Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.27 no.4 Bogotá oct./dic. 2012
Case report
(1) Gastroestudio Clínica Las Vegas in Medellín
Received: 16-05-12 Accepted: 23-10-12
Abstract
We report on two cases of inflammatory fibroid polyps in the stomach (Vanek's tumor). The first patient was a 63-year-old woman with abdominal pain for whom resection of an antral polypoid lesion was done. This polyp presented classical histopathology. The second case was a 63-year-old male patient who was admitted to the hospital with hemorrhagic shock due to hematemesis. During endoscopy a Dieulafoy's lesion was found in the cardia of the stomach and a polypoid lesion was found in the antrum. Unusual histopathology required that the diagnosis be confirmed by immunohistochemistry.
Key words
Gastric polyp, inflammatory fibroid gastric polyp, Vanek's tumor, Dieulafoy's lesion.
INTRODUCTION
Inflammatory fibroid polyps (IFPs) are infrequent pseu-dotumoral lesions which were described by Vanek in 1949 (1) and inappropriately called tumors because their structures correspond to those of benign mesenchymal polyps. IFPs originate in the submucosa and are formed of fibrous tissues and vascular structures, present prominent inflammatory infiltrate in which numerous eosinophils stand out. The mucosa that covers an IFP may be normal or may present ulceration, inflammation or regeneration secondary to erosion. IFPs tend to be unique, antral or prepyloric lesions. The clinical symptomatology of IFPs varies. Patients can be asymptomatic, but ofen present dyspepsia, non-specific abdominal pain, high digestive hemorrhaging, or intestinal obstructions from the efect of the pyloric valve (2).
CASE PRESENTATION
Case 1
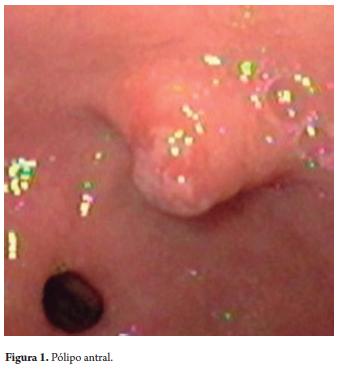
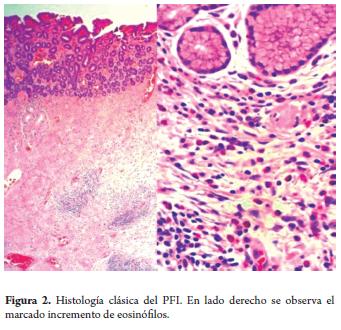
An upper digestive endoscopy of a 63 year old woman sufering from epigastric pain found a 10mm polyp with benign characteristics (Figure 1). The polyp was resected with a thermal loop. Histopathological diagnosis showed an inflammatory fibroid polyp (Figure 2).
Case 2
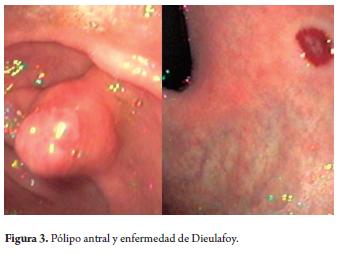
Following 8 hours of melena and hematemesis which resulted in acute anemia, a 63 year old man was admited for an emergency superior digestive endoscopy. During gastric lavage fresh blood was extracted. A 10mm polyp with a broad pedicle and no signs of recent bleeding or active hemorrhaging was found in the prepyloric region. This did not explain the massive bleeding (Figure 3). Exhaustive lavages were repeated until a subcardial lesion corresponding to Dieulafoy's disease was found and treated with sclerotherapy and the performance of posterior thermal snare polypectomy.
The pathological anatomy study showed a polypoid formation covered by antral gastric mucosa with complete and incomplete intestinal metaplasia zones and superficial erosion. A vascular proliferation of spindle cells with enlarged nuclei was subjacent to this zone. It had a notorious nucleolus and broad cytoplasm with indistinct edges which was arranged in bundles. Some of these cells had dun colored pigment in thick granules. There was also an intense inflammatory infiltrate with eosinophilic predominance.
Immunohistochemistry with HMB45, c-KIT, cytokeratin and S-100 cocktail had negative results, but the CD34 study of the cells was positive confirming the IFP diagnosis.
DISCUSSION
The incidence of gastric polyps found in the literature ranges from 0.1% to 0.8% in autopsy series. However, series based on endoscopic findings show a frequency ranging from 1% to 4% in endoscopies performed (2).
From a total of 3,531 gastric biopsies examined in our center in 2009, 54 polypoid lesions (1.5%) were found and studied. Out of these 2 corresponded to IFP, which is equivalent to a frequency of 3.7% among endoscopically diagnosed polypoid lesions which is similar to the frequency described in the literature.
IFPs were first described by Vanek. Their incidence is rare, but they occur most frequently in the prepyloric region although they may also be found anyplace in the digestive tract. IFPs have been described in the esophagogastric junction union and, less frequently, in the duodenum, jejunum and ileum (3).
Symptoms for these lesions vary directly in relation to size. There is abundant chronic bleeding in eroded polyps and mechanical obstructions or intussusceptions accompanying large polyps (4). They generally occur in the sixth decade of life (5).
A homogeneous hypoechoic mass of a submucosal origin can be observed with echoendoscopy. Histological studies show that these lesions are composed of spindle-shaped cells that intertwine with inflammatory infiltrate which has a striking abundance of eosinophils. Peripheral eosinophilia is not normally found. No correlation has been found between IFP and eosinophilic enteritis or the presence of parasites.
The histologic differential diagnosis must consider gastrointestinal stromal tumor (GIST), inflammatory myofi-broblastic polyp and eosinophilic gastroenteritis.
Immunohistochemistry plays an important role in tumoral Classification of spindle cells which test positive for CD34, vimentin and S100, and test negative for c-kit and desmin (4,5,6).
Even though the histogenesis of IFPs is controversial, the most likely origins are in dendritic cells or CD34 positive perivascular cells. Many well represented proliferative cells of myoepithelial appearance and many endothelial cells are found ultrastructurally.
IFPs present mutations in the PDGFR gene (growth factor receptor, derived from alpha platelets).
Treatment for lesions less than 3 cm has been with endoscopic thermal loop resection. If resection is incomplete, it can be concluded with an argón plasma láser (7). Gastrectomy has been used for a few patients with large tumors when a malignant tumor has been suspected during the operation or when the CT sean shows a possible invasión or malignaney (8). Up to 8% of IFPs have been described as concomitant with adenoma or carcinoma.
Even though no precise etiology is clear, it has been postulated that IFP's are related to infections, allergies, previous surgeries or trauma. When the mucosa is damaged it may incite an inflammatory response in the adjacent submucosa and stimulate the formation of a polypoid mass.
Other associated lesions include gastritis from Helicobacter pylori, ulcers, adenoma and carcinoma (9). Other studies suggest that IFP's are more frequent among patients with atrophic gastritis and pernicious gastritis.
Although some studies hold that the Helicobacter pylori cause the lesión, this has not been confirmed (10). A case report shows that a lesión decreased in size with only eradication treatment for bacteria (ll). Neither of our two patients presented Helicobacter pylori infections.
The scarcity of cases of recurrences reported in the literature indicates that recurrences are infrequent after adequate endoscopic or surgical resection.
CONCLUSIONS
1. IFP symptoms before performing superior digestive endoscopy may be:
- Antropyloric subocclusion in large polyps
- Digestive bleeding in eroded polyps.
2. Vanek's polyp is suspected in endoscopies from:
- Broad pedicle due to mesenchymal origin with healthy gastric mucosa or similar to neighboring mucosa. This leads to a diagnosis of a submucosal tumor and therefore requires resection to diferentiate it from GIST.
- The most frequent characteristic is superficial ulceration in the most prominent zone. Unlike adenomas, forcep microbiopsies cannot diagnose IFP when taken from the implantation base or the non-eroded lateral zones.
- Immunohistochemistry after resection can confirm a definitive diagnosis.
REFERENCES
1. Vanek J. Granuloma gástrica submucosa con infiltración eosinofílica. Am J Pathol 1949; 25: 397-411. [ Links ]
2. Aguirre A, Vázquez JL. Pólipos gástricos. Revista gastroenterol 2000; 4: 213-222. [ Links ]
3. Guerra Bautista JA, F Ibáñez Delgado, et al. Inflammatory fibroid polyp of the stomach. Rev Esp Enferm Dig 2006; 98(6): 482-483. [ Links ]
4. Galbfach PJ, et al. Inflammatory fibroid polyp of the stomach -a case report. Pol Merkur Lekarski 2009; 26(152): 125-126. [ Links ]
5. Odze R, Goldblum J. Surgical pathology of the GI tract, liver, biliary tract and Pancreas. Saunders; 2009. p. 439-441. [ Links ]
6. Tocchi A, G Mazzoni, et al. Inflammatory fibroid polyp of the stomach. Report a case. G Chir 1997; 18(8-9): 413-416. [ Links ]
7. Neneman B, A Gasiorowska, et al. The efficacy and safety of argon plasma coagulation (APC) in the management of polyp remnants in stomach and colon. Adv Med Sci 2006; 51: 88-93. [ Links ]
8. Cheol H, Yang KM, et al. CT Findings of a Gastric inflammatory Fibroid. J Korean Soc Radiol 2009; 60: 271-274. [ Links ]
9. Fenoglio-Preiser C. Gastrointestinal Pathology. Lippincot William & Wilkins; 2008. p. 215-216. [ Links ]
10. Shalom A, Wasserman I, et al. Inflammatory Fibroid Polyp and Helicobacter Pylori. Etiology or Coincidence? European Journal of Surgery 2000; 166(1): 54-57. [ Links ]
11. Nishiyama Y, Koyama S, Andoh A, Kishi Y, Yoshikawa K, Ishizuka I, Yokono T, Fujiyama Y. Gastric inflammatory fibroid polyp treated with Helicobacter pylori eradication therapy. Intern Med 2003; 42: 263-267. [ Links ]











 texto en
texto en 

