Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.2 Bogotá Apr./June 2013
Intestinal Lymphangiectasia associated with Hemihypertrophy: A case report
Dalia Tatiana Mora Arbeláez (1), Mónica Contreras Ramírez, MD (2), Javier Rendón Henao, MD (3)
(1) Resident in Pediatrics at CES University in Medellin, Colombia.
(2) Pediatric Gastroenterologist in the Department of Gastroenterology at the Hospital Pablo Tobón Uribe in Medellín, Colombia.
(3) Medical Pathologist in the Pathology Department at the Hospital Pablo Tobón Uribe in Medellín, Colombia.
Received: 21-01-13 Accepted: 16-04-13
Abstract
Primary intestinal lymphangiectasia is a disease characterized by dilated intestinal lymph vessels which manifests as a protein losing enteropathy. We present a patient with chronic diarrhea, recurrent rectal prolapse and left upper limb hemihypertrophy associated with lymphopenia and hypoalbuminemia. Primary intestinal lymphangiectasia was diagnosed with upper endoscopy and biopsy. Nutritional treatment was successfully begun.
Keywords
Intestinal lymphangiectasia, protein losing enteropathy, nutritional therapy.
INTRODUCTION
Primary intestinal lymphangiectasia is a rare disease characterized by dilation of the lymph nodes of the mucosa, submucosa, serosa and mesentery bowel. This leads to secondary lymphatic leakage which is responsible for the clinical condition. A wide spectrum of clinical and paraclinical manifestations can be present in patients with this diseases. The exact manifestations which present themselves are conditioned by the anatomical location and extent of the lymphatic anomaly (1). Here we report the case of a child with intestinal lymphangiectasia associated with hemihypertrophy of his upper left limb.
CASE REPORT
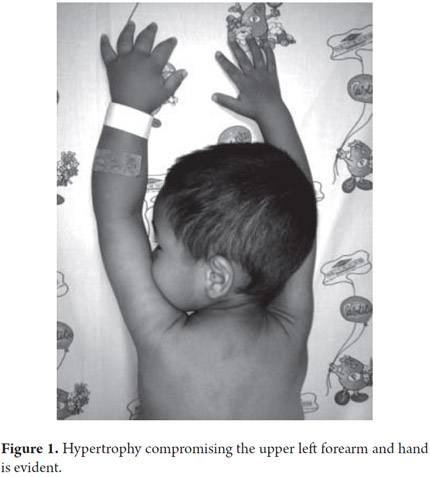
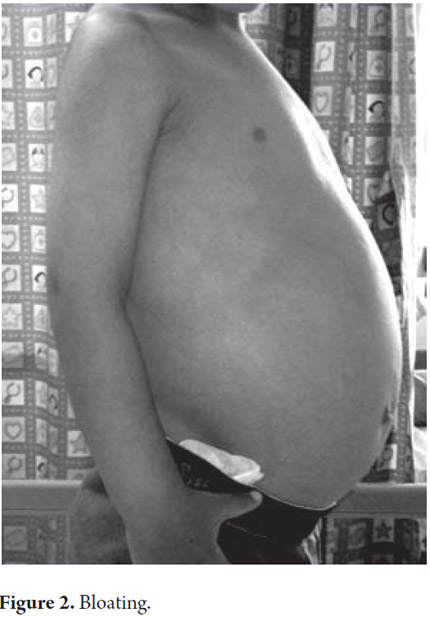
A 33 month old male patient was admitted after 9 months of recurrent rectal prolapse associated with liquid stools. Stools were not fetid and they contained no mucus or blood. Patient's frequency of defecation was five to six times per day sometimes accompanied by non-bilious vomiting. No blood was found in patient's vomit. Patient had no history of fever or other digestive or extra-digestive symptoms. Patient had been hospitalized previously for self-limiting edema with ascites, hypoalbuminemia, lymphopenia and elevation levels of transaminase which subsequently resolved. There were no other issues relevant to the current illness in the patient's personal or family history (See Figures 1 and 2).
Upon admission the patient was found to have abdominal distension, rectal prolapse and left upper limb hemihypertrophy that compromised the forearm and hand. An assessment of the patient's nutritional status was compatible with short stature. There were no other significant findings from the physical examination. Paraclinical evidence showed hypochromic microcytic anemia, lymphopenia and hypoalbuminemia (see Table 1).
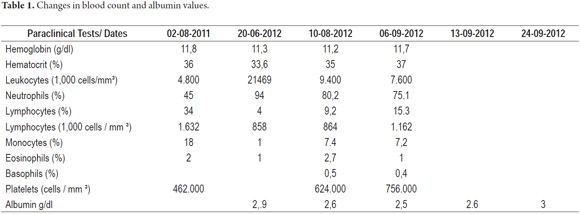
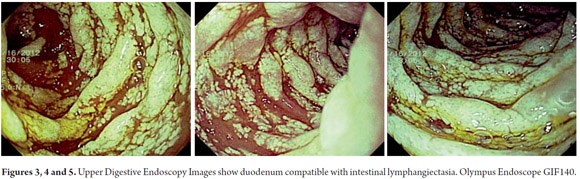
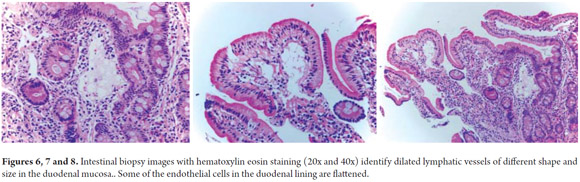
Diagnostic tests performed included a series of tests of fecal matter to rule out parasites. The serum ionogram, liver function tests, and kidney function tests were normal. Patient tested negative for iontophoresis and positive on SUDAN III staining. Patient's immunoglobulin G level was 300 mg/dl (VR: 749-2682 mg/dl). Upper endoscopy showed severe edema of intestinal folds with whitish lesions that covered 90% of the walls of the duodenal bulb and first portion of the duodenum which suggested intestinal lymphangiectasia. The diagnosis was confirmed by intestinal biopsy (See Figures 3, 4, 5, 6, 7 and 8).
Initially the patient's condition was managed with a high calorie, low-fat diet enriched with medium chain triglycerides (MONOGEN®) for 20 days. Because little changed in response to this treatment, it was decided to change diet to a formula consisting exclusively of medium chain triglycerides. After 15 days paraclinical and clinical indications showed a positive response characterized by a reduction in the number of stools to only one or two per day. Stools also became well formed. Patient gained approximately 30 grams of weight every day. Serum albumin increased to 3g/dL.
DISCUSSION
In 1961, Waldmann et al. described the first 18 cases of idiopathic hypercatabolic hypoproteinemia. These patients had hypoproteinemia and edema associated with low serum albumin and immunoglobulin levels. Daily excretion of fecal protein was twice as high as that of healthy patients. Microscopic examination of biopsies from the small intestine showed varying degrees of dilation of lymphatic vessels in the mucosa and submucosa. These authors proposed the term intestinal lymphangiectasia (2).
Intestinal lymphangiectasia is responsible for lymphatic leakage into the intestinal lumen leading to hypoalbuminemia and lymphopenia. This edema is caused by decreased oncotic pressure associated with hypoproteinemia. This condition can present through a broad spectrum of clinical and paraclinical manifestations which are conditioned on the anatomical location and extent of the lymphatic anomaly (1). The intestinal lesion can be patchy or can be located at a more or less specific site (3). There may be involvement of other structures such as the uterus, lungs and conjunctiva. Many diseases are associated with excessive loss enteric proteins from the plasma. They include intestinal lymphangiectasia, Menetrier's disease and inflammatory conditions such as lupus erythematosus (4).
Primary intestinal lymphangiectasia is typically limited to the small intestine although its etiology and prevalence are unknown. Children with primary intestinal lymphangiectasia are usually diagnosed before the age of 3.
Secondary intestinal lymphangiectasia occurs most frequently in adults and is an effect secondary to increased lymphatic pressure. Among its causes are bowel lymphoma, lymphoenteric fistulas, Whipple's disease, Crohn's disease, sarcoidosis, tuberculosis, intestinal giardiasis, radiotherapy, chemotherapy, Fontan surgery and constrictive pericarditis.
Many diseases including intestinal lymphangiectasia, Menetrier's disease and inflammatory conditions such as lupus erythematosus are associated with excessive enteric loss of plasma proteins (4).
Children with primary intestinal lymphangiectasia are usually diagnosed before the age of 3.
In 95% of cases, the principal clinical feature is edema. It is usually peripheral and symmetric with pitting and varies from moderate to severe. It is common to have pleural effusion, pericarditis and chylous ascites. It may be suspected in utero because of fetal ascites or lower limb edema (4).
Patients may exhibit fatigue, abdominal pain, nausea, vomiting and failure to thrive. The main digestive symptom is moderate and intermittent diarrhea. Mechanical ileus may also be present secondary to areas of edema in the small bowel leading to thickening of the intestinal wall and reduction of the lumen. The deficit can cause malabsorption of fat soluble vitamins.
According to some descriptions, hypocalcemia can lead to secondary tetany and seizures. There are reports that associate it with osteomalacia, osteoporosis, development of vitamin D deficiency and secondary hyperparathyroidism (5).
Patients may have iron deficiency with anemia secondary to chronic bleeding for multiple nonspecific ulcers in the small bowel. In addition there have been reports of gastrointestinal bleeding without significant paraclinical evidence of enteric protein loss. This has been associated with increased lymphatic pressure in lympho-venous anastomoses with venous vessel ruptures and secondary bleeding.
Biological abnormalities which are indirectly suggestive of intestinal lymphangiectasia include hypoproteinemia, hypoalbuminemia, hypogammaglobulinemia with low levels of IgG, IgM and IgA, low counts of CD4 and T cells, and lymphopenia.
Although these patients have severe hypogammaglobulinemia and lymphopenia, the risk of infection is not very high and opportunistic infections are rare (6). There have been reports of meningitis and disseminated cryptococcal infections. One case of severe infection by hemolytic group b streptococcus and one case of gelatinous transformation of the bone marrow have been described. Another report discussed replacement of hematopoietic cells and adipocytes by an amorphous extracellular material composed of mucopolysaccharides which was attributed to malnutrition resulting from malabsorption (4).
Several syndromes including yellow nail syndrome, Von Recklighausen Disease, Turner syndrome, Noonan syndrome, Klippel-Trenaunay Syndrome and Henekam syndrome have been described.
The following criteria have been described for diagnosis: 1) typical clinical manifestations, 2) lymphopenia, 3) simultaneous decrease of serum albumin and IgG levels, 4) pathological intestinal lymphangiectasia confirmation by either endoscopic biopsy or surgical specimen, and 5) findings compatible with digestive tract protein loss. The first three items are suggestive, but the next two are essential for establishing the diagnosis (7). However, the diagnosis is confirmed when endoscopic findings with histologic confirmation of an intestinal biopsy show the presence of intestinal lymphangiectasia.
Evidenced of macroscopic abnormalities in upper endoscopy are clear. They include yellowish cream color villi within the intestinal mucosa corresponding to lymphatic vessel dilation. In mild presentation the mucosa may appear to have edema without being creamy. Endoscopy can be negative when lesions are segmented or localized. In these cases the endoscopic video capsule is useful for detecting the presence, location and extent of intestinal lymphangiectasia. Donzelli et al. describe two types of plates on the surface of the duodenal mucosal that were detected by this means and which are associated with lymphangiectasia. One type has a diameter less than 1 mm while the other's diameter exceeded 3 mm (8).
Histological examination of biopsies from the duodenum, jejunum and ileum confirm the presence of white material with dilated lymphatic vessels located in the mucosa, submucosa and serous in the absence of inflammation. The nodes may be enlarged in many or a few villi and dilated villi can also be found in the intestinal mesentery. Intestinal biopsies may be negative in 16% of cases (4).
SUPPLEMENTARY TESTS
1. When the 24 hour alpha-1 antitrypsin clearance test shows elevated levels of alpha-1 antitrypsin it may be due to enteric protein loss. The method of the alpha-1 antitrypsin clearance test has replaced scintigraphy which is more expensive and less often available and because of the use of a human product carries risks of infection.
2. When albumin scintigraphy finds the elevated levels of human serum albumin bound to technetium-99 it can indicate intestinal protein loss in this region. Serial examinations are required within 24 hours since the loss of protein in the intestine is intermittent. This test is very sensitive and is able to identify the site of protein loss.
3. Abdominal ultrasound can demonstrate characteristics such as severe mesenteric edema, ascites, dilated bowel loops, diffuse thickening of the walls, and hypertrophy of the folds which are suggestive of primary intestinal lymphangiectasia.
4. When a CT scan with contrast shows diffuse and nodular thickening of the walls of the small intestine, edema as a result of dilated lymph nodes within the villi, and some degree of intestinal dilatation, it suggests intestinal lymphangiectasia. These findings are similar in adults and children. There is also the halo sign which is a low attenuation inner ring surrounded by an outer ring of increased attenuation which has been reported in intestinal lymphangiectasia, Crohn's disease, ulcerative colitis and radiation enteritis. Computed tomography with contrast may be useful for the detection of localized areas of lymphangiectasia.
5. An MRI with contrast can show ascites, mesenteric edema with hyperintense fluid collection around the mesenteric blood vessels, and a ring surrounding areas of markedly hyperintense thickened bowel loops.
6. A lymphatic scan can be useful for identification of abnormalities of the node tree and for confirmation of lymphedema.
We have used different treatments including dietary modifications, octreotide, antiplasmin therapy, corticosteroids, surgical resection of the affected segments and treatments of symptoms such as the infusion of albumin and paracentesis. We have also used peritoneal venous shunts and intestinal transplantation (5).
The cornerstone of treatment for primary intestinal lymphangiectasia is a reduction of long chain fatty acids derived from food combined with the addition of medium chain triglycerides (9). Dietary modifications aim at controlling symptoms and the consequences of lymphatic obstruction but do not alter the underlying disease process. (3). Exclusion of long chain fatty acids prevents the swelling and rupture of malformed nodes while medium chain triglycerides are directly absorbed into portal venous circulation. Dietary modifications are more effective in children than in adults (8) and can result in improvement in more than 50% of patients (4). Evidence of improvement in clinical and laboratory parameters in these patients such as changes in levels of albumin, immunoglobulins and lymphocyte counts should be seen within weeks. Refractory edema associated with hypoproteinemia has been described in some cases (15). For cases that do not respond or in which the clinical indications are severe total, parenteral nutrition may be required (10).
Octreotide is an analogue of somatostatin that can help reduce losses of lymph nodes through several mechanisms. It has been reported to be useful in two cases which were refractory to other treatments. A study of 11 pediatric patients diagnosed with primary intestinal lymphangiectasia who were treated with doses of 150-200 mg of subcutaneous octreotide twice a day for 3 to 37 months found decreases in the total number of stools and the requirements of albumin but only one patient revealed histopathological improvement. Acute pancreatitis was observed as an adverse effect in one case (11).
There are also case reports of patients who did not respond adequately to octreotide or dietary treatment but who responded well to antiplasmin therapy consisting of 1 gram of tranexamic acid administered orally 3 times daily (3). This treatment is based on increasing the activity of fibrinolytic plasma or tissue in these patients (9). However, the responses described with this treatment varied and improvements were partial.
Corticoid steroids have been used primarily in patients with elevated acute phase reactants. They can be used in the subset of patients with underlying inflammatory diseases such as systemic lupus erythematosus.
Surgical management has been reported primarily for adults with initial clinical indications which simulate acute abdomen. There has been one reported case of resection of the affected segment of a 7 year old patient (4). Surgery is usually not useful for patients with primary intestinal lymphangiectasia as alterations in these patients are not confined to a specific area (1).
Supportive therapy may require infusion of albumin, diuretics, thoracentesis or paracentesis to achieve temporary relief of symptoms. Albumin infusion is proposed for symptomatic treatment of patients with important serous effusions that cause discomfort. Repeated infusions of albumin may be useful to reduce edema and improve serum albumin levels, but its efficacy is temporary.
The long-term prognoses of patients with primary intestinal lymphangiectasia vary, but this is usually a chronic disease which slowly progresses albeit with intermittent clinical remission. It requires a continuous restrictive diet. It is important to make clear to parents that there is no curative intent in this treatment. The main factors affecting the quality of life of these patients are associated with lymphedema and infections (4).
REFERENCES
1. Uguralp S, Mutus M, Kutlu O, Baysal T, Mizrak B. Primary intestinal lymphangiectasia: A rare disease in the differential diagnosis of acute abdomen. J Pediatr Gastroenterol Nutr 2001; 33(4): 508-510. [ Links ]
2. Waldmann TA, Steinfeld JL, Dutcher TF, Davidson JD, Gordon RS: The role of the gastrointestinal system in "Idiopathic hypoproteinemia". Gastroenterology 1961; 42: 197-207. [ Links ]
3. Maclean J, Cohen E, Weinstein M. Primary intestinal and thoracic lymphangiectasia: A response to antiplsmin therapy. Pediatrics 2002; 109: 1177-1180. [ Links ]
4. Vignes S, Bellanger J. Primary intestinal lymphangiectasia. Orphanet J Rare Dis 2008; 3:5. [ Links ]
5. Suresh N, Ganesh r, Sankar J, Malathi S. Primary intestinal lymphangiectasia. Indian Pediatr 2009; 46: 903-906. [ Links ]
6. Jabeen S, Murthy A, Kandadai R, Meena A, Borgohain R, Uppin M. Cryptoccocal meningitis as a primary manifestation in a patient with intestinal lymphangiectasia. Ann Indian Acad Neurol 2012; 15: 218-20. [ Links ]
7. Li XP, Shen WB, Long MQ, Lian XL, Yu M. Osteomalacia and osteoporosis associated with primary intestinal lymphangiectasis. Chin Med J 2012; 125(10): 1836-38. [ Links ]
8. Oh TG, Chung JW, Kim HM, Han SJ, Lee JS, Park JY et al. Primary intestinal lymphangiectasia diagnosed by capsule endoscopy and double ballon enteroscopy. World J Gastrointest Endosc 2011; 3(11): 235-240. [ Links ]
9. Campos C, Fernández-Argüelles A, Rabat JM, Sendón A. Dietoterapia en paciente con linfangiectasia intestinal primaria y ascitis quilosa de repetición. Nutr Hosp 2007; 22(6): 723-25. [ Links ]
10. Aoyagi K, Iida M, Matsumoto T, Sakisaka S. Enteral nutrition as a primary therapy for intestinal lymphangiectasia: Value of elemental diet and polymeric diet compared with total parenteral nutrition.Digestive Diseases and Sciences. 2005; 50(8): 1467-70. [ Links ]
11. Sari S, Baris Z, Dalgic b. Primary intestinal lymphangiectasia in children: Is octreotide an effective and safe option in the treatment? J Pediatr Gastroenterol Nut 2010; 51(4): 454-7. [ Links ]
12. Gortani G, Maschio M, Ventura A. A child with edema, lower limb deformity, and recurrent diarrhea. J Peadiatrics 2012. [ Links ]
13. Holzknecht N, Helmberg T, Beuers U, Rust C, WiebeckeB, Reiser M. Cross-Sectional imaging findings in congenital intestinal lymphangiectasia. Journal of Computer Assisted Tomography 2002; 26(4): 526-528. [ Links ]
14. Perisic Vojislav, Kokai G. Bleeding from duodenal lymphangiectasia. Arch Dis Child 1991; 66: 153-154. [ Links ]
15. Dierselhuis M, Boelens JJ, F Versteegh, Weemaes C, Wulffrat N. Recurrent and opportunistic infections in children with primary intestinal lymphangiectasia. J Pediatr Gastroenterol Nut 2007: 44(3): 382-85. [ Links ]
16. McDonald K, Bears C. A preterm infant with intestinal lymphangiectasia: A diagnostic dilemma. Neonatal Network 2009; 28(1): 29-36. [ Links ]
17. Molina M, Romero S, Antón S, Prieto G, Polanco I. Linfangiectasia intestinal primaria: Evolución a largo plazo. An Esp Pediatr 2001; 54 (Supl. 3): 33-35. [ Links ]











 text in
text in