Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.3 Bogotá July/Sept. 2013
The development of compromised Swallowing in patients with Alzheimer's Disease and dysphagia
Camilo A. Manrique M., MD. (1), Julián D. Martínez, MD. (2), Martín A. Garzón O., MD. (2), Natan Hormaza, MD. (2), Jorge Lizarazo R., MD. (2), Juan C. Marulanda, MD. (2), Juan Molano V., MD. (2), Renzo Pinto C., MD. (1), Mario H. Rey T., MD. (3), Daysi Rivera H., MD. (1)
(1) Fellow in Gastroenterology at the Universidad del Rosario and the Hospital Universitario de La Samaritana. Bogotá, Colombia.
(2) Associate Professor in the Department of Medicine at the Universidad Nacional de Colombia in Bogotá, Colombia.
(3) Gastroenterologist in the Gastroenterology Service of the Hospital Universitario de La Samaritana, Post Graduate student of Gastroenterology at the Universidad del Rosario. Bogotá, Colombia.
Received: 17-08-12 Accepted: 26-06-13
Abstract
Dysphagia is a common symptom in many neurological diseases, particularly occurring after a stroke or head trauma. Another important group of patients who develop swallowing dysfunctions are older adults with progressive loss of cognitive functions such as patients with Alzheimer's disease. This type of disease is accompanied by difficulty swallowing that has been associated with increased mortality due to respiratory complications that are potentially preventable if dysphagia is recognized early so that appropriate strategies for treatment can be used.
Keywords
Dysphagia, alzheimer's disease, swallowing dysfunction, esophagus.
CASE PRESENTATION
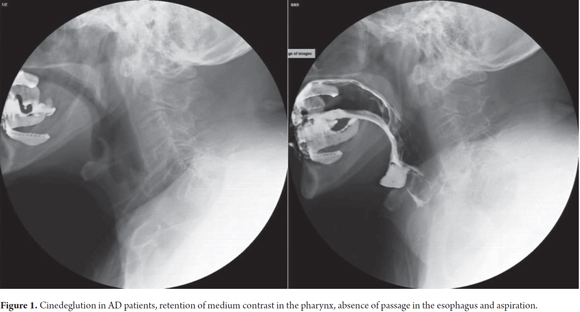
An 80 year old female with a clinical history of progressive memory loss over the previous 4 years was admitted to the hospital. Patient's ability to care for herself began to diminish 2 years after onset of memory loss and was accompanied first by progressive reduction in her ingestion of solid food followed by decreasing ingestion of liquids. The patient gradually lost weight and body mass and began to suffer muscle atrophy. One year later, the patient had obvious dysphagia, coughing and nasal regurgitation. Because of the changes in the patient´s swallowing, an esophageal motility test (cinedeglution) was performed which showed barium retention in the hypo pharynx and bronchial aspiration in contrast medium (Figure 1).
To ensure adequate nutrition of the patient as well as decreasing the risk of chronic aspiration an endoscopic gastrostomy was performed without complications.
DISCUSSION
The increase in life expectancy in the global population has generated an increasing occurrence of pathologies associated to this demographic phenomenon as well as increasing costs of health systems and the need to develop strategies to address and contain complications arising from this longevity and to adjust them according to the needs of the population.
The phenomenon of human aging goes hand in hand with the development of neurological diseases that not only impact the patient but also their families since they must make adjustments to their basic care based on the severity of the condition and the impact that this may have on each individual (1).
It is for this reason that there is an ongoing importance in the relation between degenerative neurological diseases, such as Alzheimer's disease, and gastrointestinal diseases especially in the upper digestive tract functions, as is the case of swallowing and motility disorders, that without a timely diagnosis and appropriate management can greatly increase the morbidity and mortality of patients.
In the USA it is estimated that 4.5 million Americans have this disease, of whom 200000 are under 65 years of age and it is projected that the current generation will contribute 10 million adults with this condition. The current prevalence is 11 million people and it will increase to 16 million over the next 20 years. Generating 17.4 billion working hours with costs of 200000 million dollars to chronic care homes. Furthermore it should be noted that one in seven elderly adults in this country do not have any type of caregiver (2).
In Colombia dementia disorders have a 1.8% prevalence in people over 65 years and 3.4% in those over 75 years. Results shown in an EPINEURO study reported that the highest prevalence is set in the southwestern and eastern regions of the country with 2.2% and 1.9%, these are significantly below those reported by countries in the same region with Brazil having the highest prevalence with 11% (3).
Life expectancy in 2030 will increase by approximately 30% with an estimated prevalence in the onset of swallowing disorders in elderly adults from 7% -22% . This will increase up to 40% - 50% in elderly adults living in chronic care homes (1).
Additionally, it has been reported that elderly adults held at chronic care homes have an incidence of up to 45% of presenting swallowing disorders, this increases as the sensory and motor functions worsen and this is added to those changes produced by the neuropathology de base (1).
Physiological assessment of the upper aero/digestive tract in its anatomical point of conjunction located in the mouth and pharynx has an important role in the coordination of functionality of each component in terms of breathing and swallowing for efficacy and safety (4). Coughing is an important marker of potential bronchoaspiration history (5). It is important to know that the basic movements involved in swallowing are a mosaic that involves four phases: oral preparatory, oral, pharyngeal and esophageal. These phases allow the bolus to be mixed with saliva, chewed, and moved to the pharynx where its consistency, size and taste dictate the length of the oral phase and start preparations for the next phase, the pharyngeal phase. In this phase the breath is stopped to allow passage of the bolus toward the pharynx and then make multiple biomechanical events seal the air duct allowing the passage of the bolus through the pharynx due to the activity of the pharyngeal constrictor muscle and moved through sequential pressure wave and passing through the esophagus the breathing immediately returns and movement into the stomach is produced by peristalstic movements given by esophageal circular and longitudinal muscles (1).
The events described above are commanded by a complex interaction between psychological, motor and sensory component that involve cranial nerves V - VII - IX - X - XI - XII, the cerebral cortex, cerebellum and the brain stem. These neurophysiological actions are integrally linked to macro phases of swallowing in which cortical and brainstem activities are integrated at the oral phase. These swallowing disorders fall under the term "presbyphagia", which is not often associated with the appearance of oropharyngeal dysphagia but it is associated with the use of drugs that compromise functionality of the central nervous system (4).
The structural changes involved may prevent progression of the bolus. The same changes in elderly adults compromises brain regions such as the promoter lateral cortex, insula, temporal parietal cortex, amygdala and cerebellum. This explains why patients who have these areas involved through vascular, degenerative, metabolic or traumatic factors will develop dysphagia. This occurs in 30% to 50% of elderly adults who suffer these structural conditions. It also occurs when the C1 or C2 cords are altered (5).
It is known that the range of duration of swallowing movements is similar to those of the muscles involved. The submental muscle below the chin has a reaction time of less than 740 milliseconds (ms), the rapid closure of the laryngeal vestibule takes less than 160 ms, and the quick opening of the upper esophageal sphincter takes less than 220 ms. Alterations in the previously mentioned areas of the central nervous system result in neurogenic dysphagia in which delayed responses are generated in the submental muscle which results in lengthened duration of the oropharyngeal swallowing response (OSR): It also results in a delay of the oropharyngeal reconfiguration phase between the airway and the digestive tract. Evidence in studies of patients with dysphagia of longer times of laryngeal vestibule closure (LVC) and quick upper esophageal sphincter opening (UESO) show that this time can double causing unsafe swallowing in healthy patients and bronchoaspiration in elderly patients with neurological disorders (5).
Videofluoroscopy evaluates the physiological closure length in normal older adults and the delay during food ingestion, and especially ingestion of liquids, which is evidenced in patients with degenerative changes in the central nervous system (6).
Every disorder that increases response times is conditioned by decreased sensations, decreased number of neurons and delays in the conduction of signals by the afferent neurons which become very satiated due to degenerative diseases of the central nervous system. The result is that transport of the bolus slows to a speed of about <10cm/second (7).
Despite anatomical, physiological and functional changes that can occur over time in humans, the first stage of functional compromise in healthy older adults is called presbyphagia. This consists of physiological changes related to the patient's age that do not endanger the safety of the airway or change the respiration rate (8).
These changes in the course of life are marked in the elderly. This is not only due to structural changes in the central nervous system but also to degenerative changes in the anatomical structures of the oral cavity, pharynx and larynx. These can include diminished masticatory muscles, lingual atrophy, fat infiltration, decreased diameter of lingual muscle fibers, atrophy of the thyroarytenoid muscle, slower contraction waves of the pharyngeal constrictor muscles and decreased upper esophageal sphincter tone. Some of the least studied alterations are related to reduce tactile perception of lips and tongue. These include decreased perception of viscosity, poor oral stereognosis and reduced perception of taste (9). Another condition that contributes to changes in the sensitivity of elderly adults is missing teeth that can be as severe as cases of anodontia. Replacing missing teeth with dental prosthetics increases current swallowing problems and leads to loss of sensation in the oral cavity.
COUGHING AS A MARKER FOR BRONCHIAL ASPIRATION
Aspiration may occur before, during or after swallowing without necessarily being associated with coughing. Silent aspiration (without coughing) presents risks of pneumonia for some patients that are four times greater than aspiration associated with coughing. For patients with greater oropharyngeal impairment this risk is ten times greater. Independent risk factors for aspiration are being male and over 65 years of age. The cough reflex is a tracheobronchial protector response to the presence of liquid or solid contents in the larynx. While the development of voluntary coughing not related to aspiration of food or liquid has a sensitivity of 55% to 70% and a specificity of 45% to 68%, the cough reflex is seen as a marker for aspiration with a sensitivity of 50% to 86% and a specificity of 50% - 91% (5). This generates the first approach to swallowing disorders in elderly adults whether they are caused by neurological impairments, drugs or associated conditions (11). Within this initial approach chest examinations must be included in order to determine whether there is any compromise of the pulmonary parenchyma. This is one of the important recommendations for patients with suspected silent aspiration (5).
IDENTIFYING DYSPHAGIA AND ITS RISKS
Community acquired pneumonia is a major cause of morbidity and mortality in elderly adults. Its risk increases in people over 60 years old and is five times higher in those over 75 years. This risk factor for hospitalization occurs in 33 out of every 1,000 elderly adults in chronic care homes, but only in 1.14 out of every 1,000 elderly adults with family caregivers (12). While the volume of aspiration is one risk factor for the development of pneumonia, there are other associated factors and indicators that the patient has dysphagia that influences the development of pulmonary aspiration (see Table 1).
Bacterial colonization of the pharynx due to swallowing disorders is critical in the development of pulmonary infections in elderly adults. Staphylococcus aureus, Klebsiella pneumoniae and Escherichia coli are the most common germs. To make matters worse, this group of patients tends to have poor oral hygiene and poor elimination of saliva (13). Changes in pulmonary immunity and local defense mechanisms significantly increase the risk of respiratory infections. In elderly adults these can include poor mucociliary clearance, decreased lung elasticity, decreased muscle strength and decreased functional capacity (12).
CHANGES RELATED TO DEMENTIA AND THE BEGGININGS OF DYSPHAGIA
Patients with dementia are more dependent on medical care givers which give them a higher risk of diseases, morbidity and mortality than experienced by adults that can feed themselves. This risk is directly proportional to the severity of neurological compromise and degree of dependence on others for feeding. Adequate nutrition techniques for patients with Alzheimer's disease (AD) can decrease the aspiration, weight loss and electrolyte disturbances.
The first changes to pay attention to in Alzheimer's patients are anosmia and changes in the patient's desire to eat the kinds of food that the patient usually consumes.(1,14). As the initial stages of the disease progress, these changes lead to decreased tongue movements, forgetting to eat, and "remembering" the act of eating even if the patient has not eaten. This combination is associated with the onset of dehydration, weight loss and high energy consumption due to the hyperkinesia (1-14).
NEURAL STUDIES
Teniendo objetivos fundamentales para la realización de estos estudios como la activación neuronal, niveles de oxigenación, durante las fases tempranas de la enfermedad de Alzheimer. En donde se evidencia respuestas tardías en la deglución en etapas donde no es evidente clínicamente, observándose en estudios que los niveles de oxigenación en áreas fundamentales para la deglución se afectan al comienzo de la enfermedad y que no logra compensación de la oxigenación, así como tampoco la activación de nuevas regiones circundantes (14).
Studies of the relations between subtle changes in swallowing and early changes occurring in the cerebral cortex which could help establish early treatment and support for this group are lacking even though imaging studies of the brain can identify changes relate to swallowing dysfunctions in these early stages of Alzheimer's disease. (15-16). Key objectives of such studies should be neuronal activation and oxygenation levels during the early stages of Alzheimer's disease. When evidence of delayed swallowing response exists in stages in which it has not become clinically evident, it has been observed that oxygenation levels in areas that are fundamental for swallowing are affected early in the disease and that, furthermore, neither compensation for these low levels of oxygenation nor activation of new surrounding regions occurs (14).
FROM THE INITIAL STAGES OF ALZHEIMER'S TO MODERATE AND SEVERE STAGES
The evolution of the disease in relation to development and adaptation of swallowing is important. Each individual's evolution develops in accordance to social and nutritional needs that include sensing the viscosity, consistency and volume of different types of food that allows a person to discriminate among these types of food. Disruption of these mechanisms results in the appearance of dysphagia and associated nutritional changes, social alterations and adaptation to these changes. The key role in the presentation of these changes in the ability to swallow is not played by aging but rather by the cognitive changes that lead to the development of the loss of independence and the ability to feed oneself independently. Limitations on the control of these risk factors are responsible for the limited development of strategies to compensate for them. These strategies include changes of posture and coordination between swallowing and breathing.
A study by Sheilla de Medeiros Correia et al. evaluated 50 patients with advanced stages of Alzheimer's disease (moderate CDR2 and severe CDR3) who also had swallowing disorders. Patients were classified with clinical dementia ratings (CDR) and cognitive exams which included mini-mental state examinations (MMSE) and testing of overall functionality based on indices of activities of daily life. At the same time patients' abilities to communicate were characterized with the Functional Outcome Questionnaire for Aphasia (FOQ-A). The study found that patients classified with severe Alzheimer's disease had greater deterioration in their swallowing rates than those classified with moderate Alzheimer's (4 = 22.2% in CDR2 and 13 = 40.6% in CDR3). Problems were more severe for ingestion of beverages (5 =27.8% in CDR2 and 23 = 71.9% in CDR3), delays in the swallowing process (12 = 37.5% in CDR3), and with certain foods (2 = 11.1% in CDR2 and 22= 68.8% in CDR3). Despite these results the best care provided for these patients was paradoxically found in the group that had moderate Alzheimer's disease even though they had recognized skills for taking care of themselves (17).
This shows how important it is for these patients to be under the care of well trained and qualified personnel who understand alterations in feeding and nutritional states. Fundamentally, this shows how important adequate evaluation, approach and management is for addressing AD patients in early stages in which intervention aimed at preventing future swallowing disorders should begin. This is based and supported by questionnaires that evaluate impairment of cognitive functioning in patients with manifestations of this disease. Once dysphagia has developed in an Alzheimer's patient, it becomes imperative to evaluate the care of the patient and the training of the personnel who are in charge of these elderly adults and who will characterize the progression and potential complications of the disease.
Patients with moderate stages of Alzheimer's disease are often distracted when eating and reject food because they simply forget what they are doing. Patients with more advanced stages of the disease are passive about eating and may eat too rapidly. This can be unintentionally stimulated by a caregiver who prompts the patient to eat without noticing that the patient has not yet swallowed resulting in the risk of choking. On the other hand, when a patient fails to open her or his mouth voluntarily, it can be interpreted as an absence of any desire to eat which in turn can lead to nutritional risks. In terminal stages of the disease this may manifest as spoon biting which is the emergence of primitive swallowing reflexes. In other less severe situations spoon biting is a technique to delay feeding because the patient has not yet swallowed. Food loss from the oral cavity is related to the degree of compromise of the muscles of the mouth and tongue and the severity of the disease. Similarly, changes in dentition and sensory abilities that start during the intermediate stage are reflected with greater severity in more advanced stages of the disease.
REFERENCES
1. Easterling CS, Robbins N. Dementia and Dysphagia. Geriatric Nursing 2008; 29(4): 275-285. [ Links ]
2. Alzheimer's Association. 2012 Alzheimer's disease facts and figures. Alzheimer's Association / Alzheimer's & Dementia 2012; 8: 131-168. [ Links ]
3. Ruiz de Sanchez C, Nariño D, Muñoz JF. Epidemiología y carga de la enfermedad de Alzheimer. Acta Neurológica 2010; 26: 3 87-94. [ Links ]
4. Forster A, Samaras D, Gold NA. Oropharyngeal dysphagia in older adults: A review. European Geriatric Medicine 2011; 2: 356-362. [ Links ]
5. Smith Hammond CA, Goldstein LB. Cough and Aspiration of Food and Liquids Due to Oral-Pharyngeal Dysphagia ACCP Evidence-Based Clinical Practice Guidelines. Chest 2006; 129: 154S-168S. [ Links ]
6. Rofes L, Arreola V, Almirall J, Cabre M, Campins L, García-Peris P, Speyer R, Clave P. Diagnosis and Management of Oropharyngeal Dysphagia and its Nutritional and Respiratory Complications in the Elderly. Gastroenterology Research and Practice 2011. [ Links ]
7. Robbins J, Hamilton JW, Lof GL, Kempster GB. Oropharyngeal swallowing in normal adults of different ages. Gastroenterology 1992; 103(3): 823-829. [ Links ]
8. Ianessa AH, Robbins JA. Dysphagia in the Elderly. Phys Med Rehabil Clin N Am 2008; 19(4): 853-865. [ Links ]
9. Pikus L, Levine MS, Yang YX, et al. Videofluoroscopic studies of swallowing dysfunction and the relative risk of pneumonia. AJR Am J Roentgenol 2003; 180: 1613-1616. [ Links ]
10. McCullough GH, Wertz RT, Rosenbek JC. Sensitivity and specificity of clinical/bedside examination signs for detecting aspiration in adults subsequent to stroke. J Commun Disord 2001; 34: 55-72. [ Links ]
11. Paul E. Marik, Kaplan D. Aspiration Pneumonia and Dysphagia in the Elderly. Chest 2003; 124: 328-336. [ Links ]
12. Palmer LB, Albulak K, Fields S, et al. Oral clearance and pathogenic oropharyngeal colonization in the elderly. Am J Respir Crit Care Med 2001; 164: 464-468. [ Links ]
13. Ianessa AH, McLaren DG, Kosmatka K, Fitzgerald M, Johnson, Porcar E, Kays S, Eno-Obong Umoh, JoAnne Robbins. Early deficits in cortical control of swallowing in Alzheimer's disease. J Alzheimers Dis 2010; 19(4): 1185-1197. [ Links ]
14. Priefer BA, Robbins J. Eating changes in mild-stage Alzheimer's disease: a pilot study. Dysphagia 1997; 12: 212-221. [ Links ]
15. Humbert IA, Fitzgerald ME, McLaren DG, Johnson S, Porcaro E, Kosmatka K, Hind J, Robbins J. Neurophysiology of swallowing: Effects of age and bolus type. Neuroimage 2009; 44: 982-991. [ Links ]
16. Sheilla de Medeiros Correia, Lilian Schafirovits Morillo, Wilson Jacob Filho, Leticia Lessa Mansur. Swallowing in moderate and severe phases of Alzheimer's disease. Arq Neuropsiquiatr 2010; 68(6): 855-861. [ Links ]











 text in
text in