, but the nature of the antigen is still unclear. In most cases, no specific inducer of autoimmunity has been identified. The measles virus, hepatitis virus, simple herpes virus, varicella zoster virus, cytome-galovirus and Epstein-Barr virus and medications such as oxyphenisatin, methyldopa, nitrofurantoin, diclofenac, minocycline and possibly statins have all been implicated as initiators of the disease (11). The administration of interferon may mask or induce autoimmunity and the treatment of chronic viral hepatitis with alpha interferon may induce or unmask autoimmune hepatitis (12).
Most of the evidence supports the central role of an alteration of the T-cell function in the pathogenesis of AIH, and anomalies in B cells may also be important. With loss of tolerance an escape of normal suppression occurs in the auto-reactivity of T-cells which results in inflammation and necrosis (24-27).
In Caucasians, classic AIH (type 1) is strongly associated with the HLA-DR3 and HLA-DR4 serotypes. DRB1 * 0301 and DRB3 3 0101 are common genotypes in North America and DRB1 * 1301 is the most common in South America. Tere is an association of Type 2 AIH with HLA-DRB1 * 07, HLA-DRB1*03 and DQB1 * 0201 alleles. In Japan, where HLA-DR3 occurs infrequently, there is a primary association with the HLA-DR4 serotype (DRB1 * 0405 and DQB1 * 0401 genotypes) (25-27).
CLINICAL
AIH's very broad clinical spectrum ranges from asymptomatic patients to those with a wide variety of symptoms. Symptoms include asthenia adynamia, malaise, anorexia, nausea, abdominal pain and pruritus. Patients with acute liver insuficiency present jaundice and coagulopathy.
Asymptomatic patients may be identified with routine exams where the only evidence of liver disease may be elevated transaminases. On other occasions the asymptomatic patient is discovered during abdominal surgery for various causes. At the other extreme of the spectrum are patients who present the acute form, sometimes with acute liver insuficiency, severe jaundice, prolonged coagulation and transaminase values greater than 1000 U/L (1, 2, 4). These patients may or may not have developed cirrhosis (Figure 1).
Physical examination can show normal features or it can show the presence of hepatomegaly splenomegaly stigmas of chronic liver disease and jaundice (l, 2, 4).
AIH may be associated with other autoimmune diseases including Sjógren syndrome, Crest, SLE, hemolytic anemia, idiopathic thrombocytopenic purpura, diabetes mellitus, hypothyroidism, thyroiditis, celiac disease, ulcerative colitis, and vitiligoc. One prospective study found concurrent immunological diseases present in 38 percent of 122 patients with autoimmune hepatitis while only 22 percent of 63 patients with chronic viral hepatitis had concurrent immunological diseases (30).
Because up to 70% of asymptomatic patients become symptomatic during the course of the disease, asymptomatic patients must be monitored throughout their entire lives to supervise changes in the diseases activity (28, 29).
DIAGNOSIS
The 2010 guidelines of the American Association for the Study of Liver diseases suggest the following considerations (4):
- Diagnosis must be based on clinical examination of patients, laboratory and histological tests including abnormal results of liver biochemical exams, increased total IgG or gamma-globulin levéis, serological markers (ANA, SMA, anti-LKM-1 or anti-LCl), and interface hepatitis (Figure 2).
- Other conditions that may cause chronic hepatitis must be excluded.
- The standard grading system must be used for evaluation of unclear cases.
- For cases which are negative for conventional antibodies, additional antibodies must be found. Minimally they must include atypical anti-SLA and pANCA.
- Cholangiography must be considered to exelude primary selerosing cholangitis in adults who do not respond to corticosteroid treatment within three months.
- In children, cholangiography must be considered to exelude selerosing autoimmune cholangitis.
- All patients with autoimmune hepatitis or inflammatory intestinal disease must be submited to cholangiography to exelude primary selerosing cholangitis.
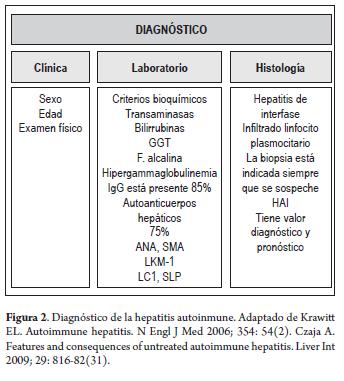
LABORATORY TESTS
Laboratory tests must include the usual liver function evaluations of aminotransferases (ALT and AST), gamma glutamyl transferase (GGT), alkaline phosphate (AP), total and differential proteins, bilirubin (conjugated and non-conjugated), serum immunoglobulin G (IgG) and protein electrophoresis.
As a general rule, elevation of transaminase levels is more striking in autoimmune hepatitis than ate elevated levels of bilirubin and alkaline phosphatase. In some cases however, autoimmune hepatitis has the appearance of cholestasis (28-29).
Characteristic laboratory results for AIH are elevated levels of serum globulins, especially gamma globulins and IgG (Figure 1). Hyperglobulinemia is generally associated with circulating antibodies which are particularly useful for identifying autoimmune hepatitis (31).
Autoantibodies, while not specific for AIH, identify patients with autoimmune hepatitis, allow Classification, and indicate appropriate treatment. Since their expression varies throughout the course of the disease, it is believed that they are not involved in the entity's pathogenesis (32-37). Consequently, a low small titer for autoantibodies does not exclude a diagnosis of autoimmune hepatitis while a high titer, in the absence of other findings, do not confirm an AIH diagnosis (38). Titer measurement in adults does, however, correlate with the severity of the disease, clinical course and approximate response to treatment. In the pediatric population of patients under 18 years of age, titer measurement is a useful biomarker for the disease's activity and may be used to monitor treatment response (34-36).
Antinuclear antibodies (ANAS) are the most common antibodies circulating in autoimmune hepatitis. They are observed in adults and children with type 1 AIH, but rarely in type 2 AIH. When the titer is considered positive depends partially on the methodology used and the age of the patient. In most laboratories, titer values ranging from 1:80 to 1:100 or greater are considered positive in adults while values of 1:20 or greater are considered positive for children. Immunofuorescence patterns are not useful for determining AIH's distinctive clinical characteristics (2, 32, 34, 38).
Anti-smooth muscle antibodies (ASMA) are the second most important class of antibodies that have proven use-ful for diagnosis of type 1 autoimmune hepatitis. Though less frequent than ANAS they are more specific, especially when present in titers of 1:180 or more in adults and titers of 1:20 or greater in children. Anti-actin antibodies (AAA) are more specific to AIH Type 1 than are other ASMA anti-bodies. Titers of 1:320 or more of ASMA generally refect the presence of AAA and may serve as a substitute marker for these antibodies (33, 39).
Liver kidney microsomal type 1 antibodies (LKM1) are the main antibodies for Type 2 autoimmune hepatitis. They are directed at the cytochrome enzyme P450 CYP2S6 (34-36).
Anti-liver cytosol antibody type 1 (anti-LC1) is a marker for type 2 autoimmune hepatitis which generally presents with LKM1 but which may also be the only autoantibody found (40). The antigen recognized by LC1 is formimino-transferase cyclodeaminase (FTCD), a metabolic enzyme specific to the liver (41).
Anti-soluble liver antigen/ liver pancreas antibodies (SLA/LP) is one of the most common markers in children for both types of AIH, but is found in only 10% to 30% of adult patients with type 1 autoimmune hepatitis. These are antibodies directed against a single enzyme which is possibly a UGA suppressor protein, a protein associated with tRNA, or a member of the transferase super family dependent on pyridoxal phosphate. Because SLA/LP is the only antibody that circulates in some patients, it was originally considered to be a different type (type 3) (42-43).
Antineutrophil cytoplasmic antibodies (ANCA) are a group of antibodies that recognize neutrophil proteins. Atypical perinuclear anti-neutrophil cytoplasmic antibodies have been identified in type 1 autoimmune hepatitis, but not in type 2 (44).
HISTOLOGY
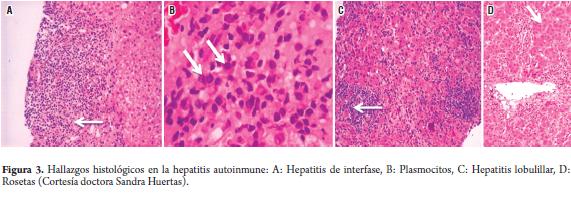
A liver biopsy is recommended at the start of any study to establish the diagnosis and guide treatment decisions (2, 4, 12, 38, 49). Autoimmune hepatitis is histologically characterized by the following non-specific findings (45):
- Portal lymphoplasmacytic infiltrate with occasional eosinophils
- Interface hepatitis or invasión of by the lymphoplasmacytic portal infiltrate into the plaque that surrounds the portal triad extending up to the lobule (periportal infiltrate)
- Occasional lobular commitment sometimes with centrizonal necrosis.
- Changes in the bile duct (destructive or non-destructive cholangitis and ductopenia) present in approximately 25% of the patients.
- Granulomas are rarely seen
- Plasmatic cell infiltrates, hepatocyte rosetes and giant multinucleated cells.
Fibrosis is present in all forms of autoimmune hepatitis, even in the mildest cases. The degree may vary from very mild to advanced. Fibrosis appears with bridging, distortion of the architecture and appearance of regeneration nodules which result in cirrhosis (46).
Histological results vary according to the evolution of the disease. Compared to patients with slow starting disease (48), patients with severe acute liver insuficiency show more interface hepatitis, lobular hepatitis, lobular disorder, hepatocyte necrosis, central less than massive necrosis, but they sufer less fibrosis and cirrhosis (47, 48).
Histological findings, including frequency of cirrhosis, are similar for both symptomatic and asymptomatic patients (45) (Figure 3).
CLASSIFICATION
Type 1 and type 2 of AIH have been recognized on the basis of serological markers (32-44) but have not been established as valid clinical or pathological entities. A third type (type 3) was proposed but has been abandoned because its serological marker (anti-SLA) is also found in 10% to 30% of type 1 and 2 AIH patients (42-43).
Type 1 (classic AIH) is characterized by the presence of ANAS and/or SMA. It constitutes 80% of all IH cases. 75% of these patients are female and peak incidence occurs among patients between 16 and 30 years oíd. 50% of the patients are older than 30 and 23% are older than 60. Associations with other autoimmune diseases are common (15-34%). At the time of diagnosis cirrhosis is present in 25% of these patients (2, 4, 38-40, 45).
Type 2 AIH is characterized by the presence of anti-LKM1 and/ or anti-LCl. The majority of patients with type 2 autoimmune hepatitis are children whose serum levéis of immunoglobulins are generally elevated. The diseases tends to be more aggressive and cirrhosis is found in up to half of the patients at the time of the diagnosis even though a severe acute form may also be present (2, 4, 5, 40, 43, 44).
SCORING SYSTEMS
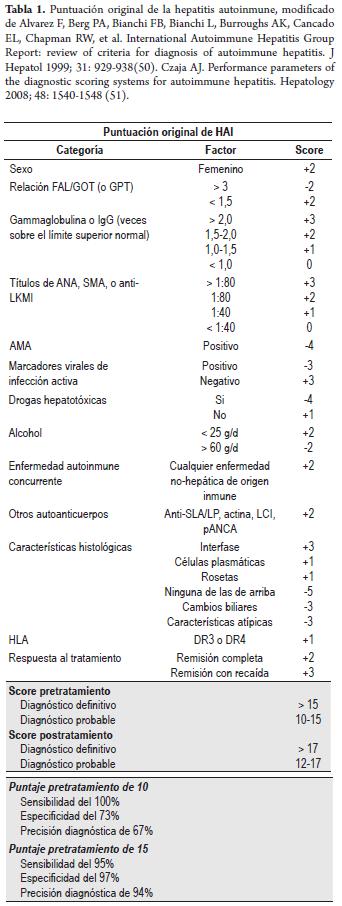
Original scoring system: Diagnostic criteria for autoimmune hepatitis for the original scoring system were developed by an international panel in 1993 as an investigation tool to standardize population studies and clinical trials (1). They were reviewed in 1999 (50) (Table 1). This system assigned scores to different laboratory and histology elements. The system can be applied before and after treatment. A pretreatment score of 10 points or more, or a score of 12 points or more after treatment, indicates "possible AIH". A score of 10 points before the treatment has a sensitivity of 100%, a specificity of 73% and diagnostic precision of 67%. A pretreatment score of 15 points, "defined AIH" has a sensitivity of 95%, a specificity of 97% and diagnostic precision of 94% (51). The clinical criteria are sufcient for diagnosing AIH as definitive or probable in most patients. The diagnostic score system may be applied in difficult cases (50).
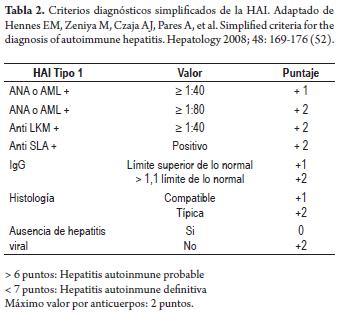
Simplified scoring system: In 2008 a system was developed with simplified criteria based on four determinations: titers of antibodies, levels of IgG, hepatic histology and exclusion of viral hepatitis (52). A probable diagnosis of autoimmune hepatitis is established with a total of 6 points and a definitive diagnosis is established with a total of 7 or more points.
A validation study conducted at 11 participating international medical centers found that the simplified scoring system with a cutof of six of more points had 88% sensitivity and 97% specificity. This compares favorably to the standard clinical and histological reference which has 81%
sensitivity and 99% specificity when a cutof point of seven or greater is used (53). A later study with a cutof point of seven or greater showed slightly lower sensitivity of 70%, but specificity remained high at 100% (54). The simplified versión of the scoring system shows a high sensitivity and specificity in the diagnosis of autoimmune hepatitis but still has not been validated in prospective studies (Table 2) (52-55).
DIFFERENTIAL DIAGNOSIS
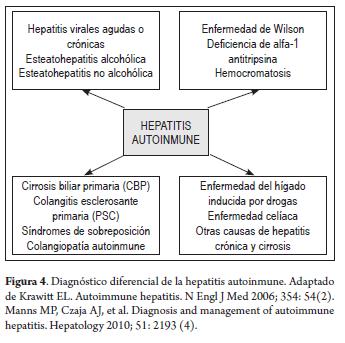
Because of the wide range of AIH characteristics mentioned which include age, appearance, clinical manifestations, and presentation in both genders, the entity must be considered in the differential diagnosis of any patient with evidence of acute or chronic liver disease (Figure 4) (2-4, 6, 38).
TREATMENT
The initial studies of glucocorticoid therapy (GCT) for AIH showed the benefit of the treatment for severe patients and justifed the use of immunosuppressives (11-13). It is known that adequate management improves quality of life, prolongs patient survival and delays the need for liver transplantation. Since cases with less serious biochemical or histological clinical indications have not been studied sufciently to determine treatment, there are not always indications for treatment when we diagnose autoimmune hepatitis (2, 4, 7, 11, 56). The decisión to treat must be individualized based on:
- The severity of the symptoms
- The degree of serum aminotransferase and IgG elevation
- Histological findings and
- The possibility of secondary effects.
AASLD guidelines (4) establish:
Absolute indications
- AST of at least 10 times the upper limit of the normal range.
- AST more than fve times the normal upper limit, along with a gamma-globulin serum level more than twice the upper normal limit.
- Histological findings of bridging necrosis or multilobular necrosis.
- Incapacitating symptoms associated with hepatic inflammation, such as fatigue and arthralgia, independerá of other severe disease indexes.
Uncertain indications for treatment
Treatment decisions for asymptomatic adults with slight laboratory and histological indications must be individualized and balanced according to treatment risks. The AASLD recommends referral of these patients to a hepatologist.
Treatment counter indications
- Inactive cirrhosis.
- Minimum or no disease activity; these patients must be monitored every three to six months.
- Patients with severe preexisting diseases or comorbidity conditions (vertebral conditions, psychosis, osteoporosis, diabetes or uncontrolled hypertension) or a previously known intolerance to prednisone, unless the disease is severe and progressive. Appropriate measures to comorbidities may be taken.
- Patients with severe pretreatment cytopenia (white blood cell count below 2,500 or plaque count under 50,000 or a known complete deficiency of thiopurine methyltransferase.)
Treatment schemes
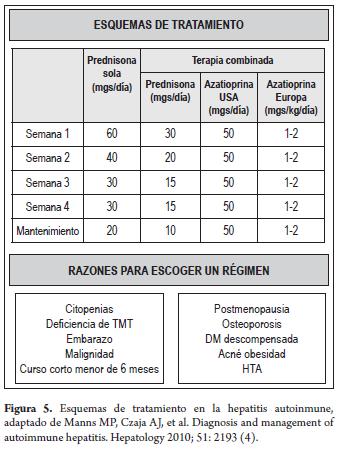
Treatment schemes are based on the use of glucocorticoids monotherapy or combination therapy with steroid sparing agents such as azathioprine which aim at decreasing adverse effects of glucocorticoids. These two schemes have not been directly compared in controlled clinical triáis with longterm follow-ups, but data and clinical experience suggest similar efciencies (2, 4, 56-61).
- Prednisone alone (60 mg a day)
- Lowest dose of prednisone (30 mg a day), along with azathioprine (50mg used in the USA or 1-2mg/kg of body weight in Europe) (Table 1).
Prednisone can be decreased to a low enough level to maintain remission. after 20mg a day doses should be reduced 5mg/week until a 10mg/day dosage is reached. Greater reductions should be 2.5mg/week until 5mg/day is reached (4).
Combined treatment is appropriate for patients who will be treated continuously for at least 6 months or who are at great risk of complications related to corticoids. Once the maintenance dose is reached, it must be continued until disease resolution, treatment failure, or medication intolerance. There is no minimum or máximum treatment duration. Duration is individualized according to the result, desired response and tolerance (2, 4, 56-61) (Figure 5).
Remission or resolution of the disease
Remission or resolution of the disease is the ideal treatment goal. It is characterized by normalization of laboratory alterations in AST, ALT, gamma-globulin, IgG G serum levels and histology. This goal is reached in 10% to 40% of patients (2, 4, 56-61). 90% of adults show improvement in AST, bilirubin, and levels of gamma-globulin by two weeks after the start of treatment (62). Histological improvement occurs three to eight months after clinical and laboratory improvement (12, 47-49). Adults rarely resolve their histological anomalies in less than 12 months and the probability of remission during the treatment decreases after 2 years. Achievement of normal laboratory values before the treatment's completion decreases relative relapse risk after drug are discontinued by three to eleven times the risk for patients who do not achieve this result (47-79). In a study, 87% of the patients that achieved longterm remission had normalized the laboratory indexes before completion of treatment (63).
It is recommended that patients be maintained on fixed daily maintenance doses until remission achieved since early atempts to adjust the dose according to the clinical response could delay or prevent histological improvement. Daily treatment rather than treatment on altérnate days or steroid pulses is recommended because altérnate day treatment may improve symptoms and laboratory results without providing histological resolution (63).
Termination of treatment must be considered after two years if liver function and immunoglobulin levéis repeatedly test normal. A liver biopsy prior to termination of treatment is the only method to assure full resolution of the disease (48, 49, 56-63).
Treatment failure
Approximately 10% of patients experience clinical and laboratory deterioration despite conventional treatment (4). Failure is characterized by sustained inflammatory activity leading to the development or worsening of cirrhosis with eventual complications and death, or the need for a liver transplant. Failure occurs more frequently in three groups of patients (22):
- People with established cirrhosis
- People who develop the disease at an early age or have had a longer duration of the disease before treatment.
- Those who possess the HLA-B8 alíele and/or HLA-DR3 phenotypes.
Optimum treatment of the persistent disease is not well established. The AASLD suggests therapy with 60mg of prednisone a day and 150 mg/day of azathioprine for at least one month after which the prednisone dose is decreased by lOmg, and the dose of azathioprine is decreased by 50 mg after each month of clinical improvement until the conventional maintenance doses are reached (4). 70% of the patients improve their clinical and laboratory results within 2 years and survival is preserved. Histological remission is achieved for only 20% of these patients. The majority of patients remain in therapy and at risk of secondary effects of the medication and/or the disease s progression (63, 64).
Incomplete response
Patients with incomplete responses improve clinically present improved laboratory test results and histological indexes, but do not experience complete resolution. They account for approximately 13% of the patients after 36 months of treatment (59-63). Alternative treatment strategies must be considered. These include long-term low doses of corticosteroids with a gradual decrease of the prednisone dose of 2.5mg a month until the lowest level (lOmg a day) is reached with normal AST or ALT. Another alternative is 2 mg/kg/day of azathioprine for people who do not tolérate corticosteroids and require more treatment (59-63).
Medication toxicity
The toxicity of the drug for 10% to 13% of patients justifies premature interruption or alteration of conventional therapy for these patients. In these cases, therapy with the tolerated agent must be kept at an adjusted dose (59-63).
Secondary effects related to treatment
Corticosteroids
- Esthetic effects produced in 80% of patients after 2 years of treatment with corticosteroids include a moon face, dorsal hump, striations, weight gain, acné, alopecia and facial hirsutism (65-66).
- The most severe systemic effects include osteopenia with vertebral compression, diabetes, psychosis, pancreatitis, opportunistic infections, arterial hypertension and malignancy In general, they occur after prolonged treatment (4-60, 65, 66).
Azathioprine
Cytopenia is the main secondary efect related to azathioprine, and its most severe consequence is bone marrow failure (65). Cytopenia's frequency among AIH patients treated with azathioprine is 46% with a 6% chance of severe hematologic anomaly (68). Patients under azathioprine treatment must have a leukocyte and plaque recount every 6 months (65). Other complications of azathioprine treatment for AIH include cholestatic hepatitis, pancreatitis, nausea, vomiting, rashes, opportunistic infections, and malignancy (65). The incidence of extrahepatic neoplasia in treated autoimmune hepatitis is 1/194 patients/year and the probability of a tumor is 3% after 3 years (67).
All adverse effects must be fully explained to patients before treatment.
Additional measures
Additional measures to reduce adverse effects of medications must be introduced according to the individual perception of risk (2, 4, 5, 11, 12, 13).
Prednisone
For patients treated with prednisone, periodical ophthal-mological assessments to evalúate cataracts and glaucoma are necessary Also, prevention of osteoporosis is important. Basal densitometry and annual lumbar spine and hip checkups are a requirement. In addition patients need regular exercise programs, vitamin D and calcium supplements and/or active agents for the bones such as bisphosphonates (63, 65, 66).
Azathioprine
At any dosage the patient must be monitored for leucopenia and thrombocytopenia at 6 month intervals (2, 4, 5, 58-61). As in other hepatopathies, patients with AIH who present negative viral markers must be vaccinated for hepatitis B (HBV) and hepatitis A (HAV) ideally before therapy (2, 4, 58-61).
Treatment alternatives
Few patient series have been published about the use of medications such as cyclosporine, tacrolimus, methotrexate, mycophenolate mofetil, and budesonide to treat patients who are refractory or intolerant to azathioprine and/or 6-MP (64, 69-73). No medications used in empirical rescue therapies have been incorporated into any standard management algorithm. Mycophenolate mofetil is currently the most promising drug. The AASLD also suggests its use in oral doses of 2g per day (4, 64, 65, 69). At this dosage it has shown improvements in 39% to 84% of patients who tolerate mycophenolate. Nevertheless, 34% to 78% of these patients suspend the medication due to intolerance (nausea, vomiting, pancreatitis, rash, alopecia, deep vein thrombosis, diarrhea and lack of normalization of hepatic function tests (69, 74).
AIH treatment for children
The evolution of the disease in children seems to be more severe than in adults perhaps due to delays in diagnosis or to other concurrent autoimmune diseases such as sclerosing cholangitis (2, 4, 5). Since more than 50% of these children have cirrhosis at the beginning, the milder parts of the disease described in adults are not typically seen in children. This makes medication therapy justifed at the time of diagnosis (2, 4, 5, 57-61). Prednisone is the fundamental pillar in all children's schemes. It is initially administered in a 1-2mg/kg/day dosages (up to 60mg a day) due to the significant long term harmful effects of high or intermediate corticoid doses during initial growth and development of bones and physical appearance. The early use of 1 to 2mg/ kg/day of azathioprine or 1.5mg/kg/day of 6-mercapto-purine is recommended for all children who do not have contraindications (2, 4, 5, 57-61).
Cirrhosis treatment for active AIH
Patients who have cirrhosis have a greater frequency of complications related to medications than do those without cirrhosis (25% vs. 8%) (2, 4, 11-13). They must be thoroughly monitored during treatment, and those with cytopenia must be evaluated for the activity of thiopurine methyltransferase before administration of azathioprine (2, 4, 11-13, 11, 65, 66, 68, 75). The response may be excellent, even in those that have experienced bleeding from esophageal varices or who have serious ascites. There is even the possibility of reversion of the cirrhosis (76). Many patients respond when treatment starts, and the 10 year survival ratefor treated patients, including those with cirrhosis, exceeds 90% (4, 61, 66, 75).
AIH treatment during pregnancy
Glucocorticoids and azathioprine are probably safe during pregnancy. However, azathioprine is in FDA category D for pregnant women because it has been associated with congenital malformations in pregnant rats and because low levels of 6-thioguanine nucleotides are detectable in newborns whose mothers have been treated for Crohn's disease (75, 77-79). Even though no increases of birth deffects have been detected in children of mothers who received this treatment, and even thought there have been no evident negative consequences from breastfeeding by treated mothers, caution is justifed when using azathioprine during pregnancy (4, 75, 79). Pregnancy among women with autoimmune hepatitis has been associated with a greater risk of prematurity, low birth weight and fetal death. Patients must be carefully monitored during pregnancy and for several months after birth due to the risk of outbreaks of the disease's activity. While estrogen levels in the blood drop, conventional therapy must be resumed in a preventive manner two weeks before birth and continued postpartum (4, 63, 64, 75, 79).
HEPATOCELLULAR CARCINOMA
Hepatocellular carcinoma develops in 4% of type 1 AIH patients. The probability this neoplasia developing within 10 years is 2.9% (81). In North America the risk of hepatocellular carcinoma is related to males, portal vein hypertension (ascites, varicose veins or thrombocytopenia), and immunosuppressive treatment for at least 3 years and to cirrhosis of at least 10 years duration (80).
A monitoring strategy based on hepatic echography at 6 month intervals is recommended for these individuals (82).
AUTOIMMUNE HEPATITIS TRANSPLANT
AIH is an indication for liver transplantation (LT) in approximately 2% to 3% of pediatric patients and 4% to 6% of adult patients in the United States of America and Europe (83, 84). LT is indicated for patients with acute liver failure. It is the treatment of choice for patients who progress to decompensated cirrhosis with a MELD score greater than or equal to 15 and for those with hepatocellular carcinoma that meet the criteria for a transplant (85-87). Untreated patients have a 10 year survival rate of less than 30% (20-22). Failed treatment that requires LT is ofen associated with HLA DRB1 * 0301 genotypes (88). Transplant for AIH has good results, with 5 to 10 year survival rates of approximately 75% (4, 85-87). Prior characteristics of patients do not seem to influence these transplant results or the recurrence of AIH (89).
REFERENCES
1. Johnson PJ, McFarlane IG. Meeting report: International Autoimmune Hepatitis Group. Hepatology 1993; 18: 998-1005. [ Links ]
2. Krawit EL. Autoimmune hepatitis. N Engl J Med 2006; 354: 54. [ Links ]
3. Mieli-Vergani G, Vergani D. Autoimmune paediatric liver disease. World J Gastroenterol. 2008; 14: 3360-3367. [ Links ]
4. Manns MP, Czaja AJ, et al. Diagnosis and management of autoimmune hepatitis. Hepatology 2010; 51: 2193. [ Links ]
5. Gregorio GV, Portman B, Reid F, Donaldson PT, Doherty DG, McCartney M, et al. Autoimmune hepatitis in childhood: a 20-year experience. Hepatology 1997; 25: 541-547. [ Links ]
6. Schramm C, Kanzler S, Galle PR. Meyer zum Büschenfelde KH, Lohse AW. Autoimmune hepatitis in the elderly. Am J Gastroenterol 2001; 96: 1587-1591. [ Links ]
7. Al-Chalabi T, Underhill JA, Portmann BC, McFarlane IG, Heneghan MA. Impact of gender on the long-term outcome and survival of patients with autoimmune hepatitis. J Hepatol 2008; 48: 140-147. [ Links ]
8. Boberg KM, Aadland E, Jahnsen J, Raknerud N, Stiris M, Bell H. Incidence and prevalence of primary biliary cirrhosis, primary sclerosing cholangitis, and autoimmune hepatitis in a Norwegian population. Scand. J. Gastroenterol 1998; 33: 99-103. [ Links ]
9. Werner M, Prytz H, Ohlsson B, Almer S, Bjornsson E, Bergquist A, et al. Epidemiology and the initial presentation of autoimmune hepatitis in Sweden: a nationwide study. Scand J Gastroenterol 2008; 43: 1232-1240. [ Links ]
10. Mistilis SP, Skyring AP, Blackburn CR. Natural history of active chronic hepatitis. Clinical features, course, diagnostic criteria, morbidity, mortality and survival. Australas Ann Med 1968; 17: 214-223. [ Links ]
11. Cook GC, Mulligan R, Sherlock S. Controlled prospective trial of corticosteroid therapy in active chronic hepatitis. Q J Med 1971; 40: 159-185. [ Links ]
12. Soloway RD, Summerskill WH, Baggenstoss AH, Geall MG, Gitnick GL, Elveback IR, Schoenfield LJ. Clinical, biochemical, and histological remission of severe chronic active liver disease: a controlled study of treatments and early prognosis. Gastroenterology 1972; 63: 820-833. [ Links ]
13. Murray-Lyon IM, Stern RB, Williams R. Controlled trial of prednisone and azathioprine in active chronic hepatitis. Lancet 1973; I: 735-737. [ Links ]
14. De Groote J, Fevery J, Lepoutre L. Long-term follow-up of chronic active hepatitis of moderate severity. Gut 1978; 19: 510-513. [ Links ]
15. Fevery J DV, DeGroote J. Long-term follow-up and management of asymptomatic chronic active hepatitis. En: S Cohen and RD Soloway (eds.). Chronic Active Liver Disease. Churchill Livingstone: New York; 1983. p. 51-64. [ Links ]
16. Crapper RM, Bhathal PS, Mackay IR, Frazer IH. 'Acute' autoimmune hepatitis. Digestion 1986; 34: 216-225. [ Links ]
17. Nikias GA, Bats KP, Czaja AJ. The nature and prognostic implications of autoimmune hepatitis with acute presentation. J Hepatol 1994; 21: 866-871. [ Links ]
18. Abe M, Hiasa Y, Masumoto T, Kumagi T, Akbar SM, Ninomiya T, Matsui H, et al. Clinical characteristics of autoimmune hepatitis with histological features of acute hepatitis. Hepatol Res 2001; 21: 213-219. [ Links ]
19. Kanda T, Yokosuka O, Hirasawa Y, Imazeki F, Nagao K, Suzuki Y, Saisho H. Acute-onset autoimmune hepatitis resembling acute hepatitis: a case report and review of reported cases. Hepatogastroenterology 2005; 52: 1233-1235. [ Links ]
20. Seaberg EC, Belle SH, Beringer KC, Schivins JL, Detre KM. Liver transplantation in the United States from 1987-1998: updated results from the Pit-UNOS Liver Transplant Registry. Clin Transpl 1998; 17-37. [ Links ]
21. Khalaf H, Mourad W, El-Sheikh Y, Abdo A, Helmy A, Medhat Y, et al. Liver transplantation for autoimmune hepatitis: a single-center experience. Transplant Proc 2007; 39: 1166-1170. [ Links ]
22. Sanchez-Urdazpal L, Czaja AJ, van Hoek B, Krom R, Wiesner RH. Prognostic features and role of liver transplantation in severe corticosteroid-treated autoimmune chronic active hepatitis. Hepatology 1992; 15: 215-221. [ Links ]
23. Campsen J, Zimmerman MA, Troter JF, Wachs M, Bak T, Steinberg T, Kaplan M, et al. Liver transplantation for autoimmune hepatitis and the success of aggressive corticosteroid withdrawal. Liver Transpl 2008; 14: 1281-1286. [ Links ]
24. Donaldson PT. Genetics of autoimmune and viral liver diseases; understanding the issues. J Hepatol 2004; 41: 327. [ Links ]
25. Doherty DG, Donaldson PT, Underhill JA, et al. Allelic sequence variation in the HLA class II genes and proteins in patients with autoimmune hepatitis. Hepatology 1994; 19: 609. [ Links ]
26. Whitingham S, Mathews JD, Schanfield MS, et al. Interaction of HLA and Gm in autoimmune chronic active hepatitis. Clin Exp Immunol 1981; 43: 80. [ Links ]
27. Krawit EL, Kilby AE, Albertini RJ, et al. Immunogenetic studies of autoimmune chronic active hepatitis: HLA, immunoglobulin allotypes and autoantibodies. Hepatology 1987; 7: 1305. [ Links ]
28. Kogan J, Safadi R, Ashur Y, Shouval D, Ilan Y. Prognosis of symptomatic versus asymptomatic autoimmune hepatitis: a study of 68 patients. J Clin Gastroenterol 2002; 35: 75-81. [ Links ]
29. Feld JJ, Dinh H, Arenovich T, Marcus VA, Wanless IR, Heathcote EJ. Autoimmune hepatitis: effect of symptoms and cirrhosis on natural history and outcome. Hepatology 2005; 42: 53-62. [ Links ]
30. Czaja AJ, Carpenter HA, Santrach PJ, Moore SB. Genetic predispositions for the immunological features of chronic active hepatitis. Hepatology 1993; 18:816. [ Links ]
31. Czaja A. Features and consequences of untreated autoimmune hepatitis. Liver Int 2009; 29: 816-823 [ Links ]
32. Czaja AJ. Autoantibodies in autoimmune liver disease. Adv Clin Chem 2005; 40: 127-164. [ Links ]
33. Czaja. The role of autoantibodies as diagnostic markers of autoimmune hepatitis. Expert Rev Clin Immunol 2006; 2: 33-48. [ Links ]
34. Czaja AJ, Homburger HA. Autoantibodies in liver disease. Gastroenterology 2001; 120: 239-249. [ Links ]
35. Czaja AJ, Norman GL. Autoantibodies in the diagnosis and management of liver disease. J Clin Gastroenterol 2003; 37: 315-329. [ Links ]
36. Strassburg CP, Manns MP. Autoantibodies and autoantigens in autoimmune hepatitis. Semin Liver Dis 2002; 22: 339-352. [ Links ]
37. Czaja AJ. Behavior and significance of autoantibodies in type 1 autoimmune hepatitis. J Hepatol 1999; 30: 394-401. [ Links ]
38. Czaja AJ. Autoimmune hepatitis. Part B: diagnosis. Expert Rev Gastroenterol Hepatol 2007; 1: 129-143. [ Links ]
39. Czaja AJ, Cassani F, Cataleta M, et al. Frequency and significance of antibodies to actin in type 1 autoimmune hepatitis. Hepatology 1996; 24: 1068. [ Links ]
40. Bridoux-Henno L, Maggiore G, Johanet C, et al. Features and outcome of autoimmune hepatitis type 2 presenting with isolated positivity for anti-liver cytosol antibody. Clin Gastroenterol Hepatol 2004; 2: 825. [ Links ]
41. Muratori L, Sztul E, Muratori P, et al. Distinct epitopes on formiminotransferase cyclodeaminase induce autoimmune liver cytosol antibody type 1. Hepatology 2001; 34: 494. [ Links ]
42. Manns M, Gerken G, Kyriatsoulis A, et al. Characterisation of a new subgroup of autoimmune chronic active hepatitis by autoantibodies against a soluble liver antigen. Lancet 1987; 1: 292. [ Links ]
43. Wächter B, Kyriatsoulis A, Lohse AW, et al. Characterization of liver cytokeratin as a major target antigen of anti-SLA antibodies. J Hepatol 1990; 11: 232. [ Links ]
44. Zauli D, Gheti S, Grassi A, et al. Anti-neutrophil cytoplasmic antibodies in type 1 and 2 autoimmune hepatitis. Hepatology 1997; 25: 1105. [ Links ]
45. Dienes HP, Popper H, Manns M, Baumann W, Toenes W, Meyer zum Büschenfelde K-H. Histologic features in autoimmune hepatitis. Z. Gastroenterol 1989; 27: 327-330. [ Links ]
46. Cooksley WGE, Bradbear R, Robinson W, Harrison M, Halliday JW, Powell LW, ng HS, et al. The prognosis of chronic active hepatitis without cirrhosis in relation to bridging necrosis. Hepatology 1986; 6: 345-348. [ Links ]
47. Burgart LJ, Bats KP, Ludwig J, Nikias GA, Czaja AJ. Recent-onset autoimmune hepatitis. Biopsy findings and clinical correlations. Am J Surg Pathol 1995; 19: 699-708. [ Links ]
48. Iwai M, Jo M, Ishii M, Mori T, Harada Y. Comparison of clinical features and liver histology in acute and chronic autoimmune hepatitis. Hepatol Res 2008; 38: 784-789. [ Links ]
49. Efe C, Ozaslan E, Purnak T, Ozseker B, Kav T, Bayraktar Y. Liver biopsy is a superior diagnostic method in some patients showing the typical laboratory features of autoimmune hepatitis. Clin Res Hepatol Gastroenterol 2012; 36(2): 185-8. [ Links ]
50. Alvarez F, Berg PA, Bianchi FB, Bianchi L, Burroughs AK, Cancado EL, Chapman RW, et al. International Autoimmune Hepatitis Group Report: review of criteria for diagnosis of autoimmune hepatitis. J Hepatol 1999; 31: 929-938. [ Links ]
51. Czaja AJ. Performance parameters of the diagnostic scoring systems for autoimmune hepatitis. Hepatology 2008; 48: 1540-1548. [ Links ]
52. Hennes EM, Zeniya M, Czaja AJ, Pares A, Dalekos GN, Krawit EL, Bitencourt PL, et al. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology 2008; 48: 169-176. [ Links ]
53. Muratori P, Granito A, Pappas G, Muratori L. Validation of simplified diagnostic criteria for autoimmune hepatitis in Italian patients. Hepatology 2009; 49: 1782-1783; author reply 1783. [ Links ]
54. Yeoman AD, Westbrook RH, Al-Chalabi T, Carey I, Heaton ND, Portmann BC, Heneghan MA. Diagnostic value and utility of the simplified International Autoimmune Hepatitis Group (IAIHG) criteria in acute and chronic liver disease. Hepatology 2009; 50: 538-545. [ Links ]
55. Mileti E, Rosenthal P, Peters MG.Validation and modification of simplified diagnostic criteria for autoimmune hepatitis in children. Clin Gastroenterol Hepatol 2012; 10(4): 417-421. [ Links ]
56. Luxon BA. Diagnosis and treatment of autoimmune hepatitis. Gastroenterol Clin North Am 2008; 37: 461-478. [ Links ]
57. Lohse A, Mieli-Vergani G. Autoimmune hepatitis Journal of Hepatology 2011; 55: 171-182. [ Links ]
58. Schaefer EA, Prat DS. Autoimmune hepatitis: current challenges in diagnosis and management in a chronic progressive liver disease. Curr Opin Rheumatol 2012; 24(1): 84-9. Review. [ Links ]
59. Vierling JM. Diagnosis and treatment of autoimmune hepatitis. Curr Gastroenterol Rep 2012; 14(1): 25-36. [ Links ]
60. Strassburg CP, Manns MP. Therapy of autoimmune hepatitis. Best Pract Res Clin Gastroenterol 2011; 25(6): 673-87. [ Links ]
61. Gleeson D, Heneghan MA; British Society of Gastroenterology. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut 2011; 60(12): 1611-29. [ Links ]
62. Czaja AJ, Rakela J, Ludwig J. Features reflective of early prognosis in corticosteroid-treated severe autoimmune chronic active hepatitis. Gastroenterology 1988; 95: 448-453. [ Links ]
63. Montano-Loza AJ, Carpenter HA, Czaja AJ. Improving the end point of corticosteroid therapy in type 1 autoimmune hepatitis to reduce the frequency of relapse. Am J Gastroenterol 2007; 102: 1005-1012. [ Links ]
64. Czaja AJ, Carpenter HA. Empiric therapy of autoimmune hepatitis with mycophenolate mofetil: comparison with conventional treatment for refractory disease. J Clin Gastroenterol 2005; 39: 819-825. [ Links ]
65. Czaja AJ. Safety issues in the management of autoimmune hepatitis. Expert Opin Drug Safety 2008; 7: 319-333. [ Links ]
66. Uribe M, Go VL, Kluge D. Prednisone for chronic active hepatitis: pharmacokinetics and serum binding in patients with chronic active hepatitis and steroid major side effects. J Clin Gastroenterol 1984; 6: 331-335. [ Links ]
67. Aguilar HI, Burgart LJ, Geller A, Rakela J. Azathioprine-induced lymphoma manifesting as fulminant hepatic failure. Mayo Clin Proc 1997; 72: 643-645. [ Links ]
68. Czaja AJ, Carpenter HA. Tiopurine methyltransferase deficiency and azathioprine intolerance in autoimmune hepatitis. Dig Dis Sci 2006; 51: 968-975. [ Links ]
69. MM, Dhawan A, Samyn M, et al. Mycophenolate mofetil as rescue treatment for autoimmune liver disease in children: a 5-year follow-up. J Hepatol 2009; 51: 156. [ Links ]
70. Baven-Pronk AM, Coenraad MJ, van Buuren HR, et al. The role of mycophenolate mofetil in the management of autoimmune hepatitis and overlap syndromes. Aliment Pharmacol Ther 2011; 34:335. [ Links ]
71. Fernandes NF, Redeker AG, Vierling JM, et al. Cyclosporine therapy in patients with steroid resistant autoimmune hepatitis. Am J Gastroenterol 1999; 94: 241. [ Links ]
72. Manns MP, Woynarowski M, Kreisel W, Oren R, Rust C, Hultcrantz R, Spengler U, et al. Budesonide 3 mg bid in combination with azathioprine as maintenance treatment of autoimmune hepatitis--final results of a large multicenter international trial. Hepatology 2008; 48: 376A-377A. [ Links ]
73. Manns MP, Bahr MJ, Woynarowski M, Kreisel W, Oren R, Gunther R, Hultcrantz R, et al. Budesonide 3 mg tid is superior to prednisone in combination with azathioprine in the treatment of autoimmune hepatitis. J Hepatol 2008; 48: S369 -S370. [ Links ]
74. Hlivko JT, Shifman ML, Stravitz RT, Luketic VA, Sanyal AJ, Fuchs M, et al. A single center review of the use of mycophenolate mofetil in the treatment of autoimmune hepatitis. Clin Gastroenterol Hepatol 2008; 6: 1036-1040. [ Links ]
75. Czaja AJ. Autoimmune hepatitis in special patient populations. Best Pract Res Clin Gastroenterol 2011; 25(6): 689-700. [ Links ]
76. Shah AM, Malhotra A, Kothari S, Baddoura W, Depasquale J, Spira R. Reversal of liver cirrhosis in autoimmune hepatitis. Hepatogastroenterology 2011; 58(112): 2115-7. [ Links ]
77. Westbrook RH, Yeoman AD, Kriese S, Heneghan MA. Outcomes of pregnancy in women with autoimmune hepatitis. J Autoimmun 2012; 38(2-3): J239-44. [ Links ]
78. Uribe M, Chavez-Tapia NC, Mendez-Sanchez N. Pregnancy and autoimmune hepatitis. Ann Hepatol 2006; 5: 187-189. [ Links ]
79. De Boer NK, Jarbandhan SV, de Graaf P, Mulder CJ, van Elburg RM, van Bodegraven AA. Azathioprine use during pregnancy: unexpected intrauterine exposure to metabolites. Am J Gastroenterol 2006; 101: 1390-1392. [ Links ]
80. Montano-Loza AJ, Carpenter HA, Czaja AJ. Predictive factors for hepatocellular carcinoma in type 1 autoimmune hepatitis. Am J Gastroenterol 2008; 103: 1944-1951. [ Links ]
81. Yeoman AD, Al-Chalabi T, Karani JB, Quaglia A, Devlin J, Mieli-Vergani G, Bomford A, et al. Evaluation of risk factors in the development of hepatocellular carcinoma in autoimmune hepatitis: Implications for follow-up and screening. Hepatology 2008; 48: 863-870. [ Links ]
82. Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology 2005; 42: 1208-1236. [ Links ]
83. European Liver Transplant Registry. 2008. http://www.eltr.org. [ Links ]
84. Scientifc Registry of Transplant Recipients. 2008. http://www.ustransplant.org. [ Links ]
85. Ahmed M, Mutimer D, Hathaway M, Hubscher S, McMaster P, Elias E. Liver transplantation for autoimmune hepatitis: a 12-year experience. Transplant Proc 1997; 29: 496. [ Links ]
86. Prados E, Cuervas-Mons V, De La Mata M, Fraga E, Rimola A, Prieto M, Clemente G, et al. Outcome of autoimmune hepatitis after liver transplantation. Transplantation 1998; 66: 1645-1650. [ Links ]
87. Vogel A, Heinrich E, Bahr MJ, Rifai K, Flemming P, Melter M, Klempnauer J, et al. Long-term outcome of liver transplantation for autoimmune hepatitis. Clin Transplant 2004; 18: 62-69. [ Links ]
88. Czaja AJ, Stretell M, Tomson LJ, Santrach P, Moore SB, Donaldson PT, Williams R. Associations between alleles of the major histocompatibility complex and type 1 autoimmune hepatitis. Hepatology 1997; 25: 317-323. [ Links ]
89. Dbouk N, Parekh S. Impact of pre-transplant Anti-nuclear antibody (ANA) and Anti-smooth muscle antibody (SMA) titers on disease recurrence and graft survival following liver transplantation in autoimmune hepatitis (AIH) patients. J Gastroenterol Hepatol 2012; 1440-1746. [ Links ]











 texto em
texto em 

