Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.3 Bogotá July/Sept. 2013
Two Case Reports of Food Protein Induced Enterocolitis
Wilson Daza, MD. (1), Silvana Dadán, MD. (2), María Carolina Uribe, MD. (3)
(1) Pediatric Gastroenterologist, Master's Degree in Clinical Nutrition, Director of Gastronutriped and of the Pediatric Gastroenterology Postgraduate Program at the Universidad El Bosque in Bogotá, Colombia.
(2) Clinical Nutritionist, Master's Degree in Clinical Nutrition, Assistant Professor in the Postgraduate Programs in Pediatrics and Pediatric Gastroenterology at the Universidad El Bosque in Bogotá, Colombia.
(3) Fellow in Pediatric Gastroenterology at the Universidad El Bosque in Bogotá, Colombia.
Received: 29-01-13 Accepted: 26-06-13
Abstract
Two case reports of patients with neonatal enterocolitis present the topic of Food Protein-Induced Enterocolitis Syndrome (FPIES). FPIES is a type of food allergy which is not mediated by IgE and which has a severe presentation. Its incidence and prevalence are unknown. Clinical suspicion, diagnosis, and timely management are important in light of likely development of severe complications which can even lead to death.
Key words
Food Protein-Induced Enterocolitis Syndrome (FPIES), oral challenge, skin prick test, food allergy, cow's milk protein allergy, introduction of solid food, breast feeding.
INTRODUCTION
In recent years, allergies have been flourishing, and allergies to diet proteins are no exception. In fact, more than 5% of children develop allergies to milk proteins or other kinds of protein (Walker-Smith, Murch, Wood). There are geographic differences in incidences of allergies to particular food allergens from one country to another. This occurs partly because of genetic variations in immune responses according to ethnic group and partly because of different dietary customs that condition different expressions of this pathology in different parts of the world.
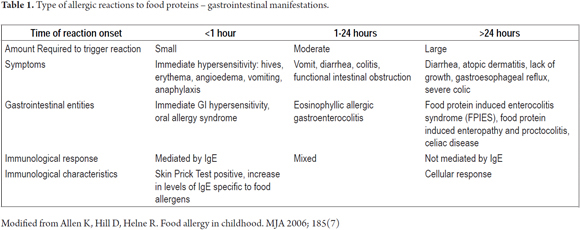
There are three kinds of reactions that mediate the physiopathological mechanisms and explain the development of a food allergy: reactions mediated by immunoglobulin E (IgE) or immediate hyper sensibility, reactions not mediated by IgE, and late and/or mixed responses which involve both to the first two types (Table 1). It is fundamental to understand this range of manifestations and mediators to be able to make a timely diagnosis considering that routine tests for allergies such as skin prick tests and blood tests for IgE may have negative results.
In this article we present two cases of Food Protein Induced Enterocolitis Syndrome (FPIES) which illustrate how allergic illnesses can be confused with common presentations of other pediatric illnesses. Within this context the temporal relation between symptomatology and food exposure provides a key guide to differential diagnosis.
Case 1
The patient was an 8 month old boy referred by pediatric surgery due to necrotizing enterocolitis (NEC). The product of a first controlled pregnancy, the child was born prematurely at 37 weeks, with a birth weight of 2,500 grams and a length of 51 cm. The mother delivered with prolonged membrane rupture. This plus baby's low birth weight led to hospitalization in the newborn unit. Baby was exclusively breastfed for 8 days followed by mixed breastfeeding with beginning formula which continued until the sixth month. During neonatal hospitalization the patient deteriorated clinically with presentation of vomiting, abdominal distension and bloody stools. An abdominal x-ray revealed intestinal perforation which led to a diagnosis of NEC III B. Patient required surgery which revealed a "cardboard colon" which required a colostomy. Biopsies taken because of suspected aganglionosis ruled out Hirschprung disease. Nevertheless, lymphoid hyperplastic follicles, lymphoplasmacytic infiltrate, mild chronic inflammation, active chronic colitis and augmented eosinophil count were found. During follow-up a barium enema showed no evidence of transition zones. The pediatric surgeon scheduled closing of the colostomy when the child was 5 months old. On the second day after surgery the patient's condition deteriorated and the patient presented colonic perforation and peritonitis. This time a biopsy revealed moderately active chronic focal colitis with ulceration. Weaning of the patient from milk began at 6 months old with the introduction of cereals. At 7 months unmodified cow's milk , fish, soy milk and peanuts (in chocolate bar form) were introduced. Due to the patient's history which included two events of enterocolitis; histopathology showing follicular hyperplasia, inflammatory infiltration and eosinophilia; finding of colitis during extra-institutional colonoscopy, a radioallergosorbent test (RAST) which was Class I positive for egg whites, premature birth, mixed milk feeding, weaning with inclusion of food allergens, bronchiolitis, two important episodes of diarrhea and persistence of chronic stool alterations (fetid Bristol Stool Scale Type 6 and rectal bleeding despite colostomy) the patient was diagnosed as having FPIES. Feeding with semi-elemental formula was begun and cow's milk and egg were removed from the patient's diet. With this new treatment the patient evolved favorably. Stool frequency diminished, stools reached normal consistency, and patient gained weight.
Case 2
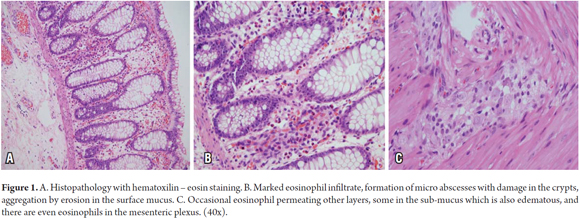
A one month old male patient was referred pediatric surgery following a colostomy. The child, the product of a first controlled pregnancy, was born vaginally, had a birth weight of 3090 grams and was 52 cm long. The child's first bowel movement occurred 48 hours after birth. On his third day of life, after one feeding of beginner's formula, the patient began to progressively deteriorate and presented bilious vomiting, scarce depositions, abdominal distension, lethargy and respiratory difficulty. The patient was taken to the emergency room where enterocolitis was suspected. An intestinal obstruction was found which required sigmoidectomy and colostomy. The biopsy report showed normal innervation, eosinophil infiltrate, formation of micro-abscesses, erosion of the mucosa, sub-mucosal edema presence of eosinophils in the mesenteric plexus (Figure 1). Paraclinical tests found peripheral eosinophilia (991 absolute recount), occult blood in feces, and 8-10 erythrocytes in each field of stool. Cystic fibrosis was ruled out. An anthropometric evaluation revealed protein- calorie malnourishment, and a physical examination showed generalized eczema with erythematous plaques in the lateral left region of the neck. FPIES was suspected. Since the child was being fed both breast milk and formula, the mother was told to restrict cow's milk, soy, dry fruit and seeds from her diet. The boy was placed on an, elimination diet plus breast milk and a complementary extensively hydrolyzed formula. At the age of 6 months complementary foods were added to the child's diet which resulted in serious vomiting following ingestion of avocado and papaya. The child was taken to the emergency room and the diet was temporarily suspended. Except for this episode, the boy's clinical evolution has been good.
DISCUSSION
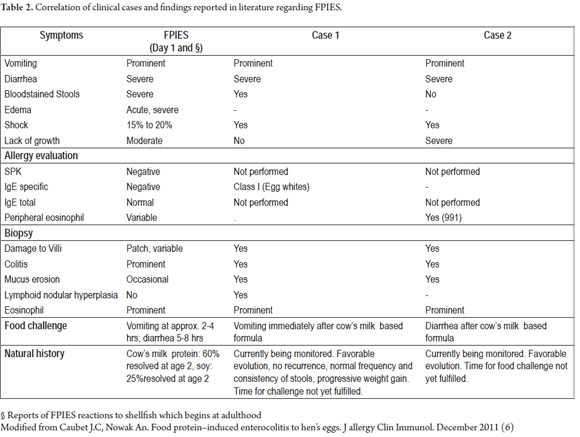
Food Protein Induced Enterocolitis Syndrome (FPIES) is a hypersensitive response which is not mediated by immunoglobulin E. It is characterized by gastrointestinal symptoms and systemic inflammatory responses and occurs in children (Table 2) (1).
This syndrome was first described by Rubin in a patient with severe bloody diarrhea which was resolved by eliminating cow's milk from the patient's diet. In 1960 Ikol referred to severe gastrointestinal reactions in response to rice and wheat intake which were consistent with FPIES. In 1967 Gryboski presented 21 children with chronic gastrointestinal allergies to milk which manifested as diarrhea (15/21), vomiting (5/21), vomiting and diarrhea (1/21) and colic (2/21). The symptoms improved when proteins causing the symptoms were eliminated from the diet and recurred when they were reintroduced. Later, Powell characterized this condition as enterocolitis induced by cow's milk and soy protein. Twenty years later, Sicherer et al. described 22 other patients who reacted to milk or soy: 31% reacted to both (4).
Since these initial descriptions, additional reports have surfaced. However, ignorance on the entity persists and it is often undiagnosed or misdiagnosed. The lack of diagnostic tests combined with atypical presentations result in improper or late diagnosis of FPIES (1).
Generally, FPIES presents in breastfed babies less than 9 months old with gastrointestinal symptoms like repeated vomiting, diarrhea or both within 24 hours of protein ingestion and without symptoms mediated by IgE like skin rashes and/or respiratory symptoms. After test elimination of cow's milk from a patient's diet, the symptoms disappear (5).
In a cohort of Israeli newborns, Katz et al. showed a 0.34% cumulative incidence of FPIES secondary to cow's milk proteins at 1 year of age. The same population has a 0.5% incidence of allergies mediated by IgE. In 65% of their cases the average age of onset was 30 days. For 16% of their patients, symptoms appeared 4 days after first exposure to cow's milk proteins. Symptoms appeared 2 to 4 weeks after exposure in the other patients who developed FPIES. Nevertheless, the scarcity of studies does not allow us to extrapolate the data to the general population.
Some research shows that FPIES is more predominant among male patients who account for 52% to 60% of the cases in those studies. The two cases presented in this article are both boys. Between 30% and 60% of those with FPIES have atopic illnesses such as dermatitis, asthma, allergic rhinitis, food allergies mediated by IgE, eosinophil esophagitis and hypersensitivity to drugs. About 50% to 80% of these patients have family histories of atopy while 20% have family histories of food allergies mediated by IgE. In addition, typical presentation of FPIES has been described in identical twins (1).
Common causal agents are the proteins in cow's milk, soy and rice. Allergies triggered by cow's milk and soy are typically diagnosed between 1 and 3 months of age. Clinical presentation may be delayed among children who are exclusively breastfed. FPIES induced by food is typically diagnosed at later ages, approximately between 4 and 8 months, in relation to the time of introduction of solids. Average age for development of FPIES is 5.5 months.
Other foods that have been correlated with FPIES are cereals like oats, barley and wheat, poultry including turkey and chicken, legumes including peas and lentils, beans, peanuts, sweet potatoes, pumpkins, egg whites, some fruit, potatoes, lamb and fish.
In a study published by Nowak-Wegrzyn et. al., 80% of children with FPIES reacted to more than one food and 65% had previous diagnoses of FPIES to milk and soy proteins. There are few reported cases of FPIES related to wheat even though this grain has historically been considered potentially allergenic because of its late introduction and has been considered more of a trigger for development of FPIES than a window of immunologic susceptibility (1,6).
Even though rice is considered a hypoallergenic food, some authors have described FPIES secondary to rice ingestion as one of the most severe forms of presentation with multiple episodes, frequent hospitalization, extensive evaluations, and late diagnosis.
Breastfeeding provides several mechanisms which protect against FPIES. These include especially IgA type antibodies and transfer of partially processed proteins to the breast feeding child. In addition, the amount of proteins transferred through milk can be less than the amount needed to trigger symptoms. Nevertheless, there are some cases of FPIES related to breast feeding. For this reason foods suspected of being FPIES triggers must be removed from the mother's diet if associated reactions occur.
Although the physiopathology of FPIES is poorly understood, it is believed that there are specific antigens against T cells, humoral responses to specific antibodies, and inflammatory cytokines that modify the permeability of the intestinal barrier involved. Endoscopic findings of friable mucosa, diffuse colitis, varied ileal compromises, rectal ulceration and bleeding are considered to be local inflammatory responses to ingestion of antigens. The resulting increased intestinal permeability and changes in fluids lead to vomiting, diarrhea, dehydration and lethargy (3).
Some studies have shown increased numbers of receptors for Tumor Necrosis Factor (TNF alpha) and reduction of transforming growth factor Beta (TGF-b) in the intestinal mucosa in patients with FPIES. Interleukin 17 has also been implicated. Nevertheless there are too few studies to prove its role in pathogenesis (2).
Symptoms include profuse vomiting and diarrhea beginning one to three hours after ingestion of the causative agent and which results in dehydration, hypotension and shock, hypothermia, pallor and lethargy (3).
Older children and adults present severe nausea, abdominal pain and vomiting after ingestion of food, especially fish and shellfish. Cases of FPIES have also been reported in adults (1).
Presentations of acute and chronic forms differ. When the triggering food is part of the regular diet, FPIES presents chronic symptoms as in the two cases presented above. Nevertheless, there are reports of severe cases in the first days of life. Chronic symptoms are solved in three to ten days after elimination of the causal agent. Acute manifestations occur when the triggering food is taken intermittently or after long periods of abstinence. Symptoms occur hours after ingestion and have even been reported at first exposure to a food (1).
Differential diagnosis of emergency case signs and symptoms must consider sepsis and viral gastroenteritis and may even require evaluation by the surgical group. Tests should include blood analysis, radiology, barium enemas and endoscopy. Frequently treatment requires intravenous rehydration and antibiotics because of presumed sepsis. In some cases patients are even treated surgically (1).
Manifestations of FPIES overlap other reactions of gastrointestinal hypersensitivity, not mediated by IgE. These include proctocolitis, food protein induced enteropathies and other eosinophillic gastrointestinal disorders (Table 1).
Although diagnostic criteria for FPIES have not been well established, those which have been proposed include age less than 9 months at the time of diagnosis, repeated exposure to food causing gastrointestinal symptoms without other justifiable cause, absence of symptoms that suggest a reaction mediated by IgE, resolution of symptoms after elimination of triggering food, and reappearance of symptoms within 4 hours (average) following re-exposure or oral challenge (1). All of these coincide with our observations in the cases presented.
Although vomiting is the most common symptom, diarrhea appears in about one of these patients and 35% develop metahemoglobinemia in which children have pale grey appearances. Metahemoglobinemia has been described in cases with severe reactions which may require treatment with bicarbonate. Although the cause is unknown, it has been proposed that intestinal inflammation leads to diminished catalytic activity and increased nitrates. In 75% of these cases the child looks ill, and in 15% to 20% the child develops hypertension and shock that require hospitalization. 50% require intravenous liquids while a few can be rehydrated orally (1).
Although not necessary for diagnosis, laboratory findings include increases in the density of neutrophils above 3,500/mm3 five to eight hours after the oral challenge begins.
There is a controversy regarding performance of the oral challenge. While some consider it necessary for confirmation of the diagnosis and resolution of FPIES, others suggest that diagnosis should be based on clinical criteria because they consider the oral challenge to be a high risk procedure since 50% of these patients require intravenous hydration. Preferred diagnosis is according to clinical criteria.
Some investigations report that hypoalbuminemia and weight gains of less than 10gr/day are predictive factors for FPIES when the patient has also ingested cow's milk protein. Patch testing has shown itself useful for tracking and predicting outcomes, although its results have yet to be validated. Many children with FPIES have negative Skin Prick Tests (SPT) for IgE and undetectable levels of specific IgE for food allergens at the time of diagnosis. Sicherer et. al. observed that children with undetectable levels of IgE have more complicated paths of development and are at risk of developing immediate symptoms mediated by IgE (1).
Other studies have demonstrated increased numbers of leukocytes in the gastric juice, but their utility must be validated with more studies (1).
Biopsies usually reveal atrophy of intestinal villi, tissue edema, cryptic abscesses, increased numbers of lymphocytes, eosinophils, mast cells and increased amounts of immunoglobulin M and A (1).
FPIES is frequently underdiagnosed. Differential diagnosis must consider acute forms of anaphylaxis, sepsis, gastrointestinal infections, metabolic disorders and surgical emergencies (5,6).
Children with bloody stools or occult bleeding require differential diagnosis that also considers common conditions like anal fissures, infectious colitis and nodular lymphoid hyperplasia. Some less common conditions must also be taken into account. They include necrotizing enterocolitis, intestinal intussusception, Henoch-Shonlein purpura, familiar Mediterranean fever, Meckle's diverticula, pancreatitis, entorocolitis secondary to aganglionosis in Hirschsprung disease, amoebic colitis and intestinal inflammatory illness. A true challenge lies in differentiating FPIES from other food protein induced gastrointestinal disorders including proctocolitis, enteropathy, and eosinophyllic gastroenteropathy. This is especially true because their severity and persistence can vary (6).
The most important step in management of FPIES is avoidance of contact and consumption of triggering food proteins. For children who are allergic to cow's milk proteins, a formula based on casein hydrolyte or serum must be used. It must also be kept in mind usage of soy-based formula is not recommended for this kind of patients since up to 60% of these children may present cross reactions or have concomitant allergies to soy. About 20% of these children require elemental formula (1).
Treatment of acute forms includes intravenous fluids and steroids (single doses); epinephrine in case of hypertension with no response to liquids. When levels of specific Immunoglobulin E for food proteins are involved, epinephrine and antihistamines may relieve the IgE mediated symptoms.
Because acute episodes of FPIES are not normally recognized as such, it is fundamental for family and/or patients to inform medical staff upon their arrival to the ER.
Once a diagnosis has been made prior to weaning a child from breast milk, it is advisable to avoid cow's milk and soy proteins during the first year of life and before the resolution of the entity. In addition, care must be taken with the choice and introduction of cereals and legumes since they are the allergens most commonly associated with this pathology.
These are empirical recommendations since there are no controlled studies that determine the optimal time for introduction of these foods to children with FPIES (1).
The food challenge is performed within 12 to 18 months after the FPIES reaction or according to the type of allergen and the time established for reintroducing the food into the diet. It must be accompanied by monitoring of the patient to determine if immunological tolerance has been established (6).
Some studies report that by 3 years of age 60% of FPIES cases secondary to milk, 27% of cases secondary to soy, 40% of cases secondary to rice and 67% of cases secondary to vegetables have resolved themselves. Other studies report higher resolution rates (1).
Type IgE specific antibody levels, when positive to diagnosis, must be checked during monitoring and before administering the oral challenge.
CONCLUSIONS
Because prevalence of FPIES is relatively high pediatricians must be alert and consider this condition within their differential diagnoses to avoid unnecessary hospitalization and/or overtreatment. FPIES must be suspected in lactating babies with risk factors for food allergies who are vomiting or have diarrhea in order to avoid complications such as hypotension, dehydration, digestive bleeding, shock and even death.
REFERENCES
1. Leonarda S, Nowak-Wegrzy. Clinical diagnosis and management of food protein induced enterocolitis síndrome. Wolters Kluwer Health 2012; 24: 1040-8703. [ Links ]
2. Shoda T, Hashimoto K, Morita. Elevation of Serum IL-17 Levels was demonstrated after Oral Food Challenge in Infants with Food Protein-Induced Enterocolitis Syndrome. J Allergy Clin Immunol 2012; 127: 2. [ Links ]
3. Katz Y, Rajuan N, Goldberg M, Cohen A. The Incidence, Manifestations and Natural Course of Food Protein Induced Enterocolitis Syndrome (FPIES). J Allergy Clin Immunol 2012; 125: 2. [ Links ]
4. Morita H, Nomura I, Matsuda A. Food protein-specific lymphocyte proliferation assay for the diagnosis of Food Protein-Induced Enterocolitis Syndrome. J Allergy Clin Immunol 2010. [ Links ]
5. Katz Y, Goldberg M, Rajuan N. The prevalence and natural course of food protein–induced enterocolitis syndrome to cow's milk: A large-scale, prospective population-based study. J Allergy Clin Immunol 2011; 127: 647-53. [ Links ]
6. Caubet JC, Nowak AN. Food protein-induced enterocolitis to hen's egg. J allergy Clin Immunol. December 2011. [ Links ]
7. Allen K, Hill D, Helne R. Food allergy in childhood. MJA 2006; 185(7). [ Links ]











 text in
text in