Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.4 Bogotá oct./dic. 2013
Clinical experience with colon capsule endoscopy (PillCam© Colon) in the study of colonic pathologies at the Clínica Universitaria San Juan de Dios in Cartagena
Fernando García del Risco, MD. (1), Elizabeth Arrieta López, MD. (2)
(1) Gastroenterologist, Associate Professor of Gastroenterology at the Universidad de Cartagena in Cartegena, Colombia and at the University of Paris VII in Paris, France. Chief of Gastroenterology at the Clínica Universitaria San Juan de Dios in Cartagena, Colombia.
(2) Second year Internal Medicine Resident at the Universidad del Sinú in the Cartagena section, Student in the Master's Degree Program in Clinical Epidemiology at the National University of Colombia in the Clínica Universitaria San Juan de Dios in Cartagena, Colombia.
Received: 14-03-13 Accepted: 27-08-13
Abstract
Introduction: In October 2006 the PillCam© Colon colonic capsule was used for the first time ever for noninvasive colon. Recent studies have shown that is diagnostic performance is good in comparison with conventional colonoscopy for the study of colonic pathologies.
Objective: The objective of this study was to compare the diagnostic yield of PillCam© Colon in detecting colonic lesions with the yield of conventional colonoscopy for patients with symptoms in the mid and lower intestinal regions in a hospital in Cartagena.
Materials and methods: This was a prospective observational study of diagnostic tests which assessed the validity (sensitivity, specificity and predictive values) of the PillCam© Colon and compared them with colonoscopy for the detection of colonic lesions in a cohort of patients at the University Clínica Universitaria San Juan de Dios in Cartagena. Patients had symptoms in the mid and lower intestinal regions. Patients were examined from June 2011 to January 2013. Analyses were performed using STATA 11.0 software.
Results: We studied 25 patients: 14 women (56 %) and 11 men (44 %). All had previously undergone colonoscopies. Panendoscopies were performed on these patients using an activated PillCam© Colon. We achieved complete colon examinations in 76% (19 patients). Colonic transit time was 2.4 hours (SD ± 1.3 hours) and oral-anal transit time was 6.2 hours (SD ± 1.18). 78.9% of the findings of the PillCam© Colon and colonoscopy agreed. The PillCam© Colon showed lesions not seen in colonoscopy in 29.2 % of patients. Its sensitivity for detecting colonic lesions was 96 % (95% CI 54.1-100) while its specificity was 46.2 % (95% CI 19.2 -74.9). The area under the ROC curve was 0.731 (95% CI .59 to .87).
Conclusion: PillCam© Colon is a highly sensitive and reliable non-invasive method for detection of lesions in the colon. It may be useful in clinical practice for early detection of colorectal cancer in high risk groups and in cases that colonoscopy is incomplete or contraindicated.
Keywords
Colon capsule endoscopy, colonoscopy, diverticula, angiodysplasia, polyps.
INTRODUCTION
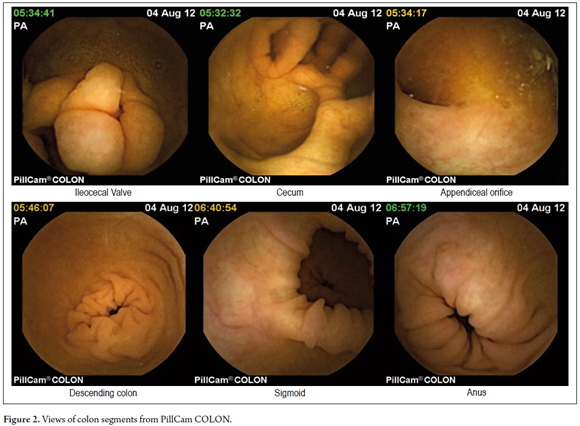
The PillCam ® COLON capsule endoscopy was developed by Given Imaging (Yoqneam, Israel), and was first used in 2006. (2) Given Imaging is the same company that invented the small bowel capsule. (1) The capsule is 31.5 mm long and 11.6 mms in diameter. It has 2 cameras, one at each end, and takes up to 4 images per second. Features include automatic control of light, enhanced optics, greater depth of field and 10 hour battery life. The capsule is activated for 3 minutes following ingestion and then goes into hibernation for 1 hour and 45 minutes to allow passage through the stomach without taking pictures until it enters the colon. The capsule is a disposable device which is expelled from the body by natural means. The duration of each study lasts from 8 to 10 hours and requires strict colon preparation for proper viewing. (3,4) In September 2009, the second generation of PillCam ® COLON was licensed in Europe. (5)
The main tasks of the capsule include early detection of colonic polyps in high-risk patients who are in programs for colorectal cancer screening, (4-8), evaluation of the colons of patients who refuse colonoscopy or for whom it is incomplete, (9) and detecting other colonic lesions such as diverticular disease and ulcerative colitis. (4)
To date no other studies have been performed in Colombia that demonstrate the validity of the PillCam ® COLON for the study of colonic disease in patients with middle and lower digestive tract symptoms. We conducted this study to compare the clinical utility and validity of noninvasive colon exploration using PillCam ® COLON for detection of colonic lesions with conventional colonoscopy which is an invasive process.
MATERIALS AND METHODS
We performed a prospective study of diagnostic tests which assessed the validity of the PillCam ® COLON for detection of colonic lesions in a cohort of patients at the Clinica San Juan de Dios of Cartagena from June 1 2011 to January 31 2013. All patients had mid and low intestine symptoms. The study's research protocol was approved by the ethics committee of the University of Cartagena. Adult patients who were selected for the study also had to meet other requirements such as capsule endoscopy because of obscure gastrointestinal bleeding, recurrent abdominal pain, colonic polyposis and abdominal tumor. All patients studied underwent panendoscopy using PillCam ® COLON to detect lesions in the small intestine and colon. Panendoscopy consisted of swallowing the capsule an hour and 45 minutes after it had been activated to allow uninterrupted exploration of the small intestine and colon. Prokinetic agents and capsule impellers were used to guarantee expulsion of the capsule before the 10 hour duration of the battery.
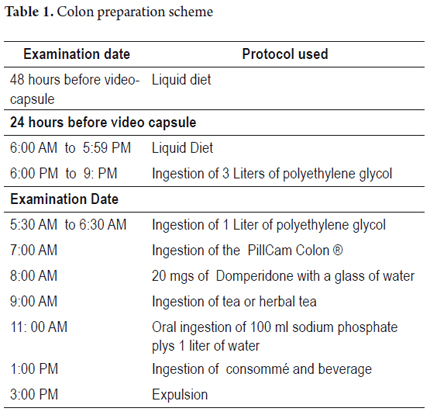
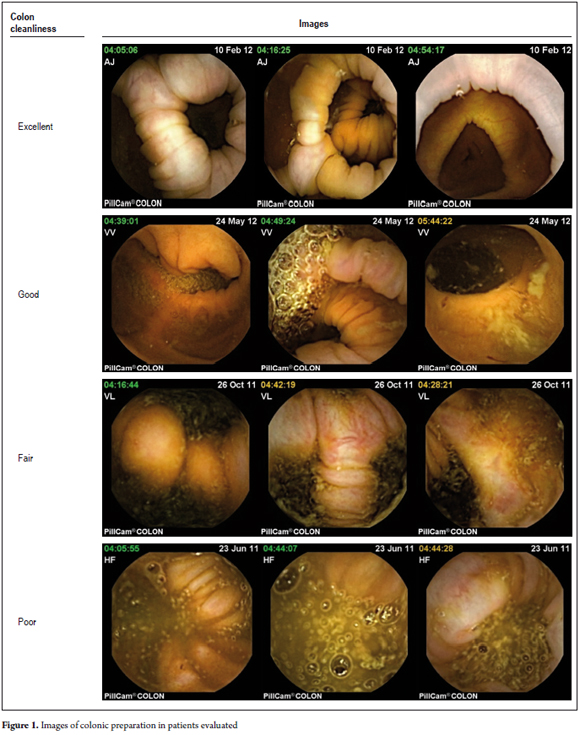
We excluded patients who were pregnant, patients who had impassable esophageal strictures, and patients who could not undergo an MRI during the study. All patients signed informed consent forms before inclusion in the study. Each patient answered a structured questionnaire before and after the procedure. Questions covered socio-demographic data, symptoms, study indications, established diagnoses, tolerance to procedures and adverse events. Results of prior colonoscopies were not known by the gastroenterologist who evaluated the capsule images. Bowel preparation for all patients was according to the European protocol for capsule colonoscopy (see Table 1). (10) The duration of each procedure was 8 hours. Each patient had eight sensors placed on the abdominal wall which were connected to a data recorder worn on a belt during the study. After the capsule was synchronized with the data recorder, the capsule was swallowed with half a glass of water. The data recorder was connected to a computer, and then images were downloaded and processed at the workstation. The resulting video of 58,000 images was studied extensively by a trained gastroenterologist. The results of the study were detailed in a written report that evaluated the quality of colon preparation in accordance with the ratings of Leyton (11) as excellent, good, fair or poor (See Figure 1). All information collected was recorded in a database to which were appended pictures and videos for further analysis. The colon examination was defined as complete when the capsule recorded all segments of the colon until anal expulsion. Exploration was defined as incomplete when the capsule did not record a colon segment because the battery died prior to removal or because the capsule was retained in some segment of the colon before expulsion.
Statistical analysis
We performed a descriptive analysis for each variable. We calculated the mean and standard deviations (± SD) for continuous variables and the median with interquartile range (IQR) for non-normally distributed variables. Categorical variables were grouped into absolute and relative frequencies and percentages were measured to describe them. Comparison of the validity of capsule colonoscopy with colonoscopy, the gold standard for studying colonic disease, was done through analyzing the results of complete studies of colon in which the capsule was expelled anally within 8 hours. We calculated the sensitivity, specificity and predictive values with 95% confidence intervals for each indicator. We evaluated the discriminatory power of the test by calculating the area under the operator-receiver curve (ROC analysis) based on the data's sensitivity and specificity. Values of the area under the curve that were close to 1.0 were considered to be adequate. The results were recorded in an Excel (Microsoft Office 2010) database of and analyzed using STATA 11.0 (Statistical Software: Release 11. College Station, TX: Stata Corp LP).
RESULTS
We studied 25 patients: 14 women (56%) and 11 men (44%). Mean patient age was 54.9 years (SD ± 17.59). The most common reason for consultation by the patients studied was melena which was present in 52% of the patients (see Table 2). The indications for the study for 22 patients were visible or occult bleeding. Indications varied for the other three patients: one was a post-treatment follow-up for a patient who had had lymphoma, one was a case of colonic polyposis, and one was a case of abdominal pain of unknown origin.
Proper preparation of the colon allowed adequate exploration in 88% of cases (Figures 1 and 2). The colon examination was complete in 76% of cases (n = 19) and incomplete in 24%. One of the incomplete examinations was complete until the right colon (4%, n = 1) and five were complete until the sigmoid colon (20%, n = 5) (See Table 2).
The average colonic transit time in patients with complete capsule examination was 2.4 hours (SD ± 1.3). Average oral-anal transit time (OATT) was 6.2 hours (SD ± 1.18). In the 6 patients with incomplete scanning of the colon, the capsule stayed in the colon for more than 8 hours. The two patients for whom exploration was complete only to the right colon expelled the capsules 10 hours after completion of the procedure. The four patients for whom exploration was complete only to the sigmoid colon took up to 20 hours (SD ± 3). Two of these four patients had histories of diabetes and the other two had severe constipation.
Toleration of preparation and procedure
Preparation tolerance was good in 76% of the cases. Six patients (24%) reported nausea during ingestion of the first 3 liters of PEG. Four patients (16%) were not able to completely consume 4 liters of PEG. The tolerance to the procedure was excellent in 100% of patients. No patient reported any complaints during ingestion or expulsion of the capsule. There were no complications during any of the procedures.
Findings from colon capsules compared with conventional colonoscopy
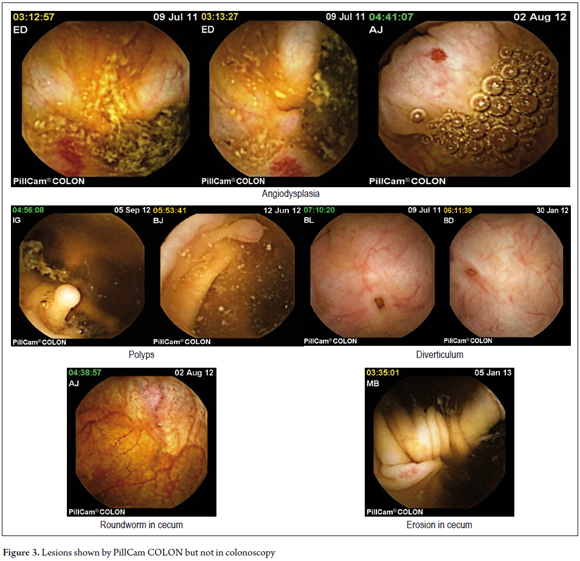
Only the 19 patients whose colon examinations were complete were included in the study. Capsule findings were abnormal in 68% of cases while colonoscopic findings were abnormal for 40% of the cases. The two studies showed similar lesions in 15 cases (78.94%). The capsule found lesions not seen in the previous colonoscopies in seven cases (29.2%) including three cases of angiodysplasia, two in the right colon and one in the left colon, two cases of small diverticula in the right colon, one case of a small polyp in the right colon, and one case of round worm (Trichuris trichiura) with erosion in the cecum (See Figure 3).
Validity of diagnoses with Colon Capsule vs. conventional colonoscopy
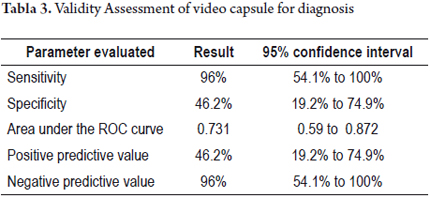
For the analysis of the validity of the capsule diagnoses, we included only 19 cases (76%) with full scans of the colon to the anus. The sensitivity of the capsule for detection of lesions was 96% (95% CI: 54.1to 100) with a specificity of 46.2% (95% CI: 19.2 to 74.9). The area under the ROC curve was 0.731 (95% CI: 0.59 to 0.87). The positive predictive value (PPV) was 46.2% and the negative predictive value (NPV) of 96% (See Table 3).
DISCUSSION
Conventional colonoscopy is a painful and invasive procedure which requires sedation. Patients tolerate the procedure poorly and often do not accept it. (12, 13) A complete scan from the colon to the cecum is not always achieved and is considered difficult in up to 20% of cases. (12) These limitations have prompted the search for less invasive techniques such as virtual colonoscopy and capsule colonoscopy. (13, 14) The capsule allows exploration of the colon in an easy and painless way even in difficult cases. In addition, it avoids exposing patients to the risk of repeated colonoscopy. (15)
Medical preparation
Although patients who ingested the capsule went on a diet very similar to the one used to prepare for standard colonoscopies, (8) preparing for the capsule study is more rigorous because its sensitivity and specificity depends on proper preparation. (16) Patients must ingest 4 liters of polyethylene glycol (PEG) in fragmented doses and 20 mgs of a prokinetic such as domperidone. Four hours after the study begins, they must also ingest substances like oral sodium phosphate to impel the capsule. (12) The colon preparations that have had the best results are two different schemes which use PEG. One is the "3 +1" scheme in which 3 liters of PEG are ingested the day before the procedure and 1 additional liter is ingested the day of the study. The other is the "2 +2" scheme in which 2 liters of PEG are ingested the day before the procedure and 2 additional liters are ingested the day of the study. (17, 18) We used the first scheme with good results (See Table 1).
A multicenter prospective study conducted by R Eliakim and colleagues which compared second-generation capsules with colonoscopy found that the medical preparation was considered excellent or good in 72% to 88% of cases. In our study, the preparation was excellent or good in 88% of cases. A very similar result was reported by Ladas and colleagues who considered the preparation excellent or good in 65% to 85% of cases. (10) Other studies, such as the one conducted by Spada and colleagues which had a protocol similar to that of Ladas, have found preparation adequate only in 42.5% of cases. (17)
Expulsion of the capsule
Using a protocol similar to ours, Spada and Eliakim reported expulsion of capsules in 69% to 84% of cases within 6 to 8 hours, (2, 17) and in 92.8% of cases at 10 hours. (5) In our study the rate of capsule expulsion was 87.5% before 8 hours. In Spada's multicenter clinical trial, they reported average colonic transit time of 2.17 ± 1.43 hours (18) which is very similar to our report of 2.4 hours (SD ± 1.3 hours).
Lesion detection and assessment of the validity of the study with CEC
In our study, the capsule showed lesions not seen in previous colonoscopy in six cases (29.2%). These lesions included three cases of angiodysplasia, three small diverticula, one polyp smaller than 6 mm, and one case of roundworm and erosion in the cecum. These findings are similar to those reported by Schoofs and colleagues in a series of 41 patients. In that study capsule colonoscopy detected seven lesions that were not seen in standard colonoscopies (6). Twenty (24%) out of the 84 patients evaluated by Eliakim were found to have polyps of 6 mm in diameter or more. Eighty percent of the patients with three or more polyps of any size were identified by standard colonoscopy, whereas 70% of these cases were identified by capsule. The rate of false identifications was considered to be insignificant. (2)
The validity of the PillCam ® COLON capsule for detection of colonic lesions has been studied by several groups around the world. Schoofs (6) reported that the overall sensitivity of PillCam ® COLON capsules for detecting significant lesions was 77%, that its specificity was 70%, that its positive predictive value was 59%, and that its negative predictive value was 84%. In 2009 Eliakim reported a sensitivity of 89% for second-generation PillCam ® COLON capsules for detection of polyps greater than or equal to 6 mm (95% CI: 70 to 97) with specificity of 76% (95% CI 72 to 78). The same study found 88% sensitivity for detection of polyps greater or equal to 10 mm (95% CI: 56 to 98) and 89% specificity (95% CI: 86 to 90). (5) In 2011 Spada, found a sensitivity of 63% and a specificity of 87% for detection of polyps ≥ 6 mm. (17) Siageap's study reported sensitivity from 63% to 88% and with specificity from 64% to 94% for the detection of polyps greater than 6 mm in diameter. (13). In our study the sensitivity was 96% (95% CI: 54.1 to 100) with a lower specificity of 46.2% (95% CI: 19.2 to 74.9) for detecting polyps and other lesions
One hundred percent of the patients in our study tolerated the procedure which was an excellent result. No patient reported any complaints during ingestion or expulsion of the capsule. We had no complications during the procedure. In a multicenter study of 582 patients, Eliakim (2) reported that 0.7% of the patients were unable to swallow the capsule. Other trials have reported no complications or complaints during the studies. (5, 18, 19)
Despite the facts that the twenty five patients in our study population is a small number, colonoscopies were not all performed by the same gastroenterologist, and they were not performed at regular intervals, the results obtained allow us to suggest that study of the colon with PillCam ® colon is a safe and effective technique for detection of colonic lesions in patients who are at high risk of colon cancer. This is especially true for those who refuse colonoscopy or for whom it is incomplete. It is essential to develop studies with larger numbers of patients to determine the diagnostic yield and cost-effectiveness of the PillCam ® colon capsule in our population.
Conflicts of interest
The authors declare that have no direct or indirect conflicts of interest in academic, scientific, financial or personal terms related to the publication of this study.
REFERENCES
1. Iddan G, Meron G, Glukhovsky A, et al. Wireless capsule endoscopy. Nature. 2000;25:405-17. [ Links ]
2. Eliakim R, Fireman Z, Gralnek IM, et al. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38:963-70. [ Links ]
3. Fernández-Urien I, Carretero C, Borda A, et al. Colon capsule endoscopy. World J Gastroenterol. 2008;14:5265-8. [ Links ]
4. Rami E. The pillcam colon capsule a promising new tool for the detection of colonic pathologies. Curr Colorectal Cancer Rep. 2008;4:5-9. [ Links ]
5. Eliakim R, Fireman Z, Gralnek IM, et al. Prospective multicenter performance evaluation of the second-generation colon capsule compared with colonoscopy. Endoscopy. 2009;41:1026-31. [ Links ]
6. Schoofs N, Devière J, Van Gossum A. PillCam colon capsule endoscopy compared with colonoscopy for colorectal tumor diagnosis: a prospective pilot study. Endoscopy. 2006;38:971-7. [ Links ]
7. Van Gossum A, Muñoz-Navas M, Fernández-Urien I, et al. Capsule endoscopy versus colonoscopy for the detection of polyps and cancer. N Engl J Med. 2009;361:264-70. [ Links ]
8. Sacher Huvelin S, Coron E, Gaudric M. et al. Colon capsule endoscopy vs colonoscopy in patients at average or increased risk of colorectal cancer. Aliment Pharmacol Ther. 2010;32:1145-53. [ Links ]
9. Gay G, Delvaux M, Frederic M, et al. Could the colonic capsule PillCam Colon be clinically useful for selecting patients who deserve a complete colonoscopy? results of clinical comparison with colonoscopy in the perspective of colorectal cancer screening. Am J Gastroenterol. 2010;105:1076-86. [ Links ]
10. Ladas SD, Triantafyllou K, Spada C, et al. European Society of Gastrointestinal Endoscopy (ESGE): recommendations (2009) on clinical use of video capsule endoscopy to investigate small bowel, esophageal and colonic diseases. Endoscopy. 2010;42:220-7. [ Links ]
11. Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam Colon capsule: a reliability study. Endoscopy. 2011;43:123-7. [ Links ]
12. Rex DK. Achieving cecal intubation in the very difficult colon. Gastrointest Endosc. 2008;67:938-44. [ Links ]
13. Sîngeap AM, Trifan A, Cojocariu C, et al. Colon capsule endoscopy compared to colonoscopy for colorectal neoplasms diagnosis: an initial experience and a brief review of the literature. Rev Med Chir Soc Med Nat Iasi. 2012;116:145-9. [ Links ]
14. Hanson ME, Pickhardt PJ, Kim DH, et al. Anatomic factors predictive of incomplete colonoscopy based on findings at CT colonography. Am J Roentgenol. 2007;189:774-9. [ Links ]
15. Riccioni ME, Urgesi R, Cianci R, et al. Colon capsule endoscopy: Advantages, limitations and expectations. Which novelties? World J Gastrointest Endosc. 2012;4:99-107. [ Links ]
16. Sieg A. Capsule endoscopy compared with conventional colonoscopy for detection of colorectal neoplasms. World J Gastrointest Endosc. 2011;3:81-5. [ Links ]
17. Spada C, Hassan C, Ingrosso M, et al. A new regimen of bowel preparation for PillCam colon capsule endoscopy: a pilot study. Dig Liver Dis. 2011;43:300-4. [ Links ]
18. Spada C, Hassan C, Ingrosso M, et al. PillCam colon capsule endoscopy: a prospective, randomized trial comparing two regimens of preparation. J Clin Gastroenterol. 2011;45:119-24. [ Links ]
19. Spada C, Hassan C, Marmo R, et al. Meta-analysis shows colon capsule endoscopy is effective in detecting colorectal polyps Clin Gastroenterol Hepatol. 2010;8:516-22. [ Links ]











 texto en
texto en