Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.3 Bogotá July/Sept. 2013
Advances in Management of Early Esophageal Carcinoma
Vitor Arantes, MD. (1), Elías Alfonso Forero Piñeros, MD. (2), Takashi Toyonaga, MD. (3)
(1) Adjunct Professor in the Department of Endoscopic Surgery at the Instituto Alfa de Gastroenterologia in the Faculty of Medicine of the Universidad Federal de Minas Gerais in Belo Horizonte, Brazil.
(2) Director of the Endoscopy and Gastroenterology Service of the Hospital de la Policía in Bogotá, Colombia.
(3) Associate Professor in the Department of Gastroenterology at the Hospital of the University of Kobe in Kobe, Japan.
Received: 12-08-12 Accepted: 26-06-13
Abstract
Squamous cell carcinoma (SCC) of the esophagus has a poor prognosis because it is generally detected in its advanced stages. Recently however, the development of high resolution endoscopy with digital chromoscopy and Lugol favors diagnosis of SCC in its initial stages. This development was made parallel to development of important endoscopic techniques for endoluminal resectioning of tumors "en bloque" from endoscopic submucosal dissection (ESD). These advances have increased the indications for minimally invasive endoscopic treatment of SCC of the esophagus providing patients with the potential of a cure. This review article aims to provide an understanding of the most recent and most important advances related to management of early SCC of the esophagus. The secondary objective of this article is to provide a detailed review of the ESD technique developed by Japanese experts. Both objectives have the aim of contributing to the diffusion of ESD and these new technologies to Latin American endoscopy and their incorporation into Latin American gastroenterological practice.
Keywords
Early esophageal carcinoma, endoscopic resection, endoscopic dissection.
INTRODUCTION
Esophageal cancer is the third leading cause of death from gastrointestinal cancer (1), and has a survival rate at five years of only 15% (2). Squamous cell carcinoma (SCC) of the esophagus is predominant in men (3.6:1) between the fifth and seventh decades of life (3). When there is an early diagnosis of esophageal SCC, the prognosis is better, with survival rates at 5 years up to 95% (4).
This review aims to describe the major recent advances which have occurred in early esophageal cancer treatment, with emphasis on the current role of high-resolution endoscopy and chromoendoscopy for the diagnosis of early forms of the disease and the importance of new Japanese techniques of endoluminal endoscopic resection called endoscopic submucosal dissection (ESD).
METHODS
A review was done of the most important original articles found in the English language literature since 1990 on Medline (PubMed). It took into account the keywords "esophageal squamous cell carcinoma," "endoscopic submucosal dissection," "management, "and" Lugol chromoscopy." For the technical description of the ESD procedure we gave priority to articles published by the co-author of this manuscript (TT), who has one of the largest personal series of ESD (4,000 cases performed up to 2012), and a line of research dedicated to developing new accessories and perfecting the ESD technique.
DIAGNOSIS
The great challenge in Latin America and in Western countries is to establish the diagnosis of esophageal cancer at an early stage when patients are asymptomatic and macroscopic changes are difficult to recognize. The best method for the detection of esophageal cancer is endoscopy, especially if it is combined with chromoscopy techniques (5, 6). Endoscopy for screening for esophageal cancer SCC in the general population is not justified because of the costs of the procedure, but population an endoscopic screening program can be cost effective in a high-risk. There is an association of esophageal SCC with certain risk factors, especially, abuse of alcohol abuse and tobacco, drinking mate, a history of SCC in the upper aerodigestive tract or caustic injury of the esophagus, megaesophagus and human papilloma virus infections (5, 6). Among all these factors, a history of SCC in the upper aerodigestive tract is most consistently associated with synchronous and metachronous neoplasia and therefore upper endoscopy for screening is routinely recommended in this population (5, 6).
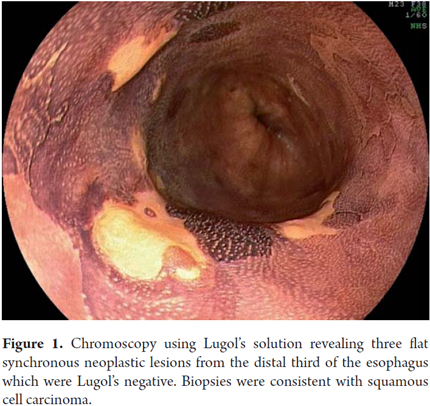
Chromoscopy using Lugol's solution is the method of choice for diagnosis of early esophageal SCC (3). Lugol's solution is a reaction dye in which iodine strongly stains esophageal squamous cells which are rich in glycogen. It does not stain dysplastic and neoplastic cells which are poor in glycogen. An important recently described aspect of chromoscopy using Lugol's solution, which is not often valued by some endoscopists, is the "pink color sign" (7, 8). This signal is the transformation of the yellow color of neoplastic lesions which are Lugol's-negative into pink after 3 to 5 minutes. This transformation is due to the low levels of glycogen existing in the neoplastic cells of the esophagus. Its appearance indicates existence of high-grade dysplasia or squamous cell carcinoma with high specificity (7, 8). In 2009, one of the authors (VA) began a screening program for cancer of the esophagus in patients with SCC in the upper aerodigestive tract. Unsedated transnasal endoscopy combined with digital chromoendoscopy using Lugol's solution was used. Among the 106 patients examined up to 2011, esophageal SCC was identified in 13 cases (12.3%) of which 77% were at early stages which allowed treatment by endoscopy in 8 cases (9). This high incidence illustrates the importance of monitoring these high-risk patients in Latin America.
CLASSIFICATION AND STAGING OF SUPERFICIAL ESOPHAGEAL CANCER
According to the endoscopic classification of superficial lesions published in the Paris Consensus (10), a surface neoplasia is defined as a lesion whose morphological appearance suggests involvement of the mucosal and submucosal layers without infiltration of the muscularis propria. In Japan the superficial lesions are conventionally referred to as type 0 of the Borrmann classification of advanced gastric tumors (12). There are three subtypes of superficial lesions: protruding (type 0-I), superficial, flat or elevated (Type 0-II) and excavated (type 0-III). Protruding lesions are further classified into pedunculated (0-Ip), sub-pedunculated (0-ISP) and sessile (0-Is). In the esophagus, flat superficial neoplasms predominate. They are subdivided into lesions that are elevated compared to adjacent mucosa (IIA), lesions that are flat (IIb) and lesions that are depressed (IIC). Protruding and excavated forms are rare (10). Superficial neoplasms are further subdivided according to transmural penetration. M1 corresponds to the epithelium and the basal layer, M2 to the lamina propria, and M3 to the muscularis mucosa. Compromises of the submucosa are also subdivided into SM1 (upper third), SM2 (average) and SM3 (lower third).
For histopathological description of superficial tumors the current recommendation is to use the Vienna classification (11), by which the cancer is classified according to the TNM-p ("p" of pathology) classification. In the absence of invasion of the lamina propria, the lesion is called low or high-grade intraepithelial neoplasia, but the term carcinoma in situ (pTis) can also be used. When there is invasion of the lamina propria, esophageal carcinoma neoplasia is called micro-invasive or intramucosal (pT1m). When there is infiltration of the submucosa, the tumor is considered to be invasive (pT1sm).
The importance of these subdivisions is due to the fact that the risk of lymph node metastasis in superficial cancer is closely related to the depth of vertical invasion of the organ wall. This is a key criterion for the selection of candidates for endoscopic therapy with curative intent. When the compromise is limited to M1 and M2 which is the neoplastic epithelial surface, the risk of lymph node involvement is close to zero, and realization of endoscopic excision is sufficient for cure (10, 12). When cancer invades the muscularis mucosa (M3) and the proximal portion of the submucosa to a depth of 200 μ (SM1), risk can conceptually vary from 9% to 19%, respectively. In cases situated at the edge of healing endoscopic treatment, further evaluation is essential. The following parameters must be carefully monitored: tumor size, presence of invasion and lymphatic vascular horizontal extension (width) of the invasion in the lamina muscularis of the mucosa. In the multivariate analysis study of Choi et al. (13), in which 190 pieces from esophagectomies were evaluated, the authors found that when the neoplastic lesion size was less than 3 cm for tumors affecting M3/SM1 focally, there was no lymph node or vascular invasion. If the compromise of the lamina muscularis of the mucosa had a width of less than 3 mm, the risk of lymph node metastases was very low (1 in 63 cases, or 1.5%). In the presence of massive infiltration of the submucosa (SM2 and SM3), there was metastasis to the lymph nodes in approximately 40% of cases.
For initial staging of esophageal cancer endoscopic
ultrasonography can be used in addition to detailed assessment of morphological
characteristics found through endoscopy. The conventional echoendoscope
operates at low frequencies (5-12 MHz) which allow division of the wall of the
gastrointestinal tract into 5 layers. Due to its greater penetration the
mediastinum and the celiac trunk can be fully appreciated in the search of
lymph nodes. High resolution miniprobes have higher frequencies (15 to 30
MHz) which allow a more detailed differentiation of the gastrointestinal tract
wall by dividing it into up to 9 layers. A fairly accurate assessment of the
vertical extent of tumor invasion can be obtained with these miniprobes.
Buskens et al. (14) analyzed the accuracy of endoscopy in the staging of
77 patients with dysplastic esophageal lesions and early carcinomas who had
undergone esophagectomies. They compared the accuracy of the method against the
gold standard of an examination of the surgical specimen. The authors
found that endoscopic ultrasound correctly demonstrated the absence of lymph
node metastases in 93% of cases. The negative predictive value of
endoscopic ultrasound for detecting the absence of submucosal invasion was
95%.
To maximize the accuracy of endoscopic ultrasonography for esophageal SCC, we
recommend combining the use of a high frequency miniprobe for T stage and a
conventional echoendoscope for evaluation of lymph nodes. The presence of
lymph node metastases suggests that neoplastic lesions cannot be cured with
only local resection. Despite the good results mentioned above (14), the
main limiting factor in endoscopic ultrasound evaluation of early esophageal
neoplasias is the desmoplastic reaction caused by the inflammatory response of
the underlying neoplasia. This can lead to over-staging of the compromise which
in turn can lead to incorrect routing of patients to treatment other than
endoscopic. Absence of invasion of the submucosal layer combined with
absence of malignant lymph nodes supports endoscopic resection. The
ultimate outcome will be dictated by systematic histological evaluation of the
resected specimen. If this assessment identifies M3 or SM invasion, lymphatic
or vascular invasion, or a tumor that is deep, the patient's treatment should
be redirected to chemotherapy and radiation therapy, or to surgery. Considering
that histological evaluation is the decisive factor in the final definition of
treatment, every effort should be made to maximize the quality of the sample
and to prevent that the esophageal neoplasia is fragmented (as in "piecemeal"
mucosectomy). Unlike complete removal of a tumor, a fragmented sample does not
allow a proper analysis of margins and prevents characterization of endoscopic
resection.
INDICATIONS FOR ENDOSCOPIC TRATMENT OF SUPERFICIAL ESPHAGEAL NEOPLASIA
The classically accepted criteria for endoscopic resection with curative intention in superficial esophageal cancer include: depth of compromise restricted to M1 and M2 layers (epithelium and lamina propria); maximum length of 3 cm, maximum lateral extent of less than ¾ of the circumference, and a maximum of 4 lesions (15). With improved techniques for endoscopic resection, especially after the advent of ESD, these criteria have tended to widen to accept the endoscopic treatment of lesions larger than 3 cm which occupy the entire circumference of the esophagus and to eliminate the limit on the number of lesions as long as they are only intramucosal.
Endoscopic Treatment modalities include esophageal cancer resection techniques (mucosal resection or ESD) and ablation techniques. Ablation methods include photodynamic therapy, argon plasma, YAG laser, multipolar electrocoagulation, and most recently radiofrequency ablation. All these methods have the drawback that they make histopathological analysis impossible because they eradicate the neoplastic lesion. However, the information obtained from such an analysis is crucial for estimating curability with the endoscopic procedure. Therefore, current ablative methods should not be used for the endoscopic treatment of esophageal SCC. In this paper, we discuss in detail the role of ESD and mucosectomy in the treatment of superficial esophageal cancer.
TECHNICAL PRINCIPLES OF ENDOSCOPIC MUCOSAL RESECTION
The gut wall is has by two main components: the mucosa and muscularis propria. These elements are joined by the submucosa which is a layer of loose connective tissue. Since the thickness of the esophageal wall is between 3.5 mm and 4 mm, removing a superficial neoplastic lesion entails a risk of unintentional damage to the muscle layer and visceral perforation. To avoid these complications, a submucosal injection is necessary to separate the lesion from the muscularis propria. A 0.9% saline solution is used for these injections. The disadvantage of saline solution is that it dissipates rapidly into the gastrointestinal wall making removal of lesions larger than 1 cm impossible. For these cases viscous solutions have been developed to promote a longer lasting bubble effect (16). The best known are sodium hyaluronate, glycerin and hydroxypropyl methylcellulose (HPMC) (16). Elevation of the neoplastic lesion after infiltration of the submucosa indicates that there is no deep invasion by cancer. After injection of a sufficient volume of solution into the submucosa, elevated lesions can be captured by loop diathermy which allows resection with an adequate margin of safety: this procedure is called mucosectomy.
There are variations of mucosectomy techniques: injection and loop; injection, cap-assisted mucosectomy with suction, and mucosectomy after application of elastic bands. Cap-assisted mucosectomy is the recommended for application in esophagus (15, 17).
Results of the esophagus mucosectomy
The most consistent results from endoscopic mucosal resection for esophageal cancer are found in the Japanese experience. Inoue (17) published a series of 142 patients with esophageal cancer who had undergone mucosectomy with curative intent who had been followed up on for up to nine years. When all the selection criteria were met, there were no cases of local recurrence or metastasis. The survival rate at 5 years was 95% and there were no deaths related to cancer. Endo et al. (18) reported the results of esophageal mucosal resection for intramucosal cancer smaller than 2 cm and occupying less than one third of the circumference. The survival rate at 5 years was 100%, local recurrences occurred in 7%. In all of these cases endoscopic treatment was repeated. Yoshida et al. (19) found no differences in 5 year survival rates between patients with esophageal cancer with involvement of M1 and M2 treated by mucosal resection (86% survival at 5 years) and similar patients treated with esophagectomy (83.2% survival at 5 years).
The efficacy of endoscopic mucosal resection for curative treatment when all inclusion criteria are met has been well demonstrated. Moreover, there is no benefit from esophagectomy for this subgroup of patients (19). However, even if certain criteria are violated, some authors have proposed alternative treatments to surgery. Shimizu et al. (20) reported a series of 16 patients who underwent endoscopic treatment of mucosal lesions that were invading the muscularis mucosa and submucosa and refused to undergo esophagectomy. These patients received adjuvant radiotherapy and chemotherapy after mucosectomy. The author compared the outcomes of these patients with another 39 patients with esophageal cancer who were treated with esophagectomy. No patients in the mucosectomy and chemotherapy/radiation therapy group presented local recurrence or metastasis. The 5-year survival rate in the group that was treated endoscopically was 100% and that of the patients who underwent surgery was 87.5%. Although this was not a randomized controlled trial, the results of this research suggest that when the criteria for curative endoscopic resection is incomplete, additional therapy with chemotherapy and radiotherapy appears to be an equally effective alternative to esophagectomy. Currently there is an ongoing Japanese multicenter study to evaluate the therapeutic efficacy of ESD associated with adjuvant chemoradiotherapy in patients with neoplasia M3 and SM1.
ENDOSCOPIC SUBMUCOSAL DISSECTION
The ESD technique was developed in Japan in the last 10 years to allow for resection of neoplastic lesions that are larger than 2 cm (21-23). ESD's secondary benefits include production of a sample that is adequate for histological study. From the clinical point of view, ESD achieves local resection with the greatest potential of healing and the lowest recurrence rate (24). This technique was originally designed for application in the stomach. Due to the smaller luminal space of the esophagus, compared to that of the stomach, the process is more complex more difficult to implement technically in the esophagus, so the use of ESD in developed more slowly in this organ. Refinement and standardization of ESD and the development of new accessories has made it possible to extend the application of this modality to the management of esophageal SCC. Besides these factors, increased detection of tumors at early stages through increasing use of high-resolution endoscopy and chromoendoscopy coupled with high morbidity and mortality associated with esophagectomy have stimulated the development and improvement of endoluminal therapeutic interventions which allow preservation of the organ and maintain the life quality of patients. Currently, ESD is the preferred method for endoscopic resection of early esophageal cancer in Japan, and this procedure is being gradually introduced and accepted in Latin America and other Western countries.
EQUIPMENT AND ACCESSORIES
- The following equipment is recommended for performance of ESD:
- A high resolution and magnification endoscope with a waterjet (a specific channel for irrigation water) for demarcation of the resection margins and therapeutic work. Double-channel endoscopes are not recommended.
- A pump for water infusion at controlled pressure.
- A CO2 insufflator.
- An electrosurgical unit especially for use in endoscopy. Currently the only validated models for use in ESD are ERBE electrocautery units (ICC 200, 200 and VIO VIO 300) which have the pulsating endocut mode in addition to software for dry-cut, soft coagulation, coagulation and swift forced coagulation. Each operator willing to perform ESD must have deep knowledge of electrosurgical properties and of the specific parameters for each step of the procedure. A number of stilettos and products developed by Japanese experts are now among the accessories used for ESD. They include:
- Stilettos: Flush-Knives (straight and ball-tipped), IT- Knives, Hook Knives, Flex Knives, Dual- Knives, Hybrid Knives, Safe Knives, and Swam Blade Knives (Figure 1).
- 25 gauge catheters for submucosal injection.
- Hemostasis: hot biopsy forceps (hot biopsy) or coagulation forceps.
- Disposable endoclips for perforation management.
- Plastic caps (fixing devices at the tip of the endoscope).
- Calipers for foreign bodies and loops with nets for retrieval of the specimen.
- Tubes with air leakage control valves.
- Solutions for submucosal injection: sodium hyaluronate (not available in Latin America), 0.4% hydroxypropyl methylcellulose, 20% mannitol, 0.9% saline solution.
ESD Technique
The patient should undergo preoperative and surgical risk evaluation since the majority of patients with esophageal cancer are chronic alcoholics and smokers. Patient should be hospitalized during treatment. In Japan, ESD procedures are performed under sedation. However, for beginners in the art, or when the estimated execution time exceeds two hours, we recommend the assistance of an anesthesiologist and the use of general anesthesia with orotracheal intubation (25). This allows the endoscopist to focus on the endoscopic procedure without risk of pulmonary aspiration. Cardiopulmonary monitoring and pulse oximetry in all cases. The use of a urinary catheter is also mandatory for prolonged procedures. Although there is no scientific evidence to justify the use of antibiotic prophylaxis for esophageal ESD, this practice is widespread in the Japanese centers where the use of second-generation intravenous cephalosporins is maintained for 3 days.
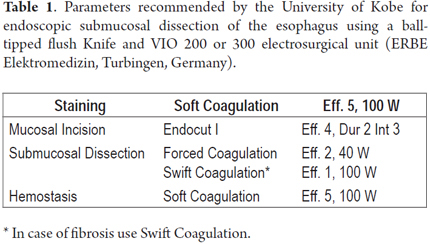
Several ESD techniques have been described. This review briefly describes two different techniques. The first technique was proposed and developed by one of the co-authors (TT) at the University of Kobe and described previously (26-29). Initially, the extent of the lesion must be thoroughly inspected using an endoscope with optical magnification and digital chromoscopy that also has a short 4mm long plastic cap attached to its distal end. Then, proceed to chromoscopy with 0.8% Lugol solution for accurate tracing of the lesion's boundaries. In the esophagus, the procedure is done with short needle stiletto that is 1.5 mm long with a rounded tip (and which was designed by one of the authors (TT)). This can be used for all the steps of the ESD: marking, incision, submucosal dissection, simultaneous injection of saline solution and hemostasis of blood vessels (Flush-Ball-tipped Knife 1.5, Fujifilm Fujinon Co., Japan) (27). Use of the 200D or 300D VIO electrosurgical unit (ERBE Elektromedizin, Turbingen, Germany) is recommended to. Table 1 shows the parameters used for surgical esophageal electrocautery through ESD at Kobe University.
Chromoendoscopy is followed by marking the limits of resection with a Flush Knife (FK) respecting the minimum margins of 2-5 mm (parameters: Soft coagulation, Outcome 5, 100 watts). In the esophagus, the proximal and distal margins must be large (5 mm), whereas the side margins can be more conservative (2 mm) to minimize the risk of circumferential esophageal stenosis secondary to resection. Next, proceed to submucosal injection with a 25 gauge needle (TOP Corporation, Japan). Initially The procedure begins by injecting a first bubble of 0.9% saline solution into the submucosa. This is followed by injection of a viscous solution of hyaluronic acid (Muco-Up®, Seikagaku, Japan) that maintains the submucosa elevated for a longer period.
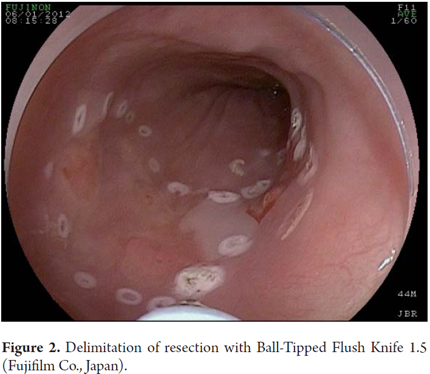
Submucosal injection should be initiated at the proximal (oral) margin of the lesion, and should continue with successive injections of 1 to 3 ml in the lateral margins (left and right). Finally, injections should be made on the distal (anal) edge. A reasonable elevation of the lesion (rising sign) should be seen. Transfixing injections in the center of the neoplastic lesion should be avoided in order to minimize the risk of the tumor seeding into the muscular layer. Next the protocol proceeds to an incision in the mucosa with an FK (Effect 4 Endocut I, cutting extent 2, cutting interval 3). The incision should create the outline of a "C" by starting at the anal margin, proceeding with a transverse incision to the lateral margin, then continuing with a longitudinal incision to the oral margin, and finally making another transverse incision to complete the "C". Once this is done, the submucosal layer should be dissected with the FK in the oral-anal direction (forced coagulation effect 2, 40 watt). This will, create an incision flap in the lateral direction towards the center of the lesion. Every electric cut should be preceded by application of more saline solution injections into the submucosa. The dissection should be performed in the deep submucosal layer between the muscular and vascular submucosal layers of the esophagus to make it more efficient and to optimize vascular control. As previously described, perfect hemostasis of submucosal vessels is essential for safe procedure and a bloodless field (30, 31). Perforated esophageal vessels should be identified and isolated, and hemostasis should be performed by applying FK Soft Coagulation, effect 5, at 100 watts for 3 to 5 seconds on each side of the vessel. This should be followed by section of the vessel with forced coagulation. If the hemostatic maneuver with the FK is not successful after 3 attempts, the practitioner should proceed to hemostasis with forceps (COAG grasper, Olympus Co. Japan). After dissection of 70% to 80% of the lesion from its outside oral edge to the anal margin, sodium hyaluronate should be injected into the submucosa on the side margin that has not yet been isolated. This should be followed by a longitudinal incision in the in the lateral margin of the mucosa in an oral-anal direction. At this point the submucosal dissection flap previously created will be completed by using the cap to expose the submucosal space. FK movements should always be parallel to the axis of the esophageal wall and never perpendicular to the muscular layer. After completion of the excision, the sample must be recovered with foreign body forceps. Take care to grasp the sample from its submucosal side in order not to damage the mucosa of the sample portion. The resection site should be reexamined. At this point protruding vessels must be coagulated and lacerations of the muscle layer must be approximately closed with clips. If the resection site's extension exceeds 75% of the circumference, a 4 ml injection of triamcinolone acetonide 10 mg/ml can be added via a catheter injector using 20 punctures with aliquots of 0.2 ml per puncture directed at both the edge and the center of the resection site. Be careful to inject only in the superficial portion of the muscle layer. This measure aims at minimizing the risk of esophageal stenosis. In a recently published study comparing 41 patients with circumferential esophageal resections that were divided into two groups (with and without injection of triamcinolone), esophageal stenosis occurred in 19% of the treated group as opposed to 75% of the control group (30). In Kobe, triamcinolone injections are repeated after 7 and 14 days. Recently, oral doses of 30 mg of prednisolone have been administered to prevent esophageal stricture after circumferential ESD. This begins on the third day following surgery, and treatment continues for 8 weeks. Preliminary results are promising (31).
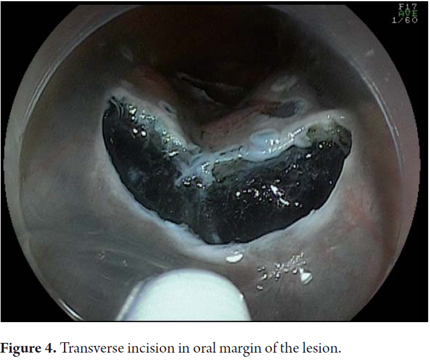
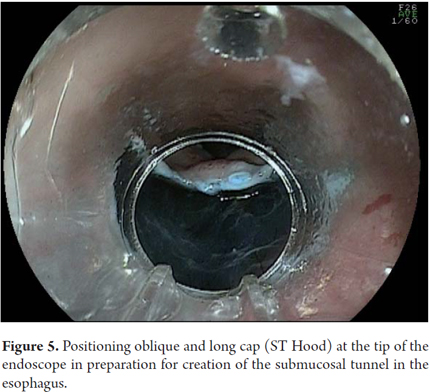
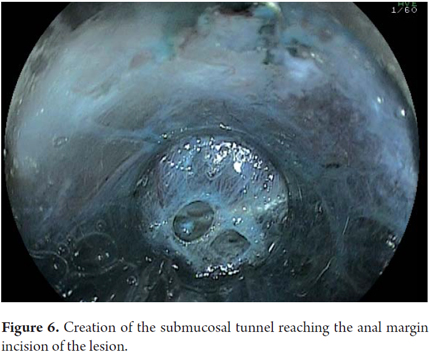
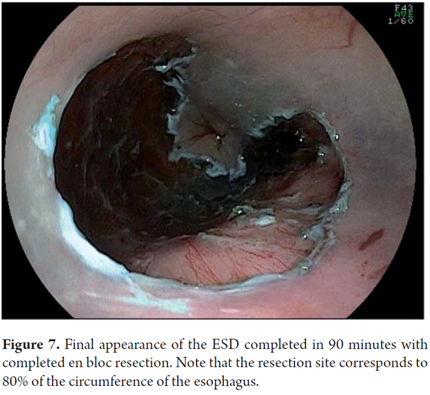
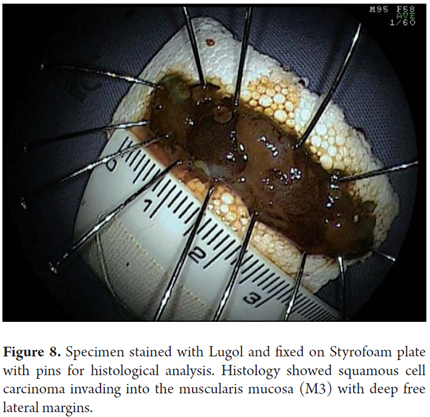
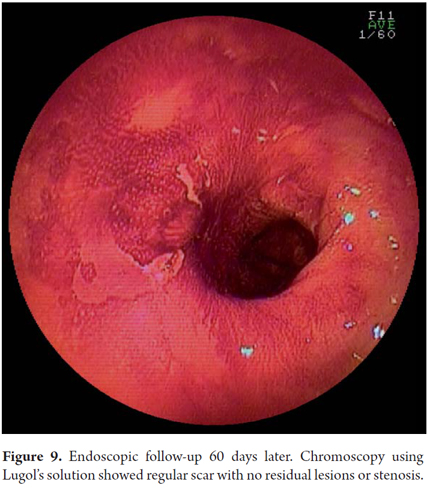
Another variant of esophageal ESD is known as the tunneling method. This technique was initially developed by Yamamoto. After training obtained by one of the authors (VA) with the inventor of this method, this technique was used in 22 consecutive patients with early esophageal SCC in Brazil. After defining the margins, the first step of the tunneling technique consists of a submucosal injection followed by FK incision in the anal margin of the lesion. This is followed by another submucosal injection and a transverse incision in the oral margin of the lesion. The incision should be deep enough to target the submucosal edge and the muscularis propria. Then the endoscope is inserted through the submucosal layer using an oblique cap over the tip of the endoscope (ST Hood, Fujinon Co., Japan). A tunnel is created in the submucosa in the oral-anal direction through visually directed dissection of submucosal fibers and clotting of perforated esophageal vessels back into the muscularis propria. After the tunnel has been created and the anal margin has been reached by the transverse incision, dissect the submucosa in the transverse direction from one end to the other of the lesion. The final steps more submucosal injections in the right and left side margins of the lesion followed by lateral incisions in an oral-anal clockwise direction to complete the procedure. Figures 1, 2, 3, 4, 5, 6, 7, 8 y 9 are representative of the tunneling technique.
Recovery and preparation of the resected specimen
This is a fundamental step in the endoscopic treatment of superficial tumors that is often ignored by Western endoscopists, but that is carried out systematically by the Japanese. The recovered sample is fixed onto Styrofoam or rubber with pins and placed in formalin. The pathologist must cut the sample into parallel fragments that are 2 mm wide. Biopsy samples should be evaluated according to the classification Vienna (11) which indicates the degree of tumor differentiation, invasion depth and whether or not resection was complete. In any case, the proximal, distal, lateral and vertical margins must be carefully evaluated. For surgical specimens containing the mucosa, submucosa, muscularis and adventitial, a semi-quantitative analysis of the submucosal invasion depth is reliable since the submucosa can be divided into three segments of equal thickness (SM1, SM2 and SM3). In pieces of endoscopic resection, the submucosa is not always complete in which case this distinction is less reliable. In these cases, a quantitative measurement is taken by measuring the invasion of the submucosa in microns (μ) from the last layer of the muscularis mucosa. A cut-off point above which it is considered there is an increased risk of metastasis in lymph nodes (SM2) must be established. In the esophagus this point is located at below 200μ (10).
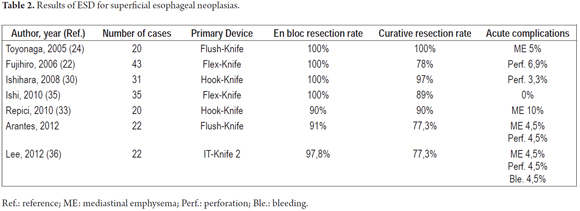
ESD results in early esophageal cancer
There are few studies in the literature on the use of ESD to resect esophageal tumors. A summary of the results of the main publications are described in Table 2. The first experiments with the use of ESD in the esophagus and colon were carried out only five years after the first use of ESD in the stomach. In 2005, Toyonaga et al. were among the first to report the use of ESD for esophageal tumors (26). The authors reported 20 patients with esophageal SCC who underwent ESD with FK. Successful resections with free margins were performed in 100% of these cases. The average diameter of the samples was 47 mm, and the average time of the procedures was 65 minutes. In this series the sole complication, mediastinal emphysema, was managed clinically. Fujishiro et al. (24) showed similar results in 43 patients. En bloc resections were performed in 100% of these cases, but in 22% of the cases endoscopic resection was not curative due to compromises at the vertical or lateral margins. There were four cases of perforations which were treated conservatively. Ishihara et. (32) Published an analysis comparing the results of ESD and mucosectomies performed on patients with esophageal cancers of less than 20 mm. The en bloc resection and curative resection rates for ESD cases were superior to those for endoscopic mucosal resection with cap and with strip biopsies (100% and 97%, 87% and 71%, 71% and 46 % respectively). There were no differences in complication rates among the three techniques. It should be noted that when the endoscopist is sufficiently trained, the ESD complication rate is similar to that for mucosectomies.
Only recently have the first two experiments with ESD for esophageal cancer been done in Europe and Brazil (Table 2) (33, 34).
At the Alfa Institute of Gastroenterology, ESD procedures in the esophagus began to be performed routinely in 2009. This began with training of the primary author (VA) of this article at the National University Hospital of Singapore and the Kobe University Hospital in Japan. So far, 22 ESD procedures have been conducted for esophageal cancer at this center. The tunneling method has been used for these procedures. It has achieved an en bloc resection rate of 91% and a curative resection rate of 77.3%. These are in line with the results found in the world's literature (24, 33, 35, 36). There were two complications in this series. One patient developed mediastinal and subcutaneous emphysema without apparent perforation. This patient was treated conservatively. The other case had esophageal perforation which was treated by applying clips and clinical management. No cases of local recurrence occurred during the follow-up periods which ranged from 3 months to 2 years (data submitted for publication).
FINAL CONSIDERATIONS
ESD is a reality in Asia where it is considered the treatment of choice for early SCC. The experience is still insufficient to conclude whether it is feasible to apply ESD for large scale treatment of superficial esophageal cancer in western centers as usually happens today in Japan and South Korea. In Western countries, especially in Latin America, the greatest challenge is to promote early diagnosis of esophageal neoplasia through proper training of endoscopists and the establishment of screening programs for high-risk patients. It is also essential to build referral centers with well-trained specialized human resources supported by a complete infrastructure so that the use of ESD can expand in these countries with the obvious benefits it can bring for quality of life and reduced morbidity and mortality.
REFERENCES
1. Blot WJ. Esophageal cancer trends and risk factors. Semin Oncol 1994; 21: 403-10. [ Links ]
2. Remontet L, Esteve J, Bouvier Am, et al. Cancer incidence and mortality in France over the period 1978-2000. Ver Epidemiol Sante Publique 2003; 51: 3-30. [ Links ]
3. Yokoyama A, Ohmori T, Makuuchi H, et al. Successful screening for early esophageal cancer in alcoholics using endoscopy and mucosa iodine staining. Cancer 1995; 76: 928-34. [ Links ]
4. Fagundes RB, de Barros SGS, Pütten ACK, et al. Occult dysplasia is disclosed by lugol chromoendoscopy in alcoholics at high risk for squamous cell carcinoma of the esophagus. Endoscopy 1999; 31: 281-5. [ Links ]
5. American Society for Gastrointestinal Endoscopy. ASGE guideline: the role of endoscopy in the surveillance of premalignant conditions of the upper GI tract. Gastrointest Endosc 2006; 63: 570-80 [ Links ]
6. Dubuc J, Legoux JL, Winnock M, et al. Endoscopic screening for esophageal squamous-cell carcinoma in high-risk patients: A prospective study conducted in 62 French endoscopy centers. Endoscopy 2006; 38: 690-5. [ Links ]
7. Shimizu Y, Omori T, Yokyama A, et al. Endoscopic diagnosis or early squamous neoplasia of the esophagus with iodine staining: High-grade intraepithelial neoplasia turns pink within a few minutes. J Gastroenterol Hepatol 2008; 23: 546-50. [ Links ]
8. Ishihara R, Yamada T, Iishi H, et al. Quantitative analysis of the color change after iodine staining for diagnosing esophageal high-grade intraepithelial neoplasia and invasive cancer. Gastrointest Endosc 2009; 69: 213-8. [ Links ]
9. Arantes V, Albuquerque W, Dias CAF, et al. Effectiveness of unseated transnasal endoscopy with white-light, FICE and lugol staining for esophageal cancer screening in high-risk patients. J Clin Gastroenterol 2012 (In press). [ Links ]
10. The Paris endoscopic classification of superficial neoplastic lesions. Gastrointest Endosc 2003; 58(suppl 6): S3-S43. [ Links ]
11. Schempler RJ, Riddel RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut 2000; 47: 251-5. [ Links ]
12. Inoue H, Fukami N, Yoshida T, Kudo S. Endoscopic mucosal resection for esophageal and gastric cancers. J Gastroenterol Hepatol 2002; 17: 382-8. [ Links ]
13. Choi JI, Park YS, Jung HY, et al. Feasibility of endoscopic resection in superficial esophageal squamous carcinoma. Gastrointest Endosc 2011; 73(5): 881-9. [ Links ]
14. Buskens CJ, Westerterp M, Lagarde SM, Bergman J, ten Kate FJW, van Lanschot JJB. Prediction of appropriateness of local endoscopic treatment for high-grade dysplasia and early adenocarcinoma by EUS and histopathologic features. Gastrointest Endosc 2004; 60: 703-10. [ Links ]
15. Inoue H, Fukami N, Yoshida T, Kudo S. Endoscopic mucosal resection for esophageal and gastric cancers. J Gastroenterol Hepatol 2002; 17: 382-8. [ Links ]
16. Arantes V, Albuquerque W, Benfica E, et al. Submucosal injection of 0,4% hydroxypropyl methylcellulose facilitates endoscopic mucosal resection of early gastrointestinal tumors. J Clin Gastroenterol 2010; 44(9): 615-9. [ Links ]
17. Inoue H. Endoscopic mucosal resection for esophageal and gastric mucosal cancers. Can J Gastroenterol 1998; 12: 355-9. [ Links ]
18. Endo M, Takeshita K, Inoue H. Endoscopic mucosal resection of esophageal cancer. Gan To Kagaku Ryoho 1995; 22: 192-5. [ Links ]
19. Yoshida M, Hanashi T, Momma K, Yamada Y, Sakaki N, Koike M, et al. Endoscopic mucosal resection for radical treatment of esophageal cancer. Gan To Kagaku Ryoho 1995; 22: 847-54. [ Links ]
20. Shimizu Y, Kato M, Yamamoto J, Nakagawa S, Tsukagoshi H, Fujita M, Hosokawa M, Asaka M. EMR combined with chemoradiotherapy: a novel treatment for superficial esophageal squamous-cell carcinoma. Gastrointest Endosc 2004; 59(2):199-204. [ Links ]
21. Ono H, Kondo H, Gotoda T, et al. Endoscopic mucosal resection for treatment of early gastric cancer. Gut 2001; 48: 225-9. [ Links ]
22. Ookuwa M, Hosokawa K, Boku N, et al. New endoscopic treatment for intramucosal tumors using an insulated-Tip diathermic knive. Endoscopy 2001; 33: 221-6. [ Links ]
23. Yamamoto H, Kawata H, Sunada K, et al. Success rate of curative endoscopic mucosal resection with circumferential mucosal incision assisted by submucosal injection of sodium hyaluronate. Gastrointest Endosc 2002; 56: 507-12. [ Links ]
24. Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal Dissection of Esophageal Squamous Cell Neoplasms. Clin Gastroenterol Hepatol 2006; 4: 688-694. [ Links ]
25. Fujishiro M, Kodashima S, Goto O, et al. Endoscopic submucosal dissection for esophageal squamous cell neoplasms. Digestive Endoscopy 2009; 21: 109-15. [ Links ]
26. Toyonaga T, Nishino E, Hirooka T, et al. Use of short needle knife for esophageal endoscopic submucosal dissection. Digestive Endoscopy 2005; 17: 246-52. [ Links ]
27. Toyonaga T, Man-I M, Fujita T, et al. The performance of a novel ball-tipped Flush Knife for endoscopic submucosal dissection: a case-control study. Aliment Pharmacol Ther 2010; 32: 908-15. [ Links ]
28. Toyonaga T, Nishino E, Hirooka T, Ueda C, Noda K. Intraoperative bleeding in endoscopic submucosal dissection in the stomach and strategy for prevention and treatment. Digestive Endoscopy 2006; 18 (suppl 1): S123-7. [ Links ]
29. Toyonaga T, Nishino E, Dozaiku T, Ueda C, Hirooka T. Management to prevent bleeding during endoscopic submucosal dissection using the flush knife for gastric tumors. Digestive Endoscopy 2007; 19 (suppl 1): S14-8. [ Links ]
30. Hashimoto S, Kobayashi M, Takeuchi M, sato Y, Narisawa R, Aoyagi Y. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointestinal Endosc 2011; 74 (6): 1389-92. [ Links ]
31. Isomoto H, Yamaguchi N, Kakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for esophageal squamous cell carcinoma. BMC Gastroenterology 2011; 11: 46. [ Links ]
32. Ishihara R, Ishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc 2008; 68: 1066-72. [ Links ]
33. Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous cell carcinoma: Results from a prospective Western series. Gastrointest Endosc 2010; 71: 715-21. [ Links ]
34. Chaves DM, Maluf Filho F, de Moura EG, et al. Endoscopic submucosal dissection for the treatment of early esophageal and gastric cancer – initial experience of a western center. Clinics 2010; 65: 377-82. [ Links ]
35. Ishi N, Horiki N, Itoh T, et al. Endoscopic submucosal dissection with acombination of small-caliber tip transparent hood and flex knife is a safe and effective treatment for superficial esophageal neoplasias. Surg Endosc 2010; 24: 335-42. [ Links ]
36. Lee CT, Chang CY, Tai CM, et al. Endoscopic submucosal dissection for early esophageal neoplasia: A single center experience in Taiwan. Journal of the Formosan Medical Association 2012, in press. [ Links ]











 text in
text in