Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.1 Bogotá jan./mar. 2013
Clostridium difficile infections in elderly patients
Ángela Blanco Pérez, MD. (1), Óscar Ruiz Morales, MD. (2), William Otero Regino MD. (3), Martín Gómez Zuleta, MD. (4)
(1) Third year resident in Geriatrics at the Universidad Nacional de Colombia in Bogotá, Colombia.
(2) Internist and Gastroenterology Fellow at the Universidad Nacional de Colombia in Bogotá, Colombia.
(3) Professor of Medicine, Gastroenterology Unit at the Universidad Nacional de Colombia, Gastroenterologist at the Clínica Fundadores in Bogotá, Colombia.
(4) Professor of Medicine, Gastroenterology Unit at the Universidad Nacional de Colombia. Gastroenterologist at the Hospital El Tunal in Bogotá, Colombia.
Received: 02-09-12 Accepted: 17-01-13
Abstract
Clostridium difficile infection is one of the most important causes of acute diarrhea in elderly patients. For over thirty years this infection has been associated with the use of antibiotics. Its incidence and severity among the elderly have progressively increased over the last decade resulting in increased risk morbidity and mortality from this infection among this population. This article aims to review disease associated with Clostridium difficile (C. difficile) among the elderly. It emphasizes the importance of early diagnosis based on risk factors, clinical manifestations, prevention and treatment before infection reaches epidemic proportions. It also emphasizes the importance of education for health care professionals and relatives of patients in order to limit the spread of this infection and associated morbidity and mortality.
Keywords
Clostridium difficile, diarrhea, pseudomembranous, colitis.
INTRODUCTION
Clostridium difficile is a Gram positive anaerobic bacillus which has become the leading cause of diarrhea in the elderly population and which has a high mortality rate in severe cases. The name of this microorganism is derived from the difficulty of isolation in culture media compared to other species of the same genus (1,2). It was first identified in 1935 as part of the normal flora of newborns, but in the 1970's it was also identified as a cause of diarrhea secondary to the use of a broad spectrum of antibiotics in the hospital environment (2).
Since 2002 new strains of C. difficile which are resistant to fluoroquinolones have been reported. This new genotype is BI/NAP1/027 strain. It produces a disease with higher incidence, greater severity and more frequent recurrence that most often affects the elderly population. It has created a significant burden in terms of morbidity, mortality, and economic cost. Its cost to the health system of the United States is over 500 million dollars per year (1-3).
The clinical manifestations of the disease range from no symptoms through diarrhea associated with C. difficile, successively pseudomembranous colitis to toxic megacolon which is considered to be the most severe presentation (3-6). The keystones of treatment have been antibiotics such as metronidazole and vancomycin. Fidaxomicin became available in 2011 when it was approved by the FDA for this purpose (4-6).
Given C. difficile's wide variety of presentations, it is a challenge to determine the true incidence and prevalence. Nevertheless, in the last 10 years there has been a consistently documented increase in the frequency of its more severe clinical presentations (2-5). This makes it imperative that health care staff implement a variety of strategies to control and prevent this infection in order to limit its potential for epidemics. Given the emergence of C. difficile as one of the most important infections in the elderly population in the twenty-first century, we decided to write this update.
METHODS
The following search methodology was used in English and Spanish with the Pubmed, Medline and Embase platforms. Key words in English included infection, Clostridium difficile, Clostridium difficile associated diarrhea and elderly, antimicrobial associated diarrhea and elderly, drug therapy, vancomycin, metronidazole, and fidaxomicin (sic), OR pathogenesis, and OR Mortality. The search was limited to metaanalyses, systematic reviews, clinical trials and review articles published in the last ten years. Publications were selected that we believe had good methodological and bibliographical support and whose authors were experienced in the subject. In addition, key references which had been key to the selected publications were identified and were then included as support for this review.
MICROBIOLOGY AND PATHOGENESIS
Clostridium difficile is a Gram positive spore-forming anaerobic bacillus which produces toxins that can exist in the vegetative or spore state. It was identified in 1935 as bacillus difficilis in the fecal flora of healthy children and recognized as a cause of antibiotic-associated colitis. In 1977, it was called C. difficile, by Hall and O'Toole to reflect the difficulty in isolating it attributable to its relatively slower growth than most other members of the genus Clostridium (1, 2, 7). Spores are resistant to physical changes and can survive high temperatures and ultraviolet light. It has been shown that when strains of C. difficile are exposed in vitro to sub-inhibitory concentrations of nonchloride disinfectants such as detergents and hydrogen peroxide, their ability to sporulate increases. This gives it the great strength that allows it to survive in isolation in the hospital environment for months and in patients for more than 40 days after hospital discharge (1, 6, 8, 9).
The infection is transmitted through the fecal-oral route (1,3). Spores of C. difficile are excreted in the feces of infected patients and then can spread through the hands of patients and healthcare workers which are the main sources of transmission. Then the spores are ingested orally (1-5). Once ingested they can survive gastric acid pH in the colon and germinate into vegetative cells or multiple trophozoites (1, 5, 6).
In healthy adults the normal bacterial flora and IgG antibodies neutralize toxin A and protect against C. difficile colonization and disease (1, 3, 6). It has been observed that IgG antitoxin antibodies are more common in patients with asymptomatic infections than those who manifest infections (10). Hydrochloric acid which decreases the number of spores and toxins and peristalsis which eliminates the bacteria and its toxins have also been identified as defense mechanisms (1, 11).
It is noteworthy that not all individuals colonized by C. difficile develop disease. This is because the pathogenicity of these bacteria is directly related to the expression of virulence factors (such as pili, flagella, proteolytic enzymes, cell surface proteins, production of A and B toxins) which contribute to the establishment of the disease at different stages during the infection process and which are associated with the competence of the host's immune system (5, 6).
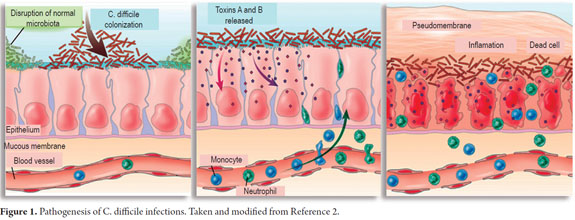
Several factors are required for C. difficile to cause disease (Figure 1) (2). The first and most important is alteration of the normal intestinal microbiota. This is usually caused by the use of antibiotics especially clindamycin, penicillins, cephalosporins and most recently fluoroquinolones although virtually all antibiotics have the potential to predispose patients to this infection (2, 7, 9, 10).
In the first stage of colonization following alteration of the normal intestinal microbiota, proteases facilitate penetration of the mucus layer of the intestinal tract by C. difficile. There it adheres to enterocytes using a repertoire of adhesins (6, 12). It then produces the release of toxins A and B which cause multiple effects among which apoptosis of epithelial cells should be highlighted since this triggers the cascade of inflammation and clinical manifestations of the disease (2, 6, 13).
Toxins A and B have monoglucoside transferase activity which mediates glycosylation of Rho family proteins (GTPases: Rho, Rac and Cdc42). These proteins normally bind to guanosine triphosphate (GTP) and are involved in signal transduction and regulation of actin filaments in the host cell (14). As a consequence of this activity the cytoskeleton becomes disorganized causing changes in cellular morphology including opening the epithelial GAP junctions responsible for maintaining tissue integrity (6, 15).
The production of Toxins A and B by C. difficile is directly linked to production of tumor necrosis factor, release of proinflammatory interleukins and increased vascular permeability. These in turn are associated with different clinical manifestations of the disease such as watery diarrhea, colitis, pseudomembranous colitis and toxic megacolon. Toxin A is an enterotoxin that causes hypersecretion of fluid and hemorrhagic inflammatory process (3, 11, 12). Toxin B is a cytotoxin and an enterotoxin that causes cell death by disruption of the cytoskeleton. It is ten times more potent than Toxin A and is the virulence factor necessary for expression of the infection (3, 11, 12, 13). Strains which produce Toxin B but not Toxin A cause the most severe forms of the infection while strains which do not produce Toxin B are not pathogenic (1, 7, 13).
In the elderly, host defenses against C. difficile are damaged by senescence of the immune response resulting from comorbidities and physiological changes associated with aging (16). Several in vitro studies have found that the neutrophils of elderly patients are not efficient at phagocytosis and killing C. difficile. It has also been determined that young adults who are infected have low serum IgG antitoxin levels as well as poorer ability to neutralize Toxins A and B (17).
EPIDEMIOLOGY
Data from the Centers for Disease Control and Prevention confirm that the incidence of C. difficile doubled from 2000 to 2005 from 5.5 cases per 10,000 to 11.2 per 10,000 people. This bacillus causes about 3 million cases of diarrhea and colitis each year (9). Approximately 3% of the colonized adults are asymptomatic, in hospital areas 20% to 30% of patients are colonized, but this percentage increases to up to 50% of patients in chronic care units (1, 2, 9).
C. difficile is responsible for 15% to 20% of cases of antibiotic associated diarrhea (AAD) and for almost all the cases of pseudomembranous colitis (18). In older patients the incidence of C. difficile infections is 5 to 10 times higher than in younger patients. Its prevalence was 69% in those over 60 years in 2008 (12, 14). The overall mortality associated with diarrhea due to infectious C. difficile is estimated at 17%, but it is higher in the elderly population (17, 19). The acquisition rate of C. difficile is estimated at 13% in patients with hospital stays of 2 weeks and at 50% for those whose stays are over 4 weeks. For a patient who shares a room with another patient who is infected with C. difficile, the infection will be acquired on average after a stay of 3.2 days (18, 20).
The change in the epidemiology of C. difficile infections has been attributed to a new hypervirulent strain: NAP1/ BI/027. This strain was first isolated in 1984 at which time it was only rarely detected in humans. Although it was initially sensitive to quinolones (1, 5), after 2000 this epidemic strain acquired increased resistance to fluoroquinolones indicating a relation between its appearance and the use of these antimicrobials (29). As a result of deletion of the Tdc gene which normally regulates toxin production, this new strain produces 16 to 26 times more toxins than other strains and is extremely virulent (5, 13, 21). The presence of this strain has been widely documented in every state in the United States, and in Canada, the UK, France, Germany, Italy, Denmark, Ireland, the Netherlands, Austria, Poland, Switzerland, Norway, Belgium, Finland, Spain, Japan, Korea and Hong Kong, and Australia (6). However, so far there has only been one publication in Latin America which described the isolation of this strain in a hospital in Costa Rica (15, 22).
C. difficile disease has caused a major burden in terms of morbidity, mortality and economic costs to health care systems. One 2011 economic model in the United States estimated costs per infection at US$9,000 and the total annual total costs at more than US$500 million (3).
RISK FACTORS
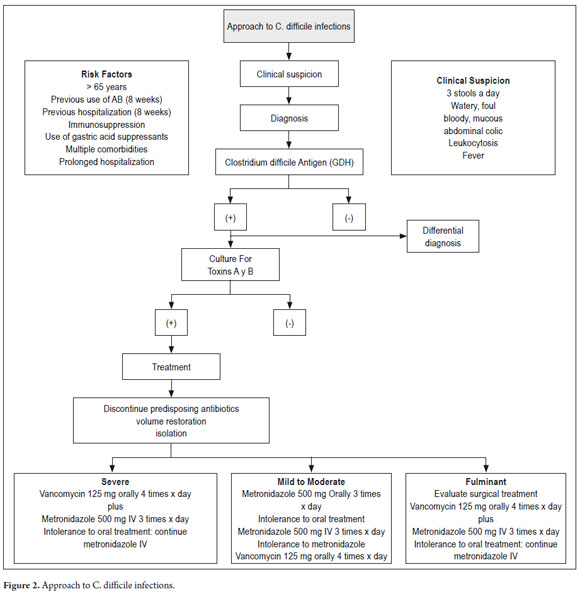
Multiple risk factors have been associated with Clostridium difficile infection. The most important of these are the use of antimicrobials, hospitalization and advanced age (2, 5, 12, 17, 18, 23). Because patients over the age of 65 years often have multiple illnesses, they are more likely to be hospitalized, more likely to receive broad-spectrum antimicrobial agents for the management of infectious processes, and more likely to have long hospital stays (Table 1) (12). A recent prospective study found that for every year of age, the risk for this infection increases 2% (23). This increased susceptibility is related to changes in fecal microbiota, reduced function of the defense system, particularly the humoral immune system, and combinations with multiple comorbidities (19, 24).
As has been mentioned, all antibiotics have the potential to promote C. difficile infections. Nevertheless, certain such as clindamycin, broad-spectrum penicillins, second and third generation cephalosporins, and fluoroquinolones disturb intestinal microbiota more than do other antibiotics. Long treatment duration and increased number of antibiotics are also risk factors for this disease (1). It has also been determined that the disease has a seasonal pattern. It is more common in winter because during the flu season unjustified use of antibiotics increases (2). Another group of drugs implicated as a risk factor by the FDA (U.S. Food and Drug Administration) for C. difficile infections is that composed of proton pump inhibitors (PPIs) when they are used at high doses for prolonged periods (13, 25, 26). Independent risk factors for severe C. difficile disease also include comorbidities such as chronic renal disease, chronic lung disease and diabetes. In general, systemic symptoms are more common in moderate to severe infections (2, 5, 13).
Infection occurs primarily in hospitals where the organism is cultured on bed rails, floors, windows, bathrooms, medical equipment and on the hands of healthcare workers who care for patients infected with C. difficile. It can persist in the wards for 40 days after the infected patients are discharged (18, 23).
CLINICAL MANIFESTATIONS
The association between high mortality rates and patients with advanced ages with this infection is a major concern. Diagnosis is difficult among the elderly because they often have atypical clinical presentations (19, 27). Usually there is no fever except in very severe cases of illness, and the initial manifestation in elderly patients may be confusion, an altered mental state or nonspecific symptoms of infection such as weakness, fatigue, anorexia, weight loss, frequent falls and loss of functional physical capacity (19, 27).
Most patients with confirmed C. difficile infections are asymptomatic. 62% to 86% of hospital patients belong to this group (7), and only about 10% of cases have severe infections (2). The most common symptom is diarrhea. Patients with mild to moderate infections have smelly watery diarrhea without blood. The frequency can be up to 10 times a day, but sometimes patients have paralytic ileus and absence of diarrhea (2, 7, 28). Other patients may have symptoms of colitis with bloody diarrhea, abdominal cramping and fecal leukocytes accompanied by mild fevers (5, 28). Paraclinical tests show hypoalbuminemia and leukocytosis with counts from 15,000 â 20,000/mL or even higher (5, 28).
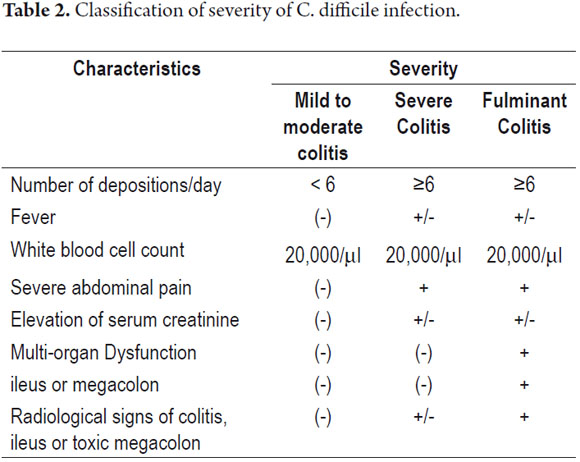
According to the guidelines of the Infectious Diseases Society of America (IDSA), severe infection is defined by a leukocyte count over 20,000/ml or creatinine levels 1.5 times above the patient's baseline value (29). Albumin levels below 2.5 mg/dl, admission to an intensive care unit, endoscopic visual identification of pseudomembrane and thickening of the colon wall are other indicators of severe disease.
Fulminant colitis due to C. difficile is a subtype of the disease that occurs in less than 5% of patients. Symptoms occur within hours of onset of the infection and last several weeks. Cases which progress rapidly have worse results (30, 33). Patients present profuse diarrhea, ileus or toxic megacolon (transverse colon diameter greater than 6 cm), and severe abdominal pain with or without signs of peritoneal irritation. These patients sometimes require surgery. They usually experience marked leukocytosis. The prognosis is poor when the leukocyte count rises above 50,000/ mL or lactate levels go above 5 mmol/L (30). Imaging studies demonstrate free air secondary to perforation in a third of these patients and diffuse colonic inflammation in 100% of these patients. Colonoscopies demonstrate diffuse inflammation and pseudomembrane (30-32). Mortality rates among these patients are close to 50% (30, 31). The most helpful predictors of mortality have been patient age over 70 years, leukocytes greater than 35,000/m L or less than 4,000/mL, bandemia greater than 10% and cardiopulmonary failure (Table 2) (31-33).
Approximately 20% of patients with C. difficile infections have recurrent occurrences. The risk of a second relapse is 40%, and the risk a third is 60% (34, 35). Recurrence may be caused by the original strain of by C. difficile or by a different strain. It has been documented that 85% of these cases are early relapses, those manifested in less than 8 weeks after initial infection. Late relapses, those which occur 8 weeks or more after the initial infection, account for up to 65% of cases (34). Risk factors for recurrence include new schemes antimicrobial exposure, advanced age (> 65 years), prolonged hospital stay, low albumin levels, and a history of previous recurrence (35).
DIAGNOSIS
Only 15% to 25% of cases of antibiotic associated diarrhea are caused by C. difficile infections. Patients should not be treated for C. difficile infections until they test positive for the bacillus unless there is rapid clinical deterioration, or if specific positive diagnostic tests are available (2, 6, 13). Furthermore, testing of the feces of asymptomatic patients and testing after antibiotic treatment has begun are not recommended (21).
C. difficile infections should be suspected in any adult with antibiotic associated diarrhea, i.e. diarrhea occurring within 8 weeks of antimicrobial use or hospitalization, especially if there is fever (12, 19, 28).
Abdominal imaging such as CT scans may show related but nonspecific colitis due to C. difficile. Indications include signs of ileus with dilated colonic segments and intestinal wall edema or inflammation (13, 14).
Colonoscopy is useful for determining the presence of pseudomembranous colitis, although depending on the clinical picture it may only reveal nonspecific colitis without pseudomembrane (11, 28, 29). In general diagnosis of C. difficile infections is based on laboratory tests to detect the organism or its toxin in fecal samples (1, 2, 29).
Several diagnostic tests exist for C. difficile infections, and there are several general principles for these tests. The gold standard for laboratory diagnosis is detection of fecal toxins using cultures of cell lines such as Vero cells (African green monkey kidney cells) (36). Although it is the most sensitive and specific method for diagnosis, the time it requires for processing makes it impractical (1, 36).
When infection is suspected because of clinical factors, testing positive test for fecal toxins A and B (sensitivity (75% 99% and specificity 92% -100), a colonoscopy, or histopathology showing disclose pseudomembranous colitis can confirm the diagnosis (1, 9, 36).
Recent clinical practice guidelines recommend a 2-step approach to diagnosis. The first step is use of an enzyme immunoassay to detection glutamate dehydrogenase (GDH) which also known as the antigen of C. difficile. Even though these tests can cross-react with other bacterial species such as clostridium sporogenes, clostridium botulinum and peptostreptococcus anaerobius (15, 36), its negative predictive value test is 99% (37), so a negative result excludes the diagnosis of clostridium difficile. If the test is positive for GDH, the second step is confirmation of C. difficile by culture and/or detection of the toxin through methods such as a cytotoxicity assay or toxigenic culture. This recommendation is considered provisional until more data is available on the GDH test's sensitivity (29, 36, 37).
TREATMENT
Although treatment of asymptomatic patients, even after diagnostic confirmation by histological methods, detection of toxins or cultures is not useful (1, 12, 38), treatment of illness due to C. difficile is a priority. Treatment includes discontinuation of antibiotics causing condition when possible, rehydration and electrolyte correction. This strategy leads to resolution of symptoms in 25% of cases (21, 36, 38, 39).
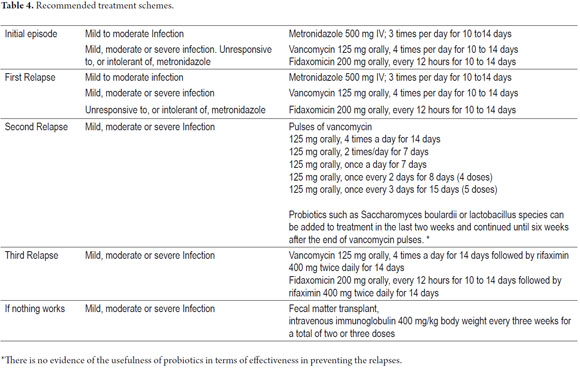
Oral vancomycin was the first antibiotic used to treat this infection: it was approved by the FDA in the 1980's. Subsequently metronidazole (29) was found to be equally effective, so now these two antimicrobials are the keystones of treatment (40). Initial management of mild to moderate forms and first recurrences should be with 500 mg of orally administered metronidazole every 6 to 8 hours for 7-14 days. 125250 mg of orally administered vancomycin every 6hours for 7-14 days is the initial choice for severe episodes and second relapses (36, 38, 39, 41). In a randomized clinical trial, treatment with vancomycin was associated with a higher cure rate than metronidazole for severe cases, but both agents were equally effective for treating mild cases (38). Another study compared the effects of three standard treatment regimens for mild C. difficile infections with respect to the risk of complications, sequelae and death from any cause within 30 days after the date of initiation of the treatment (42). The schemes were 500 mg of metronidazole administered intravenously three times a day, 500 mg of metronidazole administered orally three times a day, , and 250 mg of vancomycin administered orally four times a day. The IV metronidazole group's mortality rate was 38.1% which was much higher than the 7.4% rate of the oral metronidazole group and the 9.5% of the vancomycin group (p <0.001). The results of this study raise the probability that IV metronidazole is less effective than oral metronidazole or oral vancomycin (42).
When there is ileus, a regimen combining intravenous metronidazole and vancomycin in retention enemas is considered justified. This ensures the intraluminal effectiveness of these agents (12, 29, 35), especially since orally administered vancomycin is not absorbed systemically and reaches predictably high levels in the colon. When vancomycin is administered intravenously it has no effect on C. difficile because the antibiotic does not reach significant levels in the colon, and consequently its concentration in the colonic lumen does not reach the minimum inhibitory concentration for Clostridium difficile The recommended dosage of vancomycin is 125 mg four times a day since dosage regimens of 125 mg four times daily are equally effective with dosages of 500 mg four times daily (53, 54).
Metronidazole is considered to be the first-line therapy for patients with mild to moderate cases. Oral vancomycin is reserved for patients who are unresponsive or intolerant to metronidazole, relapse cases, and patients with severe disease (54). The use of vancomycin is reserved as a second-line treatment because of it is less cost efficient and because its indiscriminate use increases bacterial resistance rates (especially of enterococci) (53, 54). Fidaxomicin was approved in 2011 by the FDA, but the moment at which it should be used remains to be determined (1, 6, 43, 44). A systematic review found that fidaxomicin, metronidazole, and vancomycin are equally effective for curing the initial illness due to Clostridium difficile, that none of them is clearly superior to the others, and that the rate of recurrence was similar for all schemes (45).
Emergency surgery is suitable for patients who develop signs of toxic megacolon dilated more than 10 cm or for patients who have perforations. Despite surgery mortality in patients with fulminant colitis remains high (up 48%). Nevertheless subtotal colectomy can be lifesaving (46, 47). As mentioned previously, the risk of relapse is estimated at 20% (41-44). In cases of primary relapses the therapeutic recommendation is to use the initial scheme treatment following staging guidelines for severity (29, 35). Secondary relapses remain challenging. The following pulsed scheme of orally administered vancomycin is recommended: 125 mg 4 times daily for 14 days, 125 mg 2 times daily for 7 days; 125 mg once daily for 7 days, 125 mg once every 2 days for 8 days (4 doses), 125 mg every 3 days for 15 days (5 doses) (29, 49, 53, 55).
Immunotherapy and biotherapy are antibiotic therapies for treatment of recurrent C. difficile infections which are designed to strengthen the immune system and increase resistance to colonization (9, 49). Passive immunotherapy using intravenous immunoglobulin at a dose of 400 mg/kg has shown beneficial effects in uncontrolled studies and is recommended in the guidelines, but there is no significant evidence for its use (9, 29, 35).
A controlled clinical trial evaluated the use of monoclonal antibodies against toxins A and B as adjuvant treatment for C. difficile infections. It showed reduced recurrence rates compared with a control group which received placebos (25% to 7%). The benefit persisted in the subgroup of patients with a history of recurrent infections (50).
Saccharomyces boulardii is the only probiotic therapy that has shown effectiveness for treating this infection. Although it has been postulated that the S. boulardii protease may deactivate toxins of C. difficile at receptor junctions through, the evidence fails to support Saccharomyces boulardii use in routine management of this disease (9, 36, 49, 51, 52). A Cochrane review assessed the effect of probiotics in the treatment of CDI and concluded that the current evidence is insufficient to recommend routine use (4,29).
Fecal microbiota transplants are an alternative for episodes of recurrent infection by Clostridium difficile. The aim of this kind of therapy is to restore or enhance the normal microbiota and restore bacterial homeostasis which is the fundamental alteration that encourages the colonization of C. difficile (9, 52). The protocol for this form of treatment involves the instillation of a suspension of feces from a healthy donor into the colon of a patient with recurrent C. difficile (52). This type of treatment is recommended for patients who relapse and for use as rescue therapy after conventional therapy schemes have failed (54, 56).
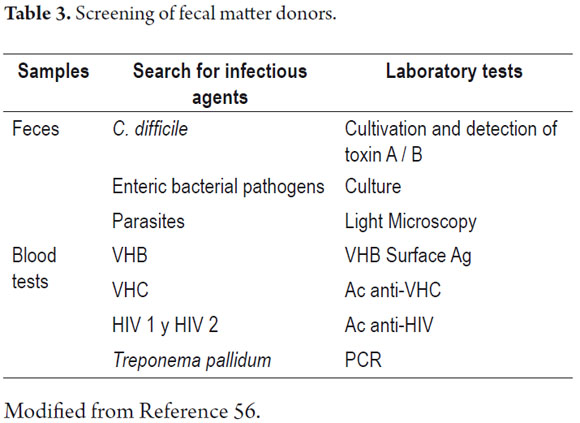
There are different methods used for fecal microbiota transplant. One of them is the application of enemas described in 2010 by M. Silverman et al. (55). The procedure was performed on 7 patients who had had multiple relapses. Prior to application of enemas patients were treated with orally administered Saccharomyces boulardii suspension for 60 days followed by antibiotic treatment for 48 to 72 hours. Enemas were made from fecal material from healthy donors who had been screened for hepatitis A, hepatitis B, hepatitis C, HIV, HTLV, syphilis, and H. pylori (Table 3). 50 ml of fecal matter were taken and then homogenized in 250 cc of saline solution. This solution was applied in an enema lasting no longer than 30 minutes the first thing in the morning. Five of the seven patients relapsed (57). Recently a study was published of a population of 70 patients with recurrent Clostridium difficile infections. Patients were grouped into those with strain 027 and those with non 027 strains. They were followed for twelve weeks. The study found that 34 (100%) of the patients with non 027 strains had total resolution of symptoms. Of the 36 patients with strain 027, 32 (89%) showed a favorable response (56). The requirements of this study's protocol were that donors had no antibiotic treatment in the preceding 6 months and an absence of gastrointestinal symptoms (diarrhea, bloating, and rectal bleeding). Donors in order of priority were relatives, individuals who had close physical contact with patients (spouses or partners), and any other healthy donor (56).
The protocol for fecal microbiota recipients followed these steps:
1. Minimum 4 days pretreatment with vancomycin or metronidazole, suspended at least 36 hours prior to transplant.
2. 20-30 ml of feces had to be obtained from the donor by no more than 6 hours prior to transplant.
3. The feces of donor had to be manually homogenized in 100-200 ml of water. Following preparation of the colon with polyethylene glycol, 100 ml of this suspension was then infused into the cecum through the biopsy channel of the colonoscope (56).
A recent systematic review of the use of fecal matter transplants to treat recurrent infections and pseudomembranous colitis showed resolution of the disease in 92% cases (4, 29). The review covered 27 series of cases which treated 317 patients. Effectiveness of treatment varied according to volume of fecal matter transplanted, donor of feces, route of administration and treatment prior to procedure. Mortality and adverse events were uncommon. Nevertheless, more studies should be performed to confirm and standardize the use of this treatment (52).
Immune therapy using a vaccine and monoclonal antibodies is under investigation and is considered an important alternative for future management of Clostridium difficile infections (39, 50).
In general, subsequent to this review we consider that the following schemes may be used to treat Clostridium difficile infections. Table 4 and Figure 2 present a general approach to diagnosis and therapeutic approach.
CONTROL AND PREVENTION
Nosocomial outbreaks of disease due to C. difficile occur quickly if the index case is not diagnosed and treated promptly. Recommended measures to prevent its spread include:
1. Isolating patient in a single room with private bathroom. If not possible, patients can be isolated in a particular area of the hospital with allocation of a specific health workforce to reduce the risk of cross contamination. Isolation should be continued until 48 hours after resolution intestinal symptoms (29, 27).
2. Anyone and everyone who comes into contact with a patient with C. difficile or potentially contaminated surfaces must wash their hands with soap and water.
3. Use of contact precautions (1, 29, 35).
4. Cleaning surfaces contaminated with feces. Patient rooms should be The disinfected with a sporicidal agent such as sodium hypochlorite in a concentration of at least 1000 ppm (29, 35, 45).
5. Limiting the use of antibiotics if they are not strictly specified. There must be a rational use of broad spectrum antibiotics whenever possible with preferance given to the lower spectrum (21, 27).
CONCLUSIONS
Overall incidence of clostridium difficile infections and incidence of its more serious forms have increased due to the appearance of a hypervirulent strain which has spread rapidly in recent years. This marked trend towards severe refractory disease and increased recurrence has led to greater participation of gastroenterologists in nosocomial cases and highlights the need for improved therapies and prevention. With increases in life expectancy and the consequent overall aging of the population, awareness that infection by this bacterium is the leading cause of diarrhea in the elderly population can prevent catastrophic consequences due to lack of thought about this etiology. All medical staff, but particularly those dedicated to care of elderly patients, should emphasize measures to prevent this condition through rational use of antibiotics and maintenance of a high index of suspicion for C. difficile infections in this age group.
1. Kee VR. Clostridium Difficile Infection in Older Adults: A Review and Update on Its Management. Am J Geriatr Pharmacother 2012; 10: 14-24. [ Links ]
2. McCollum DL, Rodríguez M. Detection, Treatment, and Prevention of Clostridium difficile Infection. Clin Gastroenterol Hepatol 2012; 10: 581-592. [ Links ]
3. McGlone SM, Bailey RR. Zimmer SM, Popovich MJ, Tian Y, Ufberg P. et al. The economic burden of Clostridium difficile. Clin Microbiol Infect 2012; 18: 282-289. [ Links ]
4. Gerding DN, Johnson S. Management of Clostridium difficile infection: thinking inside and outside the box. Clin Infect Dis 2010; 51: 1306-1313. [ Links ]
5. Sánchez AL, Otero W, Caminos JE. Enfermedades asociadas a Clostridium difficile: nuevas amenazas de un viejo enemigo. Rev Col Gastroenterol 2008; 23: 142-159. [ Links ]
6. Balassiano IT, Yates EA, Domingues RM, Ferreira EO. Clostridium difficile: a problem of concern in developed countries and still a mystery in Latin America. J Med Microbiol 2012; 61: 169-179. [ Links ]
7. Curry S. Clostridium difficile. Clin Lab Med 2010; 30: 329-342. [ Links ]
8. Huang H. Weintraub A. Fang H. Nord CE. Antimicrobial resistance in Clostridium difficile. Int J Antimicrob Agents. 2009; 34: 516-522. [ Links ]
9. Rupnik M, Wilcox MH, Gerding DN. Clostridium difficile infection: new developments in epidemiology and pathogenesis. Nat Rev Microbiol 2009; 7: 526-536. [ Links ]
10. Kyne L, Warny M, Qamar A, Kelly C, et al. Asymptomatic carriage of Clostridium difícile and serum levels of IgG antibody against toxin A. N Engl J Med 2000; 342: 390-7. [ Links ]
11. Blondeau JM. What have we learned about antimicrobial use and the risks for Clostridium difficile-associated diarrhoea? J Antimicrob Chemother 2009; 63: 238-242. [ Links ]
12. Simor AE. Diagnosis, management, and prevention of Clostridium difficile infection in long-term care facilities: a review. J Am Geriatr Soc 2010; 58: 1556 -1564. [ Links ]
13. Pant C, Sferra T, Deshpande A, Minocha A. Clinical approach to severe Clostridium difficile infection: Update for the hospital practitioner. Eur j intern med. 2011, 22: 561-568. [ Links ]
14. McFarland LV. Update on the changing epidemiology of Clostridium difficile-associated disease. Nat Clin Pract Gastroenterol Hepatol 2008; 5: 40-48. [ Links ]
15. O'Connor JR, Johnson S, Gerding DN. Clostridium difficile infection caused by the epidemic BI/NAP1/027 strain. Gastroenterology 2009; 136: 1913-1924. [ Links ]
16. Leclair MA, Allard C, Lesur O, Pepin J. Clostridium difficile infection in the intensive care unit. J Intensive Care Med 2010; 25: 23-30. [ Links ]
17. Simor AE, Bradley SF, Strausbaugh LJ, Crossley K, Nicolle L. Clostridium difficile in long-term-care facilities for the elderly. Infect Control Hosp Epidemiol 2002; 23: 696-703. [ Links ]
18. Schroeder MS. Clostridium difficile Associated Diarrhea. Am Fam Physician 2005; 71: 921-28. [ Links ]
19. Crogan NL, Evans BC. Clostridium difficile: an emerging epidemic in nursing homes. Geriatr Nurs 2007; 28: 161-164. [ Links ]
20. Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002; 346: 334-9. [ Links ]
21. Arteaga A, Santa-Olaya P, Sierra MJ, Limia A, Cortés M, Amela C. Riesgo epidémico de la enfermedad asociada a una nueva cepa de Clostridium difficile. Enferm Infecc Microbiol Clin 2009; 27: 278-284. [ Links ]
22. Quesada-Gómez C, Rodríguez C, Gamboa-Coronado M, Rodriguez-Cavallini E, Du T, Mulvey MR, et al. Emergence of Clostridium difficile NAP1 in Latin America. J Clin Microbiol 2010; 48: 669-670. [ Links ]
23. Loo VG. Bourgault AM. Poirier L. Lamothe F. Michaud S. Turgeon N. Host and pathogen factors for Clostridium difficile infection and colonization. N Engl J Med 2011; 365: 1693-1703. [ Links ]
24. Calfee DP. Clostridium difficile: A reemerging pathogen. Geriatrics 2008; 63: 10-14. [ Links ]
25. Kim JW, Lee KL, Jeong JB, Kim BG, Shin S, Kim JS, et al. Proton pump inhibitors as a risk factor for recurrence of Clostridium difficile-associated diarrhea. World J Gastroenterol 2010; 16: 3573-3577. [ Links ]
26. FDA Drug Safety Podcast for Healthcare Professionals: Clostridium difficile-associated diarrhea can be associated with stomach acid drugs known as proton pump inhibitors (PPIs). www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm290838.htm; acceso mayo 20, 2012. [ Links ]
27. Johnson S. Recurrent Clostridium difficile infection: A review of risk factors, treatments, and outcomes. J Infect 2009; 58: 403-410. [ Links ]
28. Bartlett JG, Gerding DN. Clinical recognition and diagnosis of Clostridium difficile infection. Clin Infect Dis 2008; 46(Suppl. 1): S12-S18. [ Links ]
29. Cohen SH, Gerding DN, Johnson S, Kelly C, Loo V, McDonald L, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the society for healthcare epidemiology of America (SHEA) and the infectious diseases society of America (IDSA). Infect Control Hosp Epidemiol 2010; 31: 431- 455. [ Links ]
30. Sayedy L, Kothari D, Richards RJ. Toxic megacolon associated Clostridium difficile colitis. World J Gastrointest Endosc 2010; 2: 293-297. [ Links ]
31. Sailhamer EA, Carson K, Chang Y, Zacharias N, Spaniolas K, Tabara M, et al. Fulminant Clostridium difficile colitis: patterns of care and predictors of mortality. Arch Surg 2009; 144: 433-439. [ Links ]
32. Girotra M, Kumar V, Khan JM, Damisse P, Abraham RR, Aggarwal V, et al. Clinical Predictors of Fulminant Colitis in Patients with Clostridium difficile Infection. Saudi J Gastroenterol 2012; 18: 133-139. [ Links ]
33. Karas JÁ, Enoch DA, Aliyu SH. A review of mortality due to Clostridium difficile infection. J Infect 2010; 61: 1- 8. [ Links ]
34. Kamboj M, Khosa P, Kaltsas A, Babady NE, Son C, Sepkowitz KA. Relapse versus reinfection: surveillance of Clostridium difficile infection. Clin Infect Dis 2011; 53: 1003-1006. [ Links ]
35. Gouliouris T, Brown N, Aliyu SH. Prevention and treatment of Clostridium difficile infection. Clin Med 2011; 11: 75-79. [ Links ]
36. Williams O. Martin, Spencer Robert C. The management of Clostridium difficile infection. Br Med Bull 2009; 91: 87-110. [ Links ]
37. Wren MW, Sivapalan M, Kinson R, Shetty NR. Laboratory diagnosis of Clostridium difficile infection. An evaluation of tests for faecal toxin, glutamate dehydrogenase, lactofer- rin and toxigenic culture in the diagnostic laboratory. Br J Biomed Sci 2009; 66: 1-5. [ Links ]
38. Zar FA, Bakkanagari SR, Moorthi KM, Davis MB. A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity. Clin Infect Dis 2007; 45: 302-7. [ Links ]
39. Gerding DN, Muto CA. Owens RC Jr. Treatment of Clostridium difficile infection Clin Infect Dis 2008; 46(Suppl 1): S32-42. [ Links ]
40. Teasley DG, Gerding DN. Olson MM, Peterson LR, Gebhard RL, Schwartz MJ, et al. Prospective randomised trial of metronidazole versus vancomycin for Clostridium difficile-associated diarrhoea and colitis. Lancet 1983; 2: 1043-1046. [ Links ]
41. Vardakas K, Polyzos K, Patouni K, Rafailidis P, Samonis G. Falagas M, et al. Treatment failure and recurrence of Clostridium difficile infection following treatment with vancomycin or metronidazole: a systematic review of the evidence. International Journal of Antimicrobial Agents. 2012. Article in press. [ Links ]
42. Wenisch J, Schmid D, Kuo H, Allerberger F, Michi V, Tesik P, et al. Prospective Observational Study Comparing Three Different Treatment Regimes in Patients with Clostridium difficile Infection. Antimicrob Agents Chemother 2012; 56: 1974-1978. [ Links ]
43. Cornely OA, Crook D, Esposito R, Poirier A, Somero M, Weiss K, et al. Fidaxomicin versus vancomycin for infection with Clostridium difficile in Europe, Canada, and the USA: a double-blind, non-inferiority, randomised controlled trial. Lancet Infect Dis 2012; 12: 281-89. [ Links ]
44. Lancaster J, Mathews J. Fidaxomicin: The Newest Addition to the Armamentarium against Clostridium difficile Infections. Clin Ther 2012; 34: 1-13. [ Links ]
45. Drekonja DM, Butler M, MacDonald R, Bliss D, Filice GA. Rector TS, et al. Comparative Effectiveness of Clostridium difficile Treatments. Ann Intern Med 2011; 155: 839-847. [ Links ]
46. Longo WE. Mazuski JE. Virgo KS. Lee P. Bahadursingh AN. Johnson FE. Outcome after colectomy for Clostridium difficile colitis. Dis Colon Rectum 2004; 47: 1620-1626. [ Links ]
47. Lamontagne F, Labbé AC, Haeck O, Lesur O, Lalancette M. Patino C. Impact of emergency colectomy on survival of patients with fulminant Clostridium difficile colitis during an epidemic caused by a hypervirulent strain. Ann Surg 2007; 245: 267-72. [ Links ]
48. Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med 2010; 170: 784-90. [ Links ]
49. Pillai A, Nelson R. Probiotics for treatment of Clostridium difficile-associated colitis in adults. Cochrane Database Syst Rev 2008; 1: CD004611. [ Links ]
50. Lowy I, Molrine DC, Leav BA, Blair BM. Baxter R. Gerding DN. Treatment with monoclonal antibodies against Clostridium difficile toxins. N Engl J Med 2010; 362: 197-205. [ Links ]
51. Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R. Probiotics for the Prevention and Treatment of Antibiotic-Associated Diarrhea A Systematic Review and Meta-analysis. JAMA 2012; 307: 1959-1969. [ Links ]
52. Gough E, Shaikh H, Manges R. Systematic Review of Intestinal Microbiota Transplantation (Fecal Bacteriotherapy) for Recurrent Clostridium difficile Infection. Clin Infect Dis 2011; 53: 994-1002. [ Links ]
53. Martinez F, Leffler D, Kelly C. Clostridium difficile outbreaks: prevention and treatment strategies. Risk Manag and Healthcare Policy 2012; 5: 5564. [ Links ]
54. Anilrudh A. Venugopal, Stuart Johnson. Current State of Clostridium difficile Treatment Options. Clin Infec Disease 2012; 55: 71-76. [ Links ]
55. Kelly C, Lamont J. Clostridium difficile more difficult than ever. N Engl J Med 2008; 359:1932-1940. [ Links ]
56. Mattilla E. Usittalo-Sepalla, Wuorela M, et al. Fecal Transplantation, Through Colonoscopy, Is Effective Therapy for Recurrent Clostridium difficile Infection. Gastroenterology 2012; 142: 490-496. [ Links ]
57. Silverman M, Davis I, Pillai D. Success of Self-Administered Home Fecal Transplantation for Chronic Clostridium difficile Infection. Clin Gastroenterol Hepatol 2010; 8: 471-473. [ Links ]











 texto em
texto em