Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.2 Bogotá Apr./June 2013
Randomized study comparing standard first line 10 day therapy against Helicobacter pylori including clarithromycin versus standard first line therapy with levofloxacin
Rodrigo Castaño Llano, MD (1), Mario H. Ruiz Vélez, MD (2), Germán Campuzano-Maya, MD (3), Eugenio Sanín Fonnegra, MD (4), Juan D. Puerta Díaz, MD (5), Víctor Calvo-Betancur (6), Luis Miguel Ruiz Velásquez (7), Alejandra Álvarez Diazgranados (7)
(1) Gastro-hepatology Group at the Hospital Pablo Tobon Uribe of the University of Antioquia, in Medellin, Colombia.
(2) General Surgeon and Endoscopist at the Hospital Pablo Tobon Uribe in Medellin, Colombia.
(3) Hematologist and Clinical Pathologist, Medical Director of the Clinical Laboratory and Professor at the University of Antioquia in Medellin, Colombia.
(4) Gastroenterologist in Medellin, Colombia.
(5) General Surgeon, Coloproctologist, Professor of General Surgery in the Clínica las Américas at the Universidad Pontificia Bolivariana in Medellin, Colombia.
(6) Statistician at the Institute of Advanced Medical Technology of Antioquia in Medellin, Colombia.
(7) Student at the Universidad Pontificia Bolivariana in Medellin, Colombia.
Received: 22-01-13 Accepted: 16-04-13
Abstract
Background: Evidence is mounting that the standard triple therapy against H. pylori infections has been losing clinical effectiveness, but triple therapy with levofloxacin, amoxicillin, and a proton pump inhibitor is effective and well tolerated. This scheme has been suggested as an alternative for standard first-line therapy. The purpose of this randomized, multicenter, controlled study is to compare rates of successful eradication of standard triple therapy using clarithromycin, amoxicillin and omeprazole (CAO) and triple therapy using levofloxacin, amoxicillin and omeprazole (LAO). Materials and Methods: A total of 317 patients who had been diagnosed with H. pylori infections through biopsies were randomized into two groups. One group of 160 patients was assigned a 10 day CAO treatment regimen and the other group of 157 patients was assigned a 10 day LAO treatment regimen. Eradication was assessed by optimized breath test. Adverse effects and toleration were also assessed. Results: Intention to treat analysis gave the following results: CAO, 68.8% (110/160) and LAO, 84.1% (132/157) with p = 0.0021. The CAO eradication rates was 71.9% (105/146) and the LAO eradication rate was 89.3% (125/140) with p = 0.0004. There were significant statistical differences in effectiveness between the two treatment groups, but there were no significant differences in toleration and adverse effects between the two groups. Conclusions: Treatment of H. pylori infections with triple therapy based on levofloxacin was a better alternative than clarithromycin-based triple therapy in this study.
Key words
Helicobacter pylori, therapy, claritromycin, levofloxacin.
INTRODUCTION
Helicobacter pylori (H. pylori) infection is the major cause of chronic gastritis, peptic ulcers, gastric adenocarcinoma, and mucosa-associated lymphoid tissue (MALT). It is therefore imperative to move towards effective bacterial eradication (1, 2).
Current guidelines for the eradication of H. pylori in Europe and North America recommend therapeutic regimens that achieve over 80% eradication rate by intention-to-treat analysis, preferably by using a proton pump inhibitor plus two antibiotics (clarithromycin plus amoxicillin) (3, 4).
Clarithromycin was once recognized as the most powerful antibiotic with bacteriostatic effects against H. pylori (5). Unfortunately, primary resistance to clarithromycin is increasing worldwide, and is considered to be the primary factor reducing efficacy against H. pylori (6, 7). Even with the most effective therapeutic regimens that include a proton pump inhibitor (PPI), and two antibiotics, up to 20-30% of treatments fail and H. pylori infections persist (8). H. pylori eradication prevents recurrence of duodenal ulcers, and reduces the risk of gastric cancer (9). Recently, we have shown that in our region 7 to 10 day treatment with clarithromycin did not achieve optimal levels of bacterial eradication. After almost 30 years implementing various treatments of H. pylori, the ideal treatment regimen has yet to be defined (7).
Currently, clarithromycin-based therapies seem to offer insufficient eradication rate in some regions, thus other alternatives have been evaluated. Levofloxacin presents outstanding in vitro activity against H. pylori and has emerged as a promising alternative. However, levofloxacin has been evaluated more as a second line therapy when one or more therapies against H. pylori have failed. Eradication rates achieved with this treatment have been between 75% and 86%. Other studies have evaluated small numbers of patients treated with a combination a PPI, amoxicillin, and levofloxacin as a first-line therapy (10-12). Some but not all of these studies have shown excellent results with eradication rates between 85% and 92% (12-17). Although levofloxacin offers an excellent safety profile, resistance of H. pylori to this quinolone has been reported recently, and this could jeopardize its use in the future (18-20).
The primary objective of this study is to evaluate and compare the clinical effectiveness of standard triple therapy using clarithromycin, amoxicillin, and omeprazole with an alternative regimen that replaces clarithromycin with levofloxacin as a first-line therapy. Secondary objectives are evaluation of the effectiveness of eradication by endoscopic diagnosis and histology by estimating the load of H. pylori, and to perform controlled clinical trials in patients with ulcer and non-ulcer dyspepsia one year after treatment.
METHODS
This study is a randomized open clinical trial conducted on outpatients of an urban population between January 2008 and July 2011. Study characteristics were presented in a recent publication (7).
Study population
Patients were over 18 years old and 70 years old or younger who had H. pylori infections histologically confirmed by endoscopy and detected by hematoxylin and eosin staining of samples. We excluded patients with clinical records of gastrectomy, peptic ulcer complications such as stenosis or bleeding, pregnant or nursing women, patients with endoscopic findings of MALT lymphoma or gastric cancer, and patients who had received any prior treatment for H. pylori.
To prevent interference with the treatment against H. pylori, we excluded patients with a history of use within the previous month of antibiotics, bismuth compounds, H2-receptor antagonists, probiotics or PPIs. In addition, we excluded patients with major psychiatric disorders, allergies to penicillin and concurrent histories of malignant disease, liver or kidney failure.
Clinical study design
The study was conducted on patients from various hospitals and private practices in the city of Medellin whose biopsies were positive for H. pylori. We assessed two standard 10 day therapies to eradicate H. pylori: clarithromycin, amoxicillin and omeprazole (CAO), and levofloxacin (Truxa ®), amoxicillin, and omeprazole (LAO). For sample selection we assumed a difference of population proportions was used. We assumed a 0.05 type I error, a 0.2 type II error, a 0.9 ratio of eradication effectiveness for the group treated with levofloxacin, a 0.78 ratio of eradication effectiveness for the group treated with clarithromycin, 0.12 as an absolute difference between proportions, and 1 as the allocation rate between groups. Using these parameters "Sample Size" version 1.1 calculated a sample size of 140 patients per study group.
Demographic characteristics of the patients were evaluated. Endoscopy findings of non-erosive gastritis, erosive gastritis, gastric ulcers, and duodenal ulcers were considered. Infection densities of H. pylori based on histological examination with at least 2 antral biopsies were categorized into two groups: low (1 or 2 cross matches) and heavy (3 or 4 cross matchs). Only hematoxylin and eosin staining of samples was used.
We performed a follow-up examination one to three weeks after treatment completion. It evaluated whether patients had adverse effects related to the treatment or tolerated treatment, and it evaluated whether treatment was completed. Completion was defined as patient having finished more than 70% of the prescription.
Study groups
Patients received triple therapy. They were randomly assigned into two groups. Group 1-CAO consisted of those patients who received 500 mg clarithromycin, 1 g amoxicillin, and 20 mg omeprazole for 30 days. Group 2-LAO consisted of those patients who received 500 mg Truxa ® levofloxacin, 1 g amoxicillin, and 20 mg omeprazole for 30 days. All medications in both groups were administered orally. At the time the study began the only levofloxacin available in the market was Truxa® which was used throughout the study. A randomization sequence was generated by EPIDAT® 3.1 statistical package. On the basis of that sequence treatment was assigned to each patient. Patients were asked not to smoke during treatment.
Eradication evaluation
Patients underwent an optimized breath test at the Clinical Hematology Laboratory in Medellin, Colombia from 4 to 6 weeks after completion of treatment. Optimized carbon-labeled urea (13C-urea) breath tests have 100% sensitivity, specificity, positive predictive value and negative predictive value (21).
As the primary outcome measure, eradication of H. pylori infection was defined as a negative result for a 13C-labelled urea breath test. As a secondary outcome measure, we evaluated completion of treatment defined as 70% or greater completion of prescribed treatment. This measure was evaluated at follow up appointments after the end of antibiotic therapy. Protocol and intention-to-treat analyses were also taken into account as secondary outcome measures.
Breath tests were conducted by a blinded researcher who did not know which treatment patients were receiving.
Evaluation after one year of treatment
Clinical conditions of patients were assessed at the time of prescription and one year after H. pylori eradication treatment. The assessment was based on the Rome III criteria for functional gastrointestinal disorders in adults. Clinical conditions were recorded after a year of therapy and determined to be better, the same or worse. In addition, the one year evaluation placed special emphasis on the initial endoscopic diagnosis of the patient, and the relationship of eradication of H. pylori with the patient's clinical condition at the end of the assessment year.
Statistical analysis
For the descriptive analysis, absolute and relative distributions as well as summary measures (arithmetic means and standard deviations) were used. The demographic characteristics of both groups were compared using confidence intervals for the differences in proportions of independent samples. The eradication rate of H. pylori was analyzed by intention-to- treat (ITT), and per-protocol population (PPP) analyses. All patients in the study were evaluated by ITT. The PPP was only considered for patients with full treatment. The differences in eradication rates of H. pylori, and 95% confidence intervals were calculated for IIT and PPP. The eradication of H. pylori in the CAO therapy (group 1) was expected to be lower than that in the LAO therapy (group 2). A 12% non-inferiority margin was determined on the basis of previously described controlled trials (17, 20). The incidence of adverse in the two groups was compared using the difference in proportions for independent samples under the assumption of normal statistical distributions of these differences. After one year of treatment, clinical responses were assessed by comparisons of chi-square values (P) of the two treatment groups. Values of P <0.05 were considered to be statistically significant.
Ethical issues
This research was classified according to the International Declaration of Helsinki, the Belmont Report, and Colombian Resolution 8430 of 1993 as an investigation without biological, physiological, psychological or social risks. Resolutions from 1995 to 1999 which establish standards for handling of medical records were also taken into account.
RESULTS
Patients
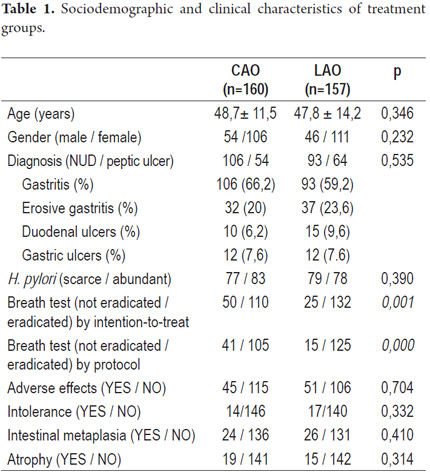
Between January 2008 and July 2011 a total of 317 patients were treated for eradication of H. pylori. The patients were diagnosed with H. pylori by endoscopic biopsies taken in the month prior to commencement of therapy. 160 out of the 317 patients were randomized to CAO treatment group (group 1), and 157 patients were randomized to LAO therapy (group 2). Endoscopic findings of gastritis, erosive gastritis, gastric ulcers and duodenal ulcers were similar for the two treatment groups. No significant demographic differences were found between the two groups (Table 1).
Eradication rates
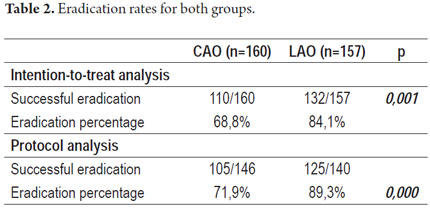
The eradication rate of patients treated with CAO was lower than that for patients treated with LAO in both IIT and PPP analyses: Nevertheless, only LAO therapy achieved optimal figures for bacterial eradication (Table 2).
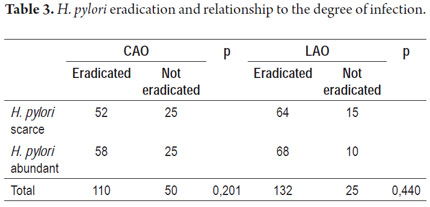
By determining whether eradication rates were related to the degree of infection by H. pylori according to biopsy reports, we found that the eradication rate was similar for both therapies regardless of bacterial load (Table 3).
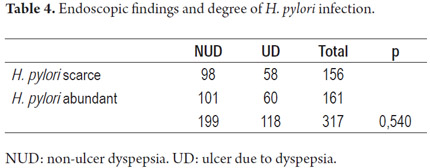
No significant differences in degrees of H. pylori infections were observed between NUD endoscopic findings and UD endoscopic findings (Table 4).
Adverse reactions
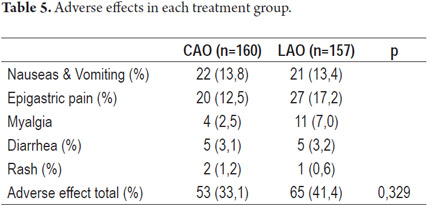
Medical treatment of 14 of the 160 patients treated with the CAO scheme was discontinued because of intolerance. Similarly, medical treatment of 14 of the 157 patients treated with the LAO scheme was discontinued because of intolerance. For PPP analysis, we included 146 patients from the CAO group, and 143 patients from the LAO group. Adverse effects were observed in 33.1% of patients treated with CAO, and 41.4% of those treated with LAO with no significant difference observed (p=0.392).
The most common symptoms for both groups were epigastric pain, nausea and vomiting (with metallic taste in patients' mouths), myalgia, diarrhea and skin rash. There were no significant differences between the two groups (Table 5).
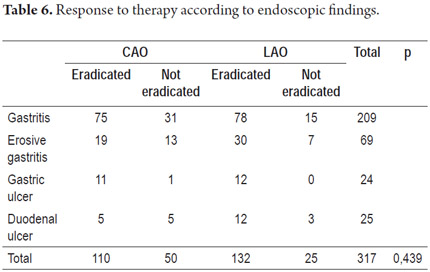
Response to eradication therapy according to initial diagnosis
There was a similar rate of eradication for both therapy regimens and for different endoscopic findings (Table 6).
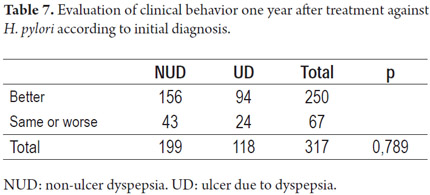
One-year follow-up
Patients were contacted in person or by telephone to review their gastrointestinal manifestations a year after receiving therapy. Gastrointestinal manifestations were classified according to the Rome III diagnostic criteria. We recorded whether symptoms had improved, worsened, or were the same one year after treatment. We emphasized whether initial diagnosis was functional dyspepsia or if endoscopy had indicated peptic acid disease with a structural component such as erosions or ulcers. No significant differences were found between clinical manifestations related to the two treatment regimens at the one-year follow-up analyses.
Nevertheless, our assessment of clinical responses in relation to the initial diagnoses found 78.4% of the 100 patients with non-ulcer dyspepsia improved while symptoms of 79.6 % of the 118 patients with ulcers due to dyspepsia (duodenal ulcers, erosive gastritis, or gastric ulcers) improved (Table 7).
DISCUSSION
This randomized, controlled, multicenter study included a total of 317 patients. It shows that in a country that has shown a high prevalence of clarithromycin resistance (greater than 15%) the rate of eradication with levofloxacin triple therapy is superior to standard triple therapy with clarithromycin with statistically significant results.
When planning treatment of an H. pylori infection, a physician should ideally be aware of the local and regional prevalence of antimicrobial resistance as well as of the efficacy of treatment in the physician's daily practice. Unfortunately, there are no studies of the sensitivity of H. pylori in Medellin however a local study has suggested that therapy with clarithromycin for 7 or 10 days is not useful (7). This could be explained by the increasing resistance of H. pylori to clarithromycin, as has been suggested by sensitivity studies elsewhere in the country (22).
While meta-analyses support the use of Levofloxacin, (10) it has been most frequently used as a second-line therapy (23-25) or even as a third-line therapy (26) when standard therapies with clarithromycin and metronidazole have failed (16, 27-29). Success has even been described with short 5 day treatment regimens. 30 However, Levofloxacin's role in first-line therapy has not been evaluated in our environment, although there are reports in the literature suggesting that it could be effective here (20, 31, 32), albeit with limitations (33) 3% primary resistance to levofloxacin has been reported elsewhere (34).
Recently, a Taiwanese study compared use of CAO and LAO regimens for first-line and second-line therapy. It showed superior effectiveness with levofloxacin alone as a second-line therapy (74% vs. 84%), although that study limited dosage to 750 mg/d and limited treatment time to 7 days (35). These rates are below the greater than 90% eradication rates for regimens with levofloxacin as first-line therapy used in countries like Italy and Holland (14-17, 36). Increased H. pylori resistance to quinolones has been shown in Belgium (16.8%), Italy (23.1%), and from 3% in 1999 to 15% in 2004 in France, Germany, and Spain (18, 19, 37-41). The October 2011 Maastricht IV -Florence consensus on treatment of H. pylori infections recommended standard triple therapy as first-line treatment in regions where the resistance of H. pylori to clarithromycin does not exceed 15% (42). In a recently published review of the role of quinolones in first-line therapy against H. pylori (43), the authors concluded that not all quinolones can be recommended as first-line therapy for eradicating H. pylori, although they can be considered as first-line therapy in specific situations primarily depending on local primary resistance to quinolones and macrolides. Studies also suggest trial implementation to assess the role of quinolones as first-line therapy.
Considering the ineffectiveness of standard therapies for eradicating H. pylori in our area, we evaluated treatment with levofloxacin and compared it to treatment with clarithromycin. We found an 89.3% eradication rate with LAO therapy using per protocol population analysis, and an 84.1% eradication rate using intention-to-treat analysis. This compares with 71.9% and 68.8% for CAO. The eradication rate for standard triple therapy with levofloxacin is higher than the recommended 80% rule. Since up to 60% of recent reports assessing triple therapy have failed to reach 80% H. pylori eradication using ITT, the eradication rate found in this study is better than expected (44).
No differences in adverse effects were found between the two lines of therapy. In addition the same number of patients (14 patients)discontinued treatment in each group. The most common adverse effects were nausea, vomiting, and epigastric pain in both treatment groups. A recent systematic review reported an 18% incidence of adverse events and a 3% incidence of serious adverse events which is consistent with our results (10). Finally, a recent meta-analysis showed a lower incidence of adverse effects with levofloxacin-based therapy combinations than with quadruple regimens (45).
Efforts should be directed towards finding first line treatments that ensure eradication rates over 90%. According to the recommendations of experts, quadruple regimens (primarily sequential and concomitant therapy) appear to be valuable alternative first-line therapies for obtaining higher eradication rates as well as for overcoming increasingly clarithromycin-resistant H. pylori (43, 44).
Our results of 84.1% of eradication rate by intention-to-treat analysis and 89.3% by protocol analysis for first-line H. pylori eradication therapy using the combination of levofloxacin, amoxicillin and PPIs for 10 days are encouraging. Other authors have reported favorable experiences with levofloxacin, but these studies have been evaluated it for second-line therapy, after one or more failed treatments (46). Other studies have evaluated the LAO regimen as first-line therapy with small numbers of patients (less than 50 patients), but with high eradication rates of 87% (16), 90% (13, 14), and 92% (15). First-line therapy with the LAO scheme has been recommended for H. pylori eradication in areas where the primary resistance to levofloxacin is less than 10% (15). Although this resistance has not been measured in our environment, clarithromycin resistance is higher than 50% (22).
Although studies show that clarithromycin-based regimens achieve a higher rate of eradication in patients with peptic ulcers than in patients with functional dyspepsia (47, 48), the cure rate in our study was similar for both entities.
Triple therapy with the LAO regimen for 10 days represents a better alternative than does standard first-line therapy based on clarithromycin. The LAO regimen meets the proposed targets for H. pylori eradication of a greater than 80% eradication rate, simplicity (twice a day administration with excellent adhesion), and safety (few and mild side effects).
REFERENCES
1. Gasparetto M, Pescarin M, Guariso G. Helicobacter pylori Eradication Therapy: Current Availabilities. ISRN gastroenterology 2012; 2012: 186734. [ Links ]
2. Varbanova M, Schulz C, Malfertheiner P. Helicobacter pylori and other gastric bacteria. Dig Dis 2011; 29: 562-9. [ Links ]
3. Malfertheiner P, Megraud F, O'Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007; 56: 772-81. [ Links ]
4. Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. The American journal of gastroenterology 2007; 102: 1808-25. [ Links ]
5. Megraud F. H. pylori antibiotic resistance: prevalence, importance, and advances in testing. Gut 2004; 53: 1374-84. [ Links ]
6. Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology 2007; 133: 985-1001. [ Links ]
7. Castaño R, Ruiz MH, Martínez C, et al. Evaluación para comparar dos esquemas de terapia estándar (7 frente a 10 días) contra el H. pylori, con seguimiento clínico a un año. Rev Col Gastroenterol 2012; 27: 80-7. [ Links ]
8. Gisbert JP, Pajares JM. Review article: Helicobacter pylori "rescue" regimen when proton pump inhibitor-based triple therapies fail. Alimentary pharmacology & therapeutics 2002; 16: 1047-57. [ Links ]
9. McColl KE. Clinical practice. Helicobacter pylori infection. The New England journal of medicine 2010; 362: 1597-604. [ Links ]
10. Gisbert JP, Morena F. Systematic review and meta-analysis: levofloxacin-based rescue regimens after Helicobacter pylori treatment failure. Alimentary pharmacology & therapeutics 2006; 23: 35-44. [ Links ]
11. Gisbert JP, Perez-Aisa A, Castro-Fernandez M, et al. Helicobacter pylori first-line treatment and rescue option containing levofloxacin in patients allergic to penicillin. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 2010; 42: 287-90. [ Links ]
12. Gisbert JP, Bermejo MF, Infante JM, et al. Levofloxacin, Amoxicillin, and Omeprazole as first-line triple therapy for Helicobacter pylori eradication. Journal of clinical gastroenterology 2009; 43: 384-5. [ Links ]
13. Cammarota G, Cianci R, Cannizzaro O, et al. Efficacy of two one-week rabeprazole/levofloxacin-based triple therapies for Helicobacter pylori infection. Alimentary pharmacology & therapeutics 2000; 14: 1339-43. [ Links ]
14. Di Caro S, Zocco MA, Cremonini F, et al. Levofloxacin based regimens for the eradication of Helicobacter pylori. European journal of gastroenterology & hepatology 2002; 14: 1309-12. [ Links ]
15. Marzio L, Coraggio D, Capodicasa S, Grossi L, Cappello G. Role of the preliminary susceptibility testing for initial and after failed therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and esomeprazole. Helicobacter 2006; 11: 237-42. [ Links ]
16. Antos D, Schneider-Brachert W, Bastlein E, et al. 7-day triple therapy of Helicobacter pylori infection with levofloxacin, amoxicillin, and high-dose esomeprazole in patients with known antimicrobial sensitivity. Helicobacter 2006; 11: 39-45. [ Links ]
17. Rispo A, Di Girolamo E, Cozzolino A, Bozzi R, Morante A, Pasquale L. Levofloxacin in first-line treatment of Helicobacter pylori infection. Helicobacter 2007; 12: 364-5. [ Links ]
18. O'Connor A, Gisbert J, O'Morain C. Treatment of Helicobacter pylori infection. Helicobacter 2009; 14 Suppl. 1: 46-51. [ Links ]
19. Cuadrado-Lavin A, Salcines-Caviedes JR, Carrascosa MF, et al. Antimicrobial susceptibility of Helicobacter pylori to six antibiotics currently used in Spain. The Journal of antimicrobial chemotherapy 2012; 67: 170-3. [ Links ]
20. Cuadrado-Lavin A, Salcines-Caviedes JR, Carrascosa MF, et al. Levofloxacin versus clarithromycin in a 10 day triple therapy regimen for first-line Helicobacter pylori eradication: a single-blind randomized clinical trial. The Journal of antimicrobial chemotherapy 2012; 67: 2254-9. [ Links ]
21. Campuzano-Maya G. An optimized 13C-urea breath test for the diagnosis of H. pylori infection. World J Gastroenterol 2007; 13: 5454-64. [ Links ]
22. Yepes CA, Rodríguez A, Ruiz A, Ariza B. Resistencia antibiótica del Helicobacter pylori en el Hospital Universitario San Ignacio de Bogotá. Acta Med Col 2008; 33. [ Links ]
23. Yahav J, Shmuely H, Niv Y, Bechor J, Samra Z. In vitro activity of levofloxacin against Helicobacter pylori isolates from patients after treatment failure. Diagnostic microbiology and infectious disease 2006; 55: 81-3. [ Links ]
24. Gisbert JP, Perez-Aisa A, Bermejo F, et al. Second-line Therapy with Levofloxacin after failure of treatment to eradicate Helicobacter pylori infection: Time Trends in a Spanish Multicenter Study of 1000 Patients. Journal of clinical gastroenterology 2012. [ Links ]
25. Gatta L, Zullo A, Perna F, et al. A 10-day levofloxacin-based triple therapy in patients who have failed two eradication courses. Alimentary pharmacology & therapeutics 2005; 22: 45-9. [ Links ]
26. Tursi A, Picchio M, Elisei W. Efficacy and tolerability of a third-line, levofloxacin-based, 10-day sequential therapy in curing resistant Helicobacter pylori infection. Journal of gastrointestinal and liver diseases. JGLD 2012; 21: 133-8. [ Links ]
27. Watanabe Y, Aoyama N, Shirasaka D, et al. Levofloxacin based triple therapy as a second-line treatment after failure of helicobacter pylori eradication with standard triple therapy. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 2003; 35: 711-5. [ Links ]
28. Bilardi C, Dulbecco P, Zentilin P, et al. A 10-day levofloxacin-based therapy in patients with resistant Helicobacter pylori infection: a controlled trial. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association 2004; 2: 997-1002. [ Links ]
29. Matsumoto Y, Miki I, Aoyama N, et al. Levofloxacin- versus metronidazole-based rescue therapy for H. pylori infection in Japan. Digestive and liver disease: official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 2005; 37: 821-5. [ Links ]
30. Federico A, Nardone G, Gravina AG, et al. Efficacy of 5-day levofloxacin-containing concomitant therapy in eradication of Helicobacter pylori infection. Gastroenterology 2012; 143: 55-61 e1; quize e13-4. [ Links ]
31. Polat Z, Kadayifci A, Kantarcioglu M, Ozcan A, Emer O, Uygun A. Comparison of levofloxacin-containing sequential and standard triple therapies for the eradication of Helicobacter pylori. European journal of internal medicine 2012; 23: 165-8. [ Links ]
32. Romano M, Cuomo A, Gravina AG, et al. Empirical levofloxacin-containing versus clarithromycin-containing sequential therapy for Helicobacter pylori eradication: a randomised trial. Gut 2010; 59: 1465-70. [ Links ]
33. Molina-Infante J, Perez-Gallardo B, Fernandez-Bermejo M, et al. Clinical trial: clarithromycin vs. levofloxacin in first-line triple and sequential regimens for Helicobacter pylori eradication. Alimentary pharmacology & therapeutics 2010; 31: 1077-84. [ Links ]
34. Romano M, Iovene MR, Russo MI, et al. Failure of first-line eradication treatment significantly increases prevalence of antimicrobial-resistant Helicobacter pylori clinical isolates. Journal of clinical pathology 2008; 61: 1112-5. [ Links ]
35. Liou JM, Lin JT, Chang CY, et al. Levofloxacin-based and clarithromycin-based triple therapies as first-line and second-line treatments for Helicobacter pylori infection: a randomised comparative trial with crossover design. Gut 2010; 59: 572-8. [ Links ]
36. Schrauwen RW, Janssen MJ, de Boer WA. Seven-day PPI-triple therapy with levofloxacin is very effective for Helicobacter pylori eradication. The Netherlands journal of medicine 2009; 67: 96-101. [ Links ]
37. Bogaerts P, Berhin C, Nizet H, Glupczynski Y. Prevalence and mechanisms of resistance to fluoroquinolones in Helicobacter pylori strains from patients living in Belgium. Helicobacter 2006; 11: 441-5. [ Links ]
38. Perna F, Zullo A, Ricci C, Hassan C, Morini S, Vaira D. Levofloxacin-based triple therapy for Helicobacter pylori re-treatment: role of bacterial resistance. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver 2007; 39: 1001-5. [ Links ]
39. Miyachi H, Miki I, Aoyama N, et al. Primary levofloxacin resistance and gyrA/B mutations among Helicobacter pylori in Japan. Helicobacter 2006; 11: 243-9. [ Links ]
40. Cattoir V, Nectoux J, Lascols C, et al. Update on fluoroquinolone resistance in Helicobacter pylori: new mutations leading to resistance and first description of a gyrA polymorphism associated with hypersusceptibility. International journal of antimicrobial agents 2007; 29: 389-96. [ Links ]
41. Glocker E, Stueger HP, Kist M. Quinolone resistance in Helicobacter pylori isolates in Germany. Antimicrobial agents and chemotherapy 2007; 51: 346-9. [ Links ]
42. Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012; 61: 646-64. [ Links ]
43. Berning M, Krasz S, Miehlke S. Should quinolones come first in Helicobacter pylori therapy? Therapeutic advances in gastroenterology 2011; 4: 103-14. [ Links ]
44. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59: 1143-53. [ Links ]
45. Saad RJ, Schoenfeld P, Kim HM, Chey WD. Levofloxacin-based triple therapy versus bismuth-based quadruple therapy for persistent Helicobacter pylori infection: a meta-analysis. The American journal of gastroenterology 2006; 101: 488-96. [ Links ]
46. Gisbert JP, Gisbert JL, Marcos S, Moreno-Otero R, Pajares JM. Third-line rescue therapy with levofloxacin is more effective than rifabutin rescue regimen after two Helicobacter pylori treatment failures. Alimentary pharmacology & therapeutics 2006; 24: 1469-74. [ Links ]
47. Calvet X, Ducons J, Bujanda L, Bory F, Montserrat A, Gisbert JP. Seven versus ten days of rabeprazole triple therapy for Helicobacter pylori eradication: a multicenter randomized trial. The American journal of gastroenterology 2005; 100: 1696-701. [ Links ]
48. Broutet N, Tchamgoue S, Pereira E, Lamouliatte H, Salamon R, Megraud F. Risk factors for failure of Helicobacter pylori therapy--results of an individual data analysis of 2751 patients. Alimentary pharmacology & therapeutics 2003; 17: 99-109. [ Links ]











 text in
text in