Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.27 no.4 Bogotá Oct./Dec. 2012
Case report
(1)Internal Medicine, Gastroenterology and Digestive Endoscopy, Coordinator of the Clínica de Enfermedad Inflamatoria Intestinal (IBS Clinica) at the Hospital Pablo Tobón Uribe, Professor at the Universidad de Antioquia and the Pontificia Universidad Bolivariana in Medellín
Correspondence: Unidad de Gastroenterología Hospital Pablo Tobón Uribe. Calle 78 B # 69-240, Medellín, Colombia. E-mail: fabianjuliao@hotmail.com. Cell phone: 315 3960499.
Received: 03-04-12 Accepted: 23-10-12
Abstract
Acute severe ulcerative colitis is a potentially life-threatening condition that requires a pro-active approach with either effective medical treatment or timely colectomy. It is very important to identify at an early stage those who are likely to fail intensive treatment. Although intravenous steroids remain the first line, for those who fail, currently available 'rescue' medical therapy with Infliximab and cyclosporine may reduce the risk of colectomy in the short term without compromising safety.
Key words
Acute severe ulcerative colitis, intravenous steroids, Infliximab, cyclosporine, colectomy.
CASE REPORT
A 44 year old male patient who is married, has two children, and who was born and still lives in Medellín, came to the Hospital Pablo Tobon Uribe (HPTU) in Medellín after sufering diarrhea for three months. Bouts of diarrhea were occurring up to 10 times a day with blood apparent in the feces. Diarrhea was associated with pushing, rectal tenesmus, asymmetric polyarthralgia in large joints (elbow and knee), skin rash on the lower limbs, fatigue, weakness, 5 kg weight loss and pallor. A colonoscopy done one month earlier had shown lef ulcerative colitis. This was confirmed with a biopsy. To manage the condition treatment was begun with 3 g/day of mesalazine and 60 mg/day of prednisone. after patient failed to respond it was decided to refer him to the IBD clinic at the HPTU for management. The patient presented no other evidence of clinical importance. Physical examination at admitance showed that the patient had generalized pallor and was feverish to touch. The patient's vital signs were as follows: blood pressure 90/60, heart rate 106/minute, respiratory rate 22/minute, temperature 38.8 ° C, and BMI 19.3. The patient had pale conjunctiva, dry mucosa, rhythmic cardiac sounds, no heart murmurs, and conserved vesicular breath sounds. The abdomen was sof without palpable masses or organomegaly. Patient felt mild pain upon palpation of the lef colic framework but without signs of peritoneal irritation. Bowel sounds were present. He showed erythematous nodular lesions in the lower limbs, pain in the lef elbow and right knee which showed litle articular efusion. Peripheral pulses were present. Neither edema nor neurological deficits were evident.
The initial clinical diagnosis was acute severe ulcerative colitis considering the number of stools, bleeding and the patient's systemic inflammatory response due to the presence of fever and tachycardia. This diagnosis was strengthened by the presence of extraintestinal manifestations such as erythema nodosum and peripheral spondyloarthritis. Given the above, the patient was hospitalized in the emergency room.
Treatment was begun with intravenous liquids containing added potassium plus 100 mg intravenous hydrocortisone every 6 hours and administration of subcutaneous enoxaparin prophylaxis followed by oral mesalazine. Tests ordered upon admission included erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), blood chemistry, simple chest and abdominal x-rays, a stool culture, toxin A and B for Clostridium difficile and a tuberculosis test. X-rays were normal, CRP was 22.2 mg/L, ESR was 66 mm, and hemoglobin (Hb) was 8.2 g/dl. As a result, two units of red blood cells were transfused. Flexible sigmoidoscopy performed within 24 hours of admission showed severe endoscopic commitment (Figure 1) and biopsies were taken to rule out infection with cytomegalovirus (CMV).
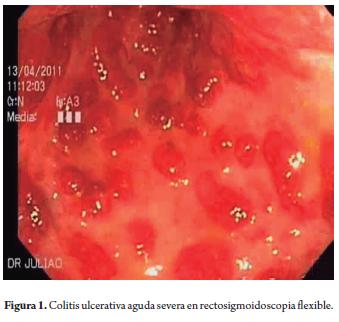
By the third day of hospitalization the patient had improved slightly, but diarrhea continued at 8 stools per day, although with less blood loss. A new CRP test showed 52.1 mg/L. Results other tests showed tuberculosis at 0 mm and patient was negative for toxins A and B for Clostridium difficile. Biopsies showed severe activity. The possibility of CMV infection was discarded. On the fifth day after admission the patient continued to have more than 10 daily bloody stools and abdominal pain. A simple abdominal radiography showed no colonic dilatation. The patient was evaluated by coloproctology due to high risk of colectomy. Prior to initiation of Infliximab, tests for antinuclear antibodies, surface antigen and anticore antibodies for Hepatitis B were requested. All tested negative. Rescue biological therapy with Infliximab (IFX, anti-tumor necrosis factor a) was begun at a dosage of 5 mg/kg intravenous infusion for 2 hours. On the seventh day after admission, the patient had improved clinically to four stools per day with litle bleeding, no fever and CRP levels of 9.8 mg/L. the patient was able to tolerate a sof diet. The atending physician decided to administrate 40 mg/day of oral prednisone. On the tenth day the patient defecated only twice without any bleeding, or arthralgia, and erythema nodosum lesions had decreased. Patient tolerated oral ingestion. CRP levels were 1.2 mg/L. It was decided to discharge the patient and manage him as an outpatient. 2 mg/kg of azathioprine were added to his medication for combination therapy, with scheduled IFX doses at 2 and 6 weeks following first infusion. Instructions were given to gradually reduce prednisone. Outpatient management of inflammatory bowel disease has continued.
DISCUSSION
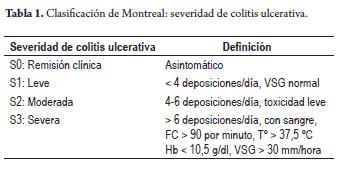
Acute severe ulcerative colitis is a medical emergency that requires high clinical suspicion by gastroenterologists in order to establish adequate and opportune management in specialized centers. According to the Montreal classification (Table 1), acute severe ulcerative colitis is defined as the presence of more than six stools per day associated with signs of systemic toxicity tachycardia (>90/min), fever (>37.8 ° C), hemoglobin <10.5 g/dl and ESR> 30 mm/h (1). Patients who meet these criteria must be hospitalized for intensive management. 15% of patients with ulcerative colitis present severe crises during the course of their illness. In addition, 20% of patients with ulcerative colitis start with severe crises at diagnosis. In our study of 202 patients with inflammatory bowel disease at the Hospital Pablo Tobon Uribe, 23.1% of patients with ulcerative colitis showed severe activity (2). The mortality rate in these individuals in the UK is 2.8%. A Scotish study found that mortality depends on the patient's age. With three year follow up times, the study found that it is 39% in patients over 65 years old, and 0% for patients under 30 years old (3). A recent publication from Oxford found that 28% of patients with acute severe ulcerative colitis were admited to emergency rooms. The colectomy rate was 19.9% for patients upon first admission and 40% after 2 admissions (4). Another study by the same group showed that when response to medical treatment is complete, the colectomy rate after 1 year is 5% while the rate after 5 years rises to 32%. However, when response to medical treatment is incomplete, colectomy rates rise to 51% and 77%, respectively (5).
Assessment and initial management
Evaluation and initial support of these patients requires multidisciplinary management support of emergency physician, internist and intensivist. Suitable intravenous fluid support with potassium substitution is necessary a until diuresis rises above 50 cc/hour while red blood cell transfusions are necessary in order to always maintain hemoglobin levels above 10 g/dL. Patients should be treated with antidiarrheal suspension, anticholinergics, anti-inflammatory drugs (NSAIDs), and opioids. They should be given enoxaparin for thromboprophylaxis. Laboratory tests such as CBC, ESR, CRP, renal function tests, serum electrolytes, albumin, blood gases, coproscopic examination, stool culture, toxin A and B for Clostridium difficile, and blood cultures for suspected infections should be ordered. In addition, an abdominal x-ray should be done to rule out colonic dilatation (toxic megacolon), and a chest x-ray and tuberculosis test should be done to rule out latent tuberculosis in case of the need for biological therapy with anti-TNF a (6). According to international guidelines (7,8) flexible sigmoidoscopy, preferably without colonic preparation, rather than total colonoscopy is preferable for patients with acute severe ulcerative colitis due to risk of perforation. Minimal insufation should always be used and biopsies should be taken to determine histological severity, CMV infection, and Clostridium difficile. This is especially important for patients with previous exposure to steroids or azathioprine.
In recent years there has been an increase in Clostridium difficile, an anaerobic Gram positive bacteria that damages the tissues and produces toxins in IBD patients that lead to a more aggressive and severe course than both in children and adults without IBD. The prevalence of this infection in IBD patients in acute crises ranges from 5% to 18% of cases. It is diagnosed by ELISA for toxin A and B in stool. In case of a negative result in the initial sample when symptoms continue and there is diagnostic uncertainty, a repetition of the test the same increases its sensitivity. Risk factors such as prior exposure to antibiotics (only 40% in IBD), the use of immunosuppressants (OR: 2.58), previous use of proton pump inhibitors, advanced age, patient comorbidities and person-to-person contamination have been described. Clostridium ddifficile infection increases time of hospitalization and the risk of colectomy (OR: 6.6). Currently, it is recommended to suspect Clostridium difficile infection in IBD patients with severe activity and presence of risk factors. In these cases the ELISA test for toxins A and B in fecal samples must be requested upon admission to make an early diagnosis and begin early treatment to prevent complications such as toxic megacolon and serious surgery. The protocols of contact isolation for infected patients are important, and it must be remembered that alcohol cannot destroy the spores of this organism. After contact with a patient or her or his environment, people should use soap and water to wash away spores (9).
On the other hand, patients with IBD have three times higher risks for thromboembolic events than does the general population. Predominantly deep venous thrombosis and pulmonary embolism, their estimated incidence is 1% to 6.7%, and these events tend to occur at earlier ages. This procoagulant state develops because of both congenital and acquired prothrombotic factors with the later being more important. Among the factors we find the most frequently are inherited mutation of factor V Leiden, homo-zygous C667T mutation in the methylenetetrahydrofolate reductase gene and gene mutation in the prothrombin 20210A. It is advisable to study these changes in IBD patients with a history of thromboembolic events. Among the acquired factors we find inflammation, prolonged immobilization, hyperhomocysteinemia (nutritional deficiencies of vitamin B12, vitamin B6 and folic acid), smoking, surgery, central venous catheters, steroid therapy and oral contraceptive use. For patients with these risk factors, prophylaxis with heparin for venous thrombosis should be administered (10, 11, 12).
Medical treatment
First-line medical treatment should be with intravenous steroids, either 100 mg c/6-8 hs hydrocortisone or 60 mg/day methylprednisolone. For patients who do not respond to intravenous steroids within 3 to 5 days of admission, rescue therapy if a single dose of 5 mg/kg of IFX, or cyclosporine at doses of 2 mg/kd/day should be ofered. All patients should be evaluated by a gastrointestinal surgeon or colorectal surgeon when high risk for colectomy is present. A recent systematic review found that the response rate to 5 days of intravenous steroids (hydrocortisone and methylprednisolone) in patients with severe ulcerative colitis admited to emergency rooms was 67%. 29% required colectomies, and the mortality rate was 1%. Continuation of treatment beyond 7 days had no additional benefits (13). In general, 6% to 7.9% of acute severe ulcerative colitis patients develop a toxic mega-colon complication with a mortality rate of about 19%. This is a higher mortality rate (41.5%) than that for patients with colonic perforation (14,15).
There are three major dilemmas for management of these individuals: how to identify patients at high risk for complications, when to start rescue therapy (IFX or cyclosporine) and when to make the decision to perform a colectomy. For patients previously exposed to azathioprine, it is preferable to use IFX cyclosporin. A randomized study of 73 patients with severe ulcerative colitis that compared doses of 2 mg/ kg/day intravenous cyclosporine rescue therapy with doses of 4 mg/kg/day found very similar 8 day response rates (83% vs. 82%) and colectomy rates (9% vs. 13%) in the two groups (16). A Scandinavian study compared an IFX dose of 5 mg/kg for the management of patients with severe ulcerative colitis with placebos and found that at 3 months of follow-up 7 out of 24 (29%) IFX patients required colectomies while 14 out of 21 (67%) placebo patients required colectomies (p: 0.017, OR: 4.9) (17). Tree year follow-ups of these patients found that 12 out of 24 (50%) Infliximab patients required colectomies while 16 out of 21 (76%) of the placebo group required colectomies, p: 0.012. None of the patients who achieved mucosal healing at 3 months required colectomies (18). The long-term results of 142 patients who avoided surgery with cyclosporine show that 88% of them require colectomy after 7 years of follow-up (19). For this reason some authors suggest that rescue therapy difers from colectomy only in a high percentage of cases, but it should become elective which would improve the prognoses of patients (7).
The Study Comparing Cyclosporine With Infliximab in Steroid-refractory Severe Atacks of Ulcerative Colitis (CYSIF) was a randomized controlled study which compared the efficacy of IFX vs. cyclosporine rescue therapy in 111 patients with acute severe ulcerative colitis who were refractory to treatment after 5 days of intravenous steroids. It found response rates on day 7 of 86% for IFX and. 84% for cyclosporine (no significant diference). At day 98, 13 patients in the IFX group and 10 patients in the cyclosporine group had required colectomies (20). Other studies have conducted sequential management with cyclosporine or IFX or vice versa. A retrospective study of 19 patients at Mount Sinai Hospital (21), achieved clinical remission at 3 months in 40% of patients with IFX after no initial response to cyclosporine, and in 33% of patients with cyclosporine after initial failure to respond to IFX. Nevertheless, 16% of patients sufered serious adverse events including death. A second similar study of 84 patients by the French GETAID group achieved a remission rate of 22% at 3 months. 10% of these patients sufered serious adverse events, including death (22). From these studies we conclude that making rescue treatment the second line is less efective and safe compared with using it as the first line therapy.
Surgical treatment
Colectomy is a good treatment option for patients who are unresponsive to medical treatment, but it is not free of complications. A retrospective study from the Mayo Clinic reported that 54% of patients required a second operation after 180 days of post-surgical follow-up. In addition both early (<30 days) and late (30-180 days) complications such as abscesses, abdominal sepsis and fstulas (23) develop frequently. The group of Leuven in Belgium found a complication rate of 27% per month of follow-up (24). Recently, a study showed that the rate of post-colectomy complications is higher when surgical treatment is delayed (25). In addition, a recent meta-analysis has shown that the risk of post-colectomy infections is higher among patients who have pre-viously received treatment with infliximab (OR 2.24) (26).
Within the established factors for prognosis we found that patients with more than 12 stools on day 2 of treatment are at risk of colectomy in 55% of cases, while those with more than 8 stools per day on day 3 have an 85% risk. One study found that at day 3, the presence of C-reactive protein levels over 45 mg/L and between 3 and 8 stools a day are at an 85% risk of colectomy. Other risk factors include albumin of less than 3.0 g/dl, fecal calprotectin over 1.922 mg/g), colonic dilatation over 5.5 cm in abdominal x-rays (75% risk of colectomy) and the presence of deep ulcers seen in colonoscopy (93% risk of colectomy) (3).
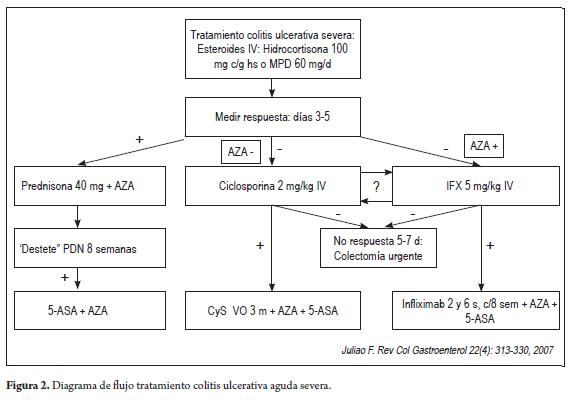
In general, we recommend starting rescue therapy in patients with severe ulcerative colitis for patients who do not respond to initial treatment with intravenous steroids after 3 to 5 days of treatment. In cases of partial response on day 3, treatment can continue for 5 to 7 days with steroids before starting rescue therapy (6). Please note the risk factors mentioned above. In the presence of these, doctors should talk with patients and their families about the possibility of management with IFX or cyclosporine. These options should also be discussed with a colorectal surgeon or gastrointestinal surgeon due to the high risk of colectomy in these cases. Sometimes, it is preferable to perform a timely colectomy rather than risk the patient's life with complications such as toxic megacolon which are associated with high mortality. A fow diagram of the treatment of patients with acute severe ulcerative colitis is shown in Figure 2.
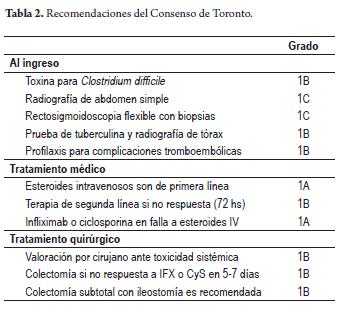
Recently, the consensus of Toronto (March 2010) (27) on treatment of severe ulcerative colitis was published. Twenty-three 23 experts from the Canadian Association of Gastroenterology voted on a series of recommendations for qualifying degrees of clinical evidence according to the GRDE (Grading of Recommendations Assessment, Development and Evaluation) criteria. A summary of these recommendations is shown in Table 2.
Acknowledgements
To my wife Lina and my daughters Paulina and Susana for their time.
REFERENCES
1. Satsangi J, Silverberg MS, Vermeire S, Colombel JF. The Montreal classification of inflammatory bowel disease: controversies, consensus, and implications. Gut 2006; 55: 749-753. [ Links ]
2. Juliao F, Ruiz MH, Florez JF, et al. Fenotipo e historia natural de la enfermedad inflamatoria intestinal en un centro de referencia en Medellín-Colombia. Rev Col Gastroenterol 2010; 25 (3): 240-251. [ Links ]
3. Travis S, Satsangi J, Lémann M. Predicting the need for colectomy in severe ulcerative colitis: a critical appraisal of clinical parameters and currently available biomarkers. Gut 2011; 60: 3-9. [ Links ]
4. Dinesen LC, Walsh AJ, Travis S, et al. The patern and out-come of acute severe colitis. Journal of Crohn's and Colitis 2010; 4: 431-437. [ Links ]
5. Bojic D, Radojicic Z, Travis S, et al. Long-term Outcome after Admission for Acute Severe Ulcerative Colitis in Oxford: Te 1992-1993 Cohort. Inflamm Bowel Dis 2009; 15: 823-828. [ Links ]
6. Hart AL, Ng SC. Review article: the optimal medical management of acute severe ulcerative colitis. Aliment Pharmacol Ther 2010; 32: 615-627. [ Links ]
7. Van Assche G, Vermeire S, Rutgeerts P. Management of acute severe ulcerative colitis. Gut 2011; 60: 130-133. [ Links ]
8. Travis SP, Stange EF, Lémann M, et al. European evidence based consensus on the management of ulcerative colitis: Current management. Journal of Crohn's and Colitis 2008; 2: 24-62. [ Links ]
9. Ananthakrishnan AN, Issa M, Binion D. Clostridium dificile and inflammatory Bowel Disease. Gastroenterol Clin N Am 2009; 38: 711-728. [ Links ]
10. Papa A, Danese S, Grillo A, Gasbarrini G, Gasbarrini A. Review Article: Inherited Trombophilia in inflammatory Bowel Disease. Am J Gastroenterol 2003; 98: 1247-1251. [ Links ]
11. Spina L, Saibeni S, Bataglioli T, et al. Trombosis in inflammatory Bowel Diseases: Role of Inherited Trombophilia. Am J Gastroenterol 2005; 100: 2036-2041. [ Links ]
12. Yoshida H, Granger DN. Inflammatory Bowel Disease: A Paradigm for the Link between Coagulation and Inflammation. Inflamm Bowel Dis 2009; 15: 1245-1255. [ Links ]
13. Turner D, Walsh C, Steinhart AH, et al. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a metaregression. Clin Gastroenterol Hepatol 2007; 5(1): 103-10. [ Links ]
14. Gan SI, Beck PL. A New Look at Toxic Megacolon: An Update and Review of Incidence, Etiology, Pathogenesis, and Management. Am J Gastroenterol 2003; 98: 2363-2371. [ Links ]
15. Autenrieth DM, Baumgart DC. Toxic Megacolon. Inflamm Bowel Dis 2012; 18: 584-591. [ Links ]
16. Van Assche G, D´Haens G, Noman M, et al. Randomized, double-blind comparison of 4 mg/kg vs. 2 mg/kg intravenous cyclosporine in severe ulcerative colitis. Gastroenterology 2003; 125: 1025-31. [ Links ]
17. Järnerot G, Hertervig E, Friis-Liby, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology 2005; 128: 1805-11. [ Links ]
18. Gustavsson A, Järnerot G, Hertervig E, et al. Clinical trial: colectomy after rescue therapy in ulcerative colitis - 3 year follow-up of the Swedish-Danish controlled infliximab study. Aliment Pharmacol Ther 2010; 32: 984-989. [ Links ]
19. Moskovitz DN, Van Assche G, Maenhout B, et al. Incidence of colectomy during long-term follow-up after cyclosporine-induced remission of severe ulcerative colitis. Clin Gastroenterol Hepatol 2006; 4:760-5. [ Links ]
20. Laharie D, Bourreille A, Branche J, et al. Cyclosporin versus Infliximab in Severe Acute Ulcerative Colitis Refractory to Intravenous Steroids: A Randomized Trial. DDW 2011 Abstract 619. [ Links ]
21. Maser E, Deconda D, Lichtiger S, et al. Cyclosporine and Infliximab as Rescue Therapy for Each Other in Patients with Steroid-Refractory Ulcerative Colitis. Clin Gastro & Hep 2008; 6:1112-1116. [ Links ]
22. Leblanc S, Allez M, Seksic P, et al. Successive Treatment with Cyclosporine and infliximab in Steroid-Refractory Ulcerative Colitis. Am J Gastroenterol 2011; 106: 771-777. [ Links ]
23. Lofus EV, Delgado DJ, Friedman HS, et al. Colectomy and the Incidence of Postsurgical Complications among Ulcerative Colitis Patients with Private Health Insurance in the United States. Am J Gastroenterol 2008; 103: 1737-1745. [ Links ]
24. Ferrante M, Declerck S, Herfogh D, et al. Outcome after Proctocolectomy with Ileal Pouch-anal Anastomosis for Ulcerative Colitis. Inflamm Bowel Dis 2008; 14: 20-28. [ Links ]
25. Randall J, Singh B, Travis S, et al. Delayed surgery for acute severe colitis is associated with increased risk of postoperative complications. British Journal of Surgery 2010; 97: 404-409. [ Links ]
26. Yang Z, Wu K, Wu D. Meta-analysis: pre-operative infliximab treatment and short-term post-operative complications in patients with ulcerative colitis. Aliment Pharmacol Ther 2010; 31, 486-492. [ Links ]
27. Biton A, Buie D, Feagan B, et al. Treatment of Hospitalized Adult Patients With Severe Ulcerative Colitis: Toronto Consensus Statements. Am J Gastroenterol 2012; 107: 179-194. [ Links ]











 text in
text in 

