Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.4 Bogotá oct./dic. 2013
Gastrostomy feeding and development of lower respiratory tract infections in adults without mechanical ventilation: a prospective cohort study
Fabián Cortés Muñoz, MSc. (1), Óscar A. Guevara Cruz, MD., MSc. (2)
(1) Nurse, MSc in Clinical Epidemiology. Assistant Professor in the Division of Research at the. Universidad El Bosque in Bogotá, Colombia. Mail: cortesfabianm@unbosque.edu.co
(2) General Surgeon, MSc in Clinical Epidemiology. Associate Professor in the Department of Surgery at the Universidad Nacional de Colombia in Bogotá, Colombia. Mail: oaguevarac@unal.edu.co
Received: 12-06-13 Accepted: 27-08-13
Abstract
Background: Nosocomial lower respiratory infections are the most frequent and expensive nosocomial infections in general medical and surgical services, and they are the ones which have the greatest impacts on patients' health. Some risk factors and some protective factors have been clearly identified while others have others have been associated since they are present in greater frequency among those affected. One of these is percutaneous endoscopic gastrostomy (PEG) feeding. Objectives: The objectives of this study were to determine the association between PEG feeding and the development of lower respiratory tract infections in adult patients without mechanical ventilation and to model risk as a function of time. Methods: This study was a prospective cohort study with follow-up of patients in the hospital and at home for 90 days. Gastrostomy fed patients and patients without PEG feeding were studied to determine whether lower respiratory infections developed. The Kaplan Meier estimator and Cox proportional hazards model were used for statistical analysis of data. Results: A total of 128 subjects, two cohorts of 64 patients each, were included. 62.5% of the patients with PEG feeding and 32.8% of those without PEG feeding developed lower respiratory infections during follow-up (p = 0.0008). PEG feeding increased the risk of developing lower respiratory infections by 180% (HR: 2.8, 95% CI: 1.64 - 4.77, p=0.0001) over the risk of patients without PEG feeding. This association increased when adjusted for confounding variables and interaction (HR: 4.6, 95% CI: 1.95 - 8.42, p=0.0000). Conclusion: PEG feeding represents a risk factor for the development of lower respiratory tract infections in adults without mechanical ventilation. This risk varies over time.
Keywords
Cohort studies, nutritional support, gastrostomy, cross-infection, respiratory tract infections, PEG feeding.
INTRODUCTION
According to estimates, more than 1.4 million people worldwide are affected by healthcare-associated infections (HAI). These are also the main causes of in-hospital mortality and increased patient morbidity. (1) A prevalence survey funded by the World Health Organization and conducted in 55 hospitals in 14 countries found that 8.7% of patients who are hospitalized develop some type of nosocomial infection. (2) However, this varies significantly between developed and developing countries.
Economic costs for the world's health systems which are attributable to HAI are huge. This is due principally to an increase of 8.2 days in average hospital stays with subsequent direct costs estimated to be in the range of $28.4 to $33.8 trillion in US hospitals in 2007. (3) This was accompanied by increased probability of patient death of between 20% and 80% for patients in intensive care units-ICU according to the WHO. (2) There are about 100,000 deaths due to HAI in US hospitals annually. (4)
Studies conducted in various clinical settings have reported that airway infections are responsible for a significant proportion of total HAI. (5) It is estimated that about 47% of HAI are respiratory tract infections, and that of these 79% are located in the lower respiratory tract. Lower respiratory tract infections account for 36.2% of all healthcare-associated infections. Their prevalence is highest prevalence in intensive care units (25%). They account for 12% of HAI in surgical services and 9% of HAI in general hospitals. (6)
Lower respiratory infections are a particular problem in intensive care units where death rates can range from 20% to 55%. (7) Research based on these events has focused on this population because of the frequency and the associated costs and mortality. For this reason little information exists about the determinants of occurrence and dynamics in other medical settings such as general medical services where frequency and cost of lower respiratory infections are highest.
Some factors clearly modify the risk of developing this type of infections, but these factors have been studied primarily among ICU patients and only to a limited extent ink general hospital services. (5) In the latter, certain factors have tried to be associated with a higher probability of dying as they are found at a higher frequency in affected patients, a situation that has occurred in patients whose feeding is via gastrostomy. (8) To date, no study with an analytical design has been conducted to evaluate how this risk behaves as a function of time and duration of exposure.
Patients often have a gastrostomy tube or nasogastric intubation to facilitate the gastrointestinal decompression or to facilitate feeding, among others. Among patients with nasogastric tubes, it has been shown an increased frequency of aspiration of pharyngeal and stomach contents to lower airways, which have a consequent risk of developing infections in this anatomical location. However for gastrostomy tubes, still no conclusions on this phenomenon have been described, neither the risk for developing low respiratory infections. (9, 10) Removal of feeding via gastrostomy is not possible for patients, but a better understanding of its etiology would allow alternative therapies that involve lower risks.
MATERIALS AND METHODS
Type of Study
Quantitative, analytical observational and prospective group study with closed characteristics.
Population and sample
Adults admitted to general and surgical medical services (excluding ICUs) of the University Hospital of Clínica San Rafael (San Rafael Clinic), and Clínica Nuestra Señora de la Paz (Our Lady of Peace Clinic) in Bogotá DC from April 1st to September 30th of 2011.
Once the eligible population was identified, two groups were formed: one exposed and one unexposed. The exposed group consisted of adults from general and surgical services who underwent gastrostomy as a feeding support before the study, and who met the selection criteria. Adults without feeding support, being able to feed themselves, formed the group of unexposed patients. They were from the same clinical service as the exposed group, and were admitted the same day. These parameters were important in order to ensure that both groups were homogeneous on certain features, and have the same opportunity to develop the event of interest. The exposed/unexposed ratio was 1:1 in each of the institutions where the study was conducted. The subjects were studied until the end of the recruitment phase.
Patients aged ≥ 18 years who had a minimum of 24 hours of hospital stay, who agreed to participate, and signed the informed consent (or had a guardian authorization) were included in the study. Individuals from the exposed group underwent nutritional support by gastrostomy tube. This procedure had to be performed during the study, ensuring no incidents or prevalence. Unexposed individuals needed oral tolerance, and no enteral nutritional support.
The following patients were excluded from the study: patients with diagnosed chronic obstructive pulmonary disease (COPD), lung cancer at any stage, history of lower respiratory infections 15 days prior the recruitment, patients referred from intensive care units with a history of mechanical ventilation 72 hours before the gastrostomy and/or recruitment, diagnostic HIV/AIDS, total gastrectomy, tube thoracostomy, presence of hemo/hydro/pyothorax and/or pleural effusion during or a month before the recruitment, and abdominal, head, and neck surgery postoperative conditions less than 30 days at recruitment.
Sample size calculation was performed using the formula for two-censored independent groups, assuming that parameters observed by Madariaga et al.,: 0.05 as a type I error, 0.2 as a type II error, 1:1 ratio between exposed/unexposed, and a maximum value of losses of 10%. (8) The number of subjects in each group was 66, with a total study population of 132 subjects.
In both groups, the outcome variable corresponded to the time when lower respiratory infections (bronchitis, tracheobronchitis, bronchiolitis, tracheitis, and pneumonia) appeared. Outcomes set out in the study were multiple, however, we only took into account the first episode of infection. The diagnosis of these events met the criteria defined by the Centers for Disease Control non-immunocompromised adults. (11)
Patients were examined every 7 days up to a total of 90 days from the date of recruitment. First, medical records, and other health care records gave the possible outcomes. Using the contact information (personal and from two close relatives) collected during recruitment, discharged patients were contacted by phone in order to gather related information to events of morbidity different from the ones occurred during the hospital discharge until the end of the study. This collection of data used a standardized format of anamnesis signs, and symptoms consistent with lower respiratory infections (designed from the CDC criteria?). If there was evidence supporting one of the outcomes and a health facility or a private physician did not diagnose the subject, a home visit was conducted by a nursing professional in order to collect more information, and classify it.
The following censures were considered: suspension of feeding by gastrostomy greater than 48 hours, death, loss of the follow-up, and completion of the follow-up period of the study subjects.
The research was approved for implementation by the ethics committee of the Faculty of Medicine of the National University of Colombia, and by institutional ethics committees of University Hospital of San Rafael Clinic, and Our Lady of Peace Clinic.
STATISTICAL ANALYSIS
To describe individual characteristics of the subjects and the general variations, measures of central tendency (mean) and dispersion (standard deviation) for quantitative variables were used after verification of normal distribution with Shapiro-Wilk tests. If normal distribution was not confirmed, median and interquartile ranges were used.
Qualitative variables were measured and analyzed using proportions. For comparison between two groups, a one-way analysis of variance (ANOVA) was used when normal distribution of data otherwise non-parametric statistics (Kruskal-Wallis) was used. Z test was used for difference between proportions in qualitative variables.
Time of the study was considered from the days gastrostomy was performed (or admission to the health facility, in the case of the unexposed) to the date on which signs and symptoms of lower respiratory infections appeared or disappeared. The behavior of the risk as a function of time was modeled using survival analysis Kaplan Meier, stratified by each of the groups. Log-rank and Wilcoxon tests were used to find differences between these survival curves.
Association between enteral nutrition via gastrostomy tubes and the risk of lower respiratory infections was estimated by Hazard Ratio (HR), with confidence intervals of 95% as a crude estimate, and multivariate analysis with Cox proportional-hazards unconditioned models, adjusted for covariates. Independent variables were selected from a full model that included all variables described as effect modifiers according to the literature, including those that were statistically different between subjects in both groups. Additionally, possible interactions between variables using a stepwise technique with 10% of input probability, and 15% of output were used as a final model with variables that were statistically significant. In this process, we found that the variables that were different between subjects with and without enteral nutrition by gastrostomy tubes provided little or no explanatory power to the final model, which is why they were excluded from the reported model.
Specification of the partnership model was evaluated with link tests, as the no violation of proportional hazards assumption by graphical (log-log plots) and residual methods (Schoenfeld) for overall assessments and for each of the variables. Model fitting was evaluated by plotting the residuals from Cox-Snell versus cumulative risk function, and Martingale residuals were used to determine the functional form of the variables included in the final model.
Due to the selection system of the unexposed group, it was necessary to prove the independence of observations from both groups using a proportional hazards model using Cox regression with shared frailty, assuming in advance a theoretical gamma distribution with mean (1) and variance (θ).
Statistical tests were considered significant at p<0.05, and relevant using confidence intervals of 95%. Statistical analysis was performed using STATA software (version 10 SE, Stata Corporation, College Station, Texas).
RESULTS
128 subjects were included in the study, 64 in each group. Due to administrative problems, it was not possible to recruit 66 people, since one of the health institutions where the research was being conducted, stopped performing gastrostomies.
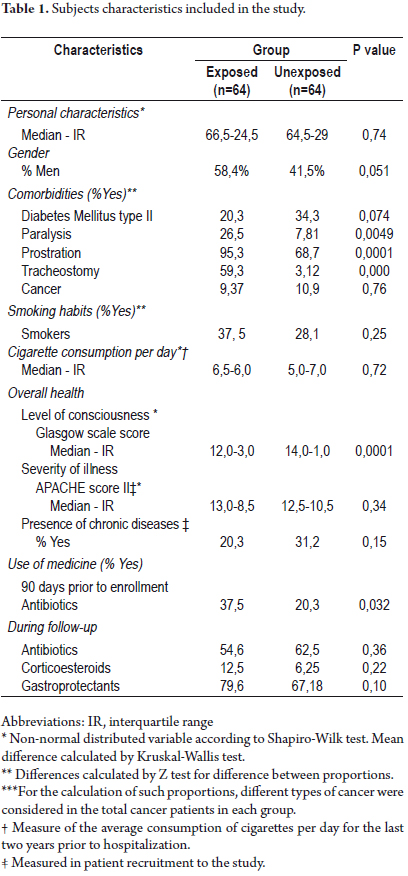
Table 1 describes the characteristics of the subjects included in each of the groups, and the differences and similarities between the statistics.
No statistically significant differences between the two groups with respect to age and gender were found. Regarding comorbidities of such patients, diabetes mellitus type II and cancer were the most frequent among unexposed subjects, although these differences were not statistically significant. However, statistical significance of this type was found in the frequency of paralysis, prostration, and tracheostomy, which were more common among the exposed group. Frequency of smoking and number of cigarettes consumed on average over the last two years prior to hospital admission were similar in both groups with no significant differences.
For the baseline measurement of overall health, levels of awareness were lower among subjects who underwent gastrostomy (exposed) compared to the subjects who didn't (unexposed) (p=0.0001). No differences between both groups were found regarding the severity of the disease as the recruitment patients with APACHE II scale, and presence of chronic diseases.
The use of antibiotics 90 days prior the study was significantly higher among subjects in whom gastrostomy was performed. No statistically significant differences regarding the use of antibiotics, corticosteroids, and gastroprotectants (H2 antagonists, proton-pump inhibitors, prostangladins, and sucralfate) for hospital and home monitoring were found.
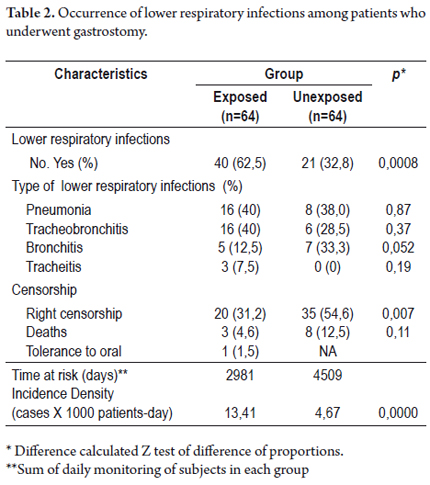
62.5% of the exposed subjects, and 32.8% of unexposed presented lower respiratory infections during follow-up, with statistically significant differences between both groups (p = 0.0008) (see Table 2). Among patients who underwent gastrostomy, the most frequent lower respiratory infections were pneumonia and tracheobronchitis with 40% each, followed by bronchitis and tracheitis with 12.5% and 7.5% respectively. In the unexposed group, pneumonia ranked first with 38% of all infections, followed by bronchitis and tracheobronchitis with 33.3% and 28.5% respectively. In the unexposed group no tracheitis was observed. Finally, no significant differences were found between the groups regarding the frequency in which these types of respiratory infections were presented.
The incidence rate of lower respiratory infections among patients with gastrostomy tubes was 13.4 cases per 1000 patient-day (95% CI: 9.84-18.29) compared to the unexposed group where it was 4.65 cases per 1000 patient-day (95% CI: 3.03-7.14). This means that there is a higher rate of occurrence of this disease among exposed subjects than among patients without gastrostomy tubes (p=0.0000). Deaths during follow-up were also more frequent in the exposed group although no statistically differences were found (p=0.11).
COMPARISON OF SURVIVAL CURVES
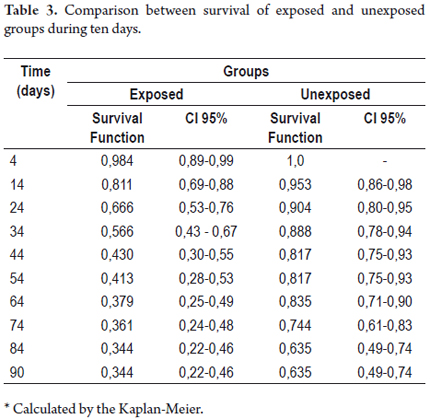
Lower respiratory infections occurred earlier in the exposed group than in the unexposed. In the exposed group, such infections occurred from day 4 after gastrostomy, and in the unexposed group, the first of these events occurred after day 8 during follow-up. Table 3 shows the survival function, and 95% confidence intervals for each group at different times.
Survival functions were significantly lower in almost every day of follow-up, except for the top 10 subjects who underwent gastrostomy compared to the subjects who were not exposed to this procedure. At day 45, the probability of not presenting any kind of lower respiratory tract infection in the group of patients with gastrostomy tubes was 43.0%, and 81.7% in the unexposed group. At day 90, these probabilities were reduced to 34.4% and 63.5% respectively.
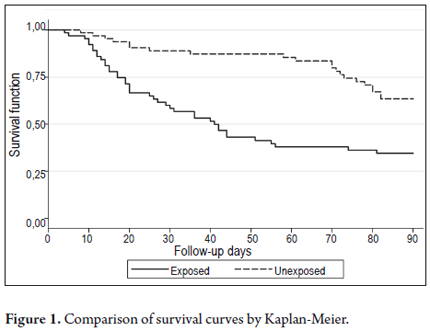
The trend described above is reflected in a plot of Kaplan-Meier survival curves (see Figure 1), which were statistically different when testing a hypothesis of equality by the log-rank (p = 0.0001) and Wilcoxon methods (p = 0.0000).
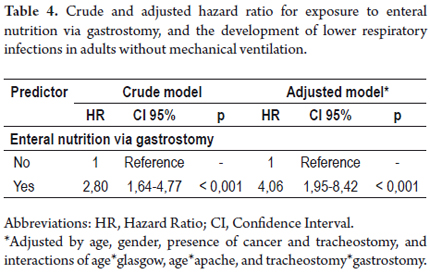
A model of Cox proportional-hazards regression was built to assess the association between the exposure and the studied outcome. Table 4 reports the crude and adjusted HR of such associations. Enteral nutrition via gastrostomy tubes increased the risk of developing lower respiratory infections by 180%, and increased to 306% after adjusting confounding and interaction variables.
Diagnosis model and evaluation of the proportional hazards assumption
The final model showed an adequate specification and enforcement of the proportional hazards assumption for each of the variables included in the study. A proper fit was observed in the evaluation.
Statistical Power
Since it was not possible to recruit 66 patients in each group, we calculated the statistical power based on the parameters of the study, hypothesis, and differences between groups. The statistical power of the study was 99.19%. Despite failing to achieve adequate sample size, the study had enough statistical power to detect clinical important differences.
Independence of observations
Likelihood test with no significant correlation was observed for the origin of exposed and unexposed subjects (p=0.62). Thus, it is concluded that the statistical methods used are adequate, and the groups are independent.
DISCUSSION
This study found that the incidence of lower respiratory infections in non-ventilated adults was significantly higher in those receiving enteral nutrition by gastrostomy compared to those unexposed to this feeding support. 62.5% of the exposed subjects had some type of lower respiratory tract infection during follow-up compared with 32.8% of the unexposed. These results are higher than those reported in other studies. (7, 12) Patients were followed during 90 days until the appearance of symptoms or until the censorship. The follow-up time for this study was higher than the conventionally used in other studies (fixed until patient discharge), a condition that would increase the possibility of capturing the failures presented in individuals beyond hospital discharge, which somehow explain the observed incidents.
Although no different studies from ours describes the impact of different types of lower respiratory infections according to the presence or absence of feeding via gastrostomy tube, research conducted internationally have reported 34.5% of pneumonia, and 65.5% of bronchitis among patients diagnosed with low respiratory infections in hospital patients (including patients from intensive care units). (13) Since frequencies of pneumonia and bronchitis found in our study are lower in each of the groups compared with international reports, this situation is possibly attributed to the fact that the subjects were selected from general medical services and not from ICUs.
Exposition to gastrostomy tubes increased 180% the risk of developing lower respiratory infections, which increased after adjustment for confounding and interaction variables. Similar associations have been described in longitudinal studies made by Madariag et al, (OR?: 4.8, 95% CI 1.9 - 12.2), and Merchant et al, (OR?: 75.7, 95% CI 22-285). (8, 12) However, there are no studies with a similar study design.
This information is consistent with other information available. In a clinical trial comparing the incidence of pneumonia among patients with gastrostomy tubes versus other types of feeding such as transpyloric tubes, reported an OR? of 0.3 (95% CI: 0.1 to 0.7, p=0.01) in favor of other types of feeding. The authors explain that this type of feeding presents a lower aspiration risk since they deal with the intestine and not with the stomach, like gastrostomy tubes. (14) Other clinical and observational trials have reported similar results. (15, 16)
In most of the cases, lower respiratory infections result from microbial invasion of lung parenchyma by micro aspiration of oropharyngeal contents, which is generally colonized by gram-negative enteric pathogens in non-ventilated patients or patients with gastric secretions. (17, 18) The main role of the stomach in the development of these events varies depending on the conditions of the patient and the presence of therapeutic or prophylactic interventions. In healthy people, few bacteria entering the stomach survive under low gastric pH (less than 2), but when it increases (>4) microorganisms survive and multiply. (17) This condition occurs in elder patients, patients with achlorhydria, and patients who receive antacids or inhibitors of histamine and enteral nutrition. (19) This last factor causes an increase in gastric pH levels, which can lead to colonization by gram-negative bacilli. (20) Besides, gastric reflux and aspiration can occur by high intragastric volume and pressure.
Among patients with gastric tube, an increased frequency of aspiration of stomach and pharynx contents to lower airways has demonstrated to have a consequent risk of developing such infections in that location. In a clinical trial in which radioactive colloid Technetium was administered directly into the stomach of patients, an increased aspiration of stomach contents into pharynx and trachea was demonstrated independent of the size of the tube. (21) Among patients who underwent gastrostomy, no clear conclusions are based on such a phenomenon. (9, 10) Migration of gastric material to different airways mediated by other factors could be explained by the increased risk of lower respiratory infections observed in subjects who underwent gastrostomy compared to the unexposed subjects. Also, foreign bodies such as endotracheal tubes, nasogastric and gastrostomy tubes are a source of colonization, and promote physical migration of lower respiratory tract pathogens to adhere to the surface and form a biofilm that protects them from antimicrobial or immune response. (22, 23)
Seven factors were significant in the relationship between the presence of gastrostomy and development of lower respiratory infections: age, gender, presence of cancer, tracheostomy, and interactions of age*glasgow, age*apache, and tracheostomy*gastrostomy. In a case-control study conducted in patients who developed nosocomial pneumonia in general medical services and intensive care units, associations were found between over 60 years of age with the development of the disease (OR?: 4.6, 95% CI: 2.5-8.5). (24) Other authors have even found a associations between over 50 years of age with the development of the event in patients coming from general medical services (OR?: 4.49, 95% CI: 1.55-13.5). (12) According to other studies, gender did not modify the association between exposure and the outcome, contrary to our results. (8, 13) Regarding the malignancies, there is no evidence that can increase or decrease the risk of lower respiratory infections (OR?: 1.74, 95% CI: 0.99-2.92). (25)
Tracheostomy procedures have shown divergent results in its association with the development of lower respiratory infections. Madariaga et al., found a statistically significant association (OR: 7.0, 95% CI 0.8-159). Contrary to results found by M. Merchant et al., in patients coming from general medical services not ICUs (Crude OR: 30.28, 95% CI: 3.55-215.3). (12) Our results suggest a positive association (HR: 5.32, 95% CI: 1.16-24.6). Unconsciousness has been reported as a factor associated with the development of lower respiratory infections (adjusted OR: 2.96, 95% CI: 1.68-4.61), as a ≤ 8 Glasgow value (unadjusted OR: 10.5, 95% CI: 3.35-321), condition which could increase the possibility of gastric content microaspiration into the lower respiratory tract, and it is related to reduced mobilization of secretions.
Since this is an observational study, unknown confounding factors and some already known that were not covered (history of alcoholism, position of the head during hospitalization, etc.) could be distributed unevenly among groups, constituting a limitation related to the methodological design. By the nature of the exposure, it was not possible to blindly follow-up because exposure would be evident in the direct interview, review of medical records, and telephone follow-up after hospital discharge. Also, by not considering time-dependent variables, the study may fall into imprecise estimates to not approach in a dynamic manner to variables that change over time (such as level of consciousness, severity of illness, etc). Such limitations will be taken into account in future research to allow the construction of predictive models different from our partnership model.
Previous studies have approached the exposure and outcome of this research subject differently. They have used their case-control designs and cross section with its own methodological limitations. However, some of these limitations were solved studying subjects just exposed to the medical procedures, and designing a prospective group study with survival analysis. This resulted in a more robust methodological design. Additionally, data analysis with Cox proportional hazards model allowed to understand that these kinds of events in order to demonstrate that the risk varies depending on the exposure time.
Our results showed that lower respiratory infections are frequent events in adult patients without mechanical ventilation, and feeding by gastrostomy tubes substantially increases the risk of developing this type of infections. In a realistic manner, the elimination of this type of feeding support is still difficult, but a better understanding of its complications will allow the selection of therapeutic alternatives that are less hazardous, and adjusted to the particular needs of each patient. Likewise, susceptible control intervention factors in individuals with this type of feeding support, and the environment around them will decrease the risk of lower respiratory infections. Most of epidemiological studies and etiology studies of lower respiratory infections focus on mechanically ventilated patients because of its high incidence and mortality, but limited studies focus on patients coming from general medical services but not UCI 's. Hence, information available for this kind of events in this specific population remains low.
Acknowledgements
National University of Colombia funded this study in the third frame cut supporting DIB to graduate thesis (code: 13182). We really appreciate this valuable aid for research development. To the Clinic University Hospital and Clinic San Rafael, and Our Lady of Peace Clinic for allowing us to conduct research in their facilities and provide the means to execute it.
REFERENCES
1. Pittet D, Allegranzi B, Storr J, et al. Infection control as a major World Health Organization priority for developing countries. J Hosp Infect. 2008;68:285-92. [ Links ]
2. PAHO/CDC/CSR/EPH. Prevención de las infecciones intrahospitalarias. Guía práctica de la Organización Mundial de la Salud. 2da ed. Ginebra: OMS; 2002. [ Links ]
3. Scott RD II. The direct medical cost of healthcare. Associated infection in U.S hospitals and the benefits of prevention. Atlanta Center for Disease Control and Prevention; 2009. [ Links ]
4. Klevens RM, Edwards JR, Richards CL Jr, et al. Estimating health care associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122:160-6. [ Links ]
5. Craven DE, Steger KA. Hospital-acquired pneumonia: perspectives for the healthcare epidemiologist. Infect Control Hosp Epidemiol. 1997;18:783-95. [ Links ]
6. Pittet D, Harbarth S, Ruef C, et al. Prevalence and risk factors for nosocomial infections in four university hospitals in Switzerland. Infect Control Hosp Epidemiol. 1999;20:37-42. [ Links ]
7. Greenaway CA, Embil J, Orr PH, et al. Nosocomial pneumonia on general medical and surgical wards in a tertiary-care hospital. Infect Control Hosp Epidemiol. 1997;18:749-56. [ Links ]
8. Madariaga MG, Thomas A, Preston B. Risk factors for nursing home-acquired pneumonia. Clin Infect Dis. 2003;37:148-50. [ Links ]
9. Holzapfel L, Chevret S, Madinier G, et al. Influence of long-term oro- or nasotracheal intubation on nosocomial maxillary sinusitis and pneumonia: results of a prospective, randomized clinical trial. Crit Care Med. 1993;8:1132-8. [ Links ]
10. Magné N, P. Y Marcy, C Foa, M. N Falewee, M. Schneider, F Demard, et al., Comparison between nasogastric tube feeding and percutaneous fluoroscopic gastrostomy tube feeding in advanced head and neck cancer patients. Eur Arch Otorhinolaryngol 2001; 258:89–92. [ Links ]
11. Horan TC, Andrus M, Dudeck MA. Surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Atlanta, Georgia: CDC/NHSN; 2004. [ Links ]
12. Merchant MK, Kanbur AA. Incidence of nosocomial pneumonia in a medical intensive care unit and general medical ward patients in a public hospital in Bombay, India. J Hosp Infect. 1998;39:143-8. [ Links ]
13. Kofteridis D, Papadakis J, Bouros D, et al. Nosocomial lower respiratory tract infections: prevalence and risk factors in 14 Greek hospitals. Eur J Clin Microbiol Infect Dis. 2004;23:888-91. [ Links ]
14. Acosta Escribano J, Fernández Vivas M, Grau Carmona T, et al. Gastric versus transpyloric feeding in severe traumatic brain injury: a prospective, randomized trial. Intensive Care Med. 2010;36:1532-9. [ Links ]
15. Montecalvo M, Steger K, Farber H, et al. Nutritional outcome and pneumonia in critical care patients randomized to gastric versus jejunal tube feedings. Crit Care Med. 1992;20:1377-87. [ Links ]
16. Metheny NA, Stewart BJ, Mcclave SA. Relationship between feeding tube site and respiratory outcomes. JPEN J Parenter Enteral Nutr. 2011;35:346-52. [ Links ]
17. Tablan O, Anderson L, Besser R, et al. Guidelines for preventing health-care–associated pneumonia, 2003: recommendations of the CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53:1-36. [ Links ]
18. Flanders Scott A, Collard Harold R, Saint S. Nosocomial pneumonia: State of the science. Am J Infect Control. 2006;34:84-93. [ Links ]
19. Du Moulin G, Paterson D, White J, et al. Aspiration of gastric bacteria in antacid-treated patients: a frequent cause of postoperative colonization of the airway. Lancet. 1982;2:242-5. [ Links ]
20. Chang R, Jacobs S, Bartlett F, et al. Continuous enteral feeding: a major cause of pneumonia among ventilated intensive care unit patients. J Parent Enter Nutr. 1990;14:353-6. [ Links ]
21. Ferrer M, Torsten T, Torres A, et al. Effect of nasogastric tube size on gastroesophageal reflux and microaspiration in intubated patients. Ann Intern Med. 1999;130:991-4. [ Links ]
22. American Thoracic Society. Guidelines for the management of adult with hospital acquired, ventilator-associated and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388-416. [ Links ]
23. Inglis TJ, Millar MR, Jones JG, et al. Tracheal tube biofilm as a source of bacterial colonization of the lung. J Clin Microbiol. 1989;27:2014-8. [ Links ]
24. Celis R, Torres A, Gatell J, et al. Nosocomial pneumonia: a multivariate analysis of risk and prognosis. Chest. 1988;93:318-24. [ Links ]
25. Kampf GP, Wischnewski S, Schumacher D, et al. Analysis of risk factors for nosocomial infections - results from the first national prevalence survey in Germany (NIDEP Study, Part 1). J Hosp Infect. 1997;37:103-12. [ Links ]











 texto en
texto en