Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.4 Bogotá out./dez. 2013
A practical approach to histopathologic diagnosis of microscopic colitis
Mario Alexander Melo Uribe, MD. (1), Elías Augusto Castilla Puentes, MD., FACP., FASCP. (2)
(1) Medical Pathologist at Compensar and the Universidad de La Sabana in Bogotá, Colombia.
(2) Medical Pathologist, FACP, FASCP, Department of Pathology at Bethesda North Hospital in Montgomery, Ohio USA.
Received: 16-10-13 Accepted: 27-08-13
Abstract
Lymphocytic colitis and collagenous colitis are two histologic forms of microscopic colitis, a condition which was first recognized over 30 years ago. It is often found in adults with chronic, watery diarrhea although endoscopic examination of the colon is frequently normal. The diagnosis is based on microscopic examination of colonic biopsies. The aim of this review is to familiarize general surgical pathologists with the morphologic features of lymphocytic and collagenous colitis. In additional, this review emphasizes good communication with the endoscopist to allow correct recognition and ensure appropriate treatment.
Keywords
Colitis, microscopic colitis, collagenous colitis, lymphocytic colitis.
INTRODUCTION
Microscopic colitis (MC) is a term used to describe a medical condition characterized by chronic watery diarrhea but with an almost normal endoscopic appearance of the mucosa of the colon and typical histopathological changes. (1) Two histological types of MC have been described: lymphocytic colitis (LC) and collagenous colitis (CC). Each has its own epidemiology and associated diseases but both follow similar clinical course. In addition, they share similar histological features such as inflammatory cells and damage to the superficial epithelium.
The term microscopic colitis has been used since 1980. (1) At that time its incidence was very low, but recent literature shows an increasing number of MC cases which now account for between 9.5% and 10.2% of the patients with chronic watery diarrhea who have undergone endoscopy and have had biopsies. (2, 3)
The etiologies and pathogenesis of LC and CC are not yet very clear. However, observational investigations suggest an association between MC and several autoimmune diseases such as autoimmune thyroiditis, rheumatoid arthritis, celiac disease, and other diseases such as Intestinal Inflammatory Disease (IBD). (4-6) Use of medicines including NSAIDs, (7) proton pump inhibitors, (8) and histamine receptor inhibitors (ranitidine and cimetidine) (9.10) have been associated with both LC and CC.
Colon biopsies are essential in the diagnosis of MC. In general, recognition of typical forms of LC and CC can be achieved in most cases, and there are excellent rate of inter observer and intra agreements among pathologists trained in gastrointestinal pathology. (11) Nevertheless, the less typical forms are difficult to recognize. The aims of this review are to familiarize pathologists who do not have specific gastrointestinal training with the morphologies of LC and CC, to suggest standardization of reports, and to highlight the importance of good communication with the endoscopist for correct diagnosis of these entities.
This article is based on a review of original and review articles on the topic published in English and Spanish in indexed journals from the PubMed and SciELO databases with the addition of personal contributions from the authors. Keywords used in both English and Spanish were "Colitis", "microscopic colitis", "lymphocytic colitis" and "collagenous colitis".
LYMPHOCYTIC COLITIS
Epidemiology
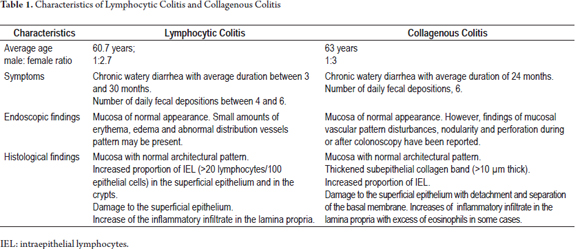
The annual incidence of LC in Europe ranges from 1.1/100,000 people to 3.1/100,000 people with a prevalence ranging from 10/100,000 to 15.7/100,000 people. (2) In North America, the incidence is 5.5/100,000 people with a prevalence of 63.7/100,000 people at the end of 2001. (12) In Latin America, isolated figures for the prevalence of MC exist for countries like Uruguay, (13) but we found no publications on the epidemiology of MC in Colombia. The average age of patients at diagnosis is 60.7 years: MC is up to 2.7 times more common among women than men. (14)
Symptoms
Patients with LC present chronic watery diarrhea with an average duration before diagnosis of three and a half month. They have between four and six fecal depositions per day. (15) Other symptoms like abdominal pain, incontinence, urgency, flatulence and weight loss have also been associated with LC. (3, 16)
Endoscopic findings
The mucosae of the majority of patients have standard endoscopic appearances. A small proportion may have erythema, edema and an abnormal distribution pattern of vessels. (17) Changes in LC may be irregular in their distribution in the different portions of the colon, but are sometimes more severe in the right colon. (18) It is recommended that biopsies be taken from the ascending colon, the transverse colon, the descending colon and the rectosigmoid colon and that each biopsy be place in a separate container. (19)
Histological findings
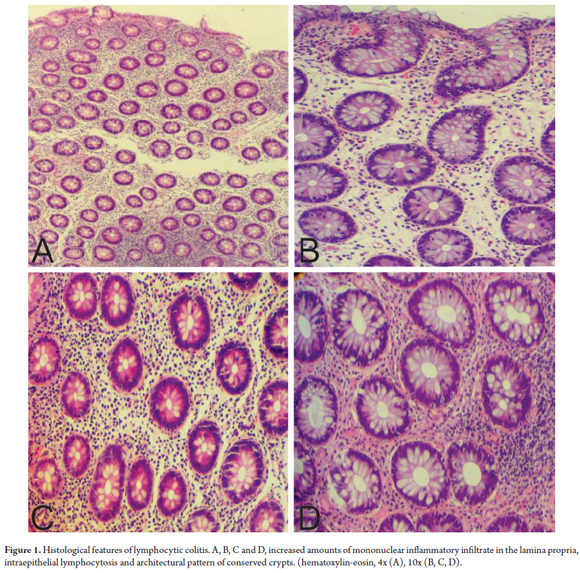
LC is characterized by an increased proportion of intraepithelial lymphocytes (IEL) (more than 20 lymphocytes/100 epithelial cells) in the superficial epithelium and in the epithelium of the crypts. (20) The mucosa has a standard architectural pattern accompanied by damage to the superficial epithelium and a mixed mononuclear inflammatory infiltrate (plasma cells, lymphocytes) with few eosinophils in the lamina propria and absence of subepithelial collagen deposits (Figure 1). (21) The damage to the superficial epithelium is associated with infiltration of lymphocytes and with degenerative characteristics such as cytoplasmic vacuoles, loss of mucin, and irregularities of the cell nuclei including pyknosis and flattening. (20)
The IEL have small circular nuclei and are generally surrounded by a clear halo. (Figure 2) Intraepithelial lymphocytosis is evident, but since most pathologists have a good idea of the normal pattern of distribution of the inflammatory cells in the colonic mucosa there is usually no need for a cell count. However, when there is any doubt about whether the number of IELs has increased, a lymphocyte count should be done. It is necessary to note that increased numbers of IELs is a normal finding for superficial epithelium overlying lymphoid aggregates and should not be interpreted as a LC. Consequently, in the context of MC, IEL counts are not recommended in those areas. (18)
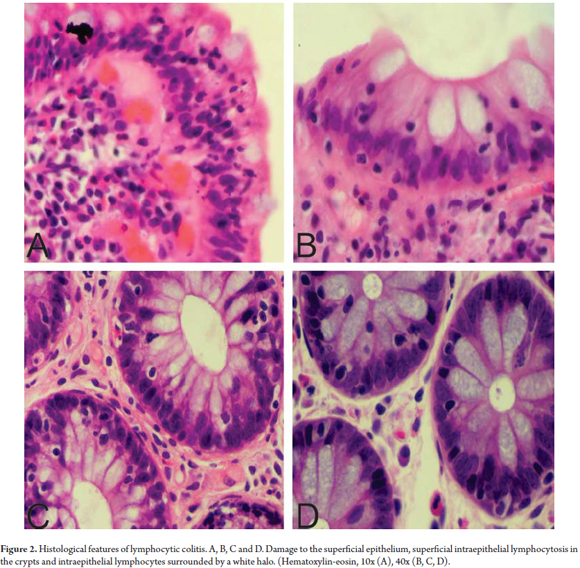
The use of immunohistochemical techniques is not of vital importance for the diagnosis of LC because diagnosis can be done with routine hematoxylin-eosin (HE) staining. However, there are reports that conclude that in the appropriate clinical setting, the use of CD3 may have additional value for diagnosis of LC. (22)
In the literature two variants of histopathological LC have been described, although there are very few reported cases in both variants, moreover they are without apparent prognostic significance for patients. The first is paucicellular LC which has been described as having symptoms similar to those already discussed but which histologically has raised foci, slightly elevated levels of IEL and lymphoplasmacytic infiltrate in the lamina propria separated by fragments of normal mucosa. (23) The second variant is LC with giant cells. This refers to the presence of giant subepithelial cells in the context of typical LC. (24)
Differential diagnosis
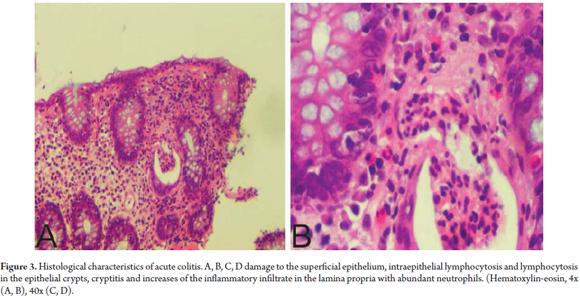
Among the most common entities requiring differential diagnosis from LC are resolved acute active colitis, collagenous colitis , inflammatory bowel disease (IBD) and enteropathy associated with T-cells lymphoma. The presence of neutrophils indicates acute colitis which allows us to differentiate between LC, active self-limited colitis in the process of resolving itself, and active colitis overlapping with LC (Figure 3).
Both entities have increased IEL levels, but patients may be more motivated to see a doctor when chronic diarrhea has recently worsened (active infectious overlapped colitis). In cases in which this pattern of inflammation is present, and in the appropriate clinical context, it is recommended that the diagnosis be made in a descriptive way that indicates to the attending physician that this may be a case of LC and overlapping active colitis or it may be the resolution phase of self-limited colitis (Table 1). If symptoms persist, repetition of the colonoscopy/biopsy in two to three months can be considered in order to establish a definitive diagnosis.
An accurate assessment of the subepithelial collagen band helps differentiate between LC and colon cancer. However, in cases with borderline features a Masson trichrome stain may be helpful (see below). Differentiating between LC and IBD is simple because in cases of IBD the architecture of the crypts is abnormal. However, it can be difficult in the context of LC with IBD because of focal features or because of true coexistence of the two conditions which has also been reported. The overall assessment of the endoscopic and clinical contexts and the predominant lesion pattern shown in the biopsies help differentiate between these two entities. (25)
Intraepithelial lymphocytosis associated with T cell lymphoma is bly linked to celiac disease and primarily involves the small intestine. Nevertheless, it can also affect the colon. Histologically, an infiltration of atypical small lymphocytes is observed occupying the intestine wall. In most cases these lymphocytes present the following immunohistochemical profile: CD45 (+), CD3 (+), CD7 (+) CD4 (-), CD8 (-), TIA-1 (+), granzyme B (+) perforin (+) and clonal rearrangements of TCR β and γ (26).
COLLAGENOUS COLITIS
Epidemiology
The annual incidence of CC in Europe ranges from 0.6/100,000 people to 5.2/100,000 people, while its prevalence ranges from 10/100,000 people to 15.7/100,000 people. (2). In the United States the annual incidence was 3.1/100,000 people and the prevalence was 39.3/100,000 people at the end of 2001. (12) The average age of patients at diagnosis is 63 years old with a range between 29 and 93 years old. (14) It is quite unusual among children (27) and it is three times more common in women than in men. (14)
Symptoms
Patients with CC present symptoms similar to those of LC, in particular they have chronic watery diarrhea which lasts an average of 24 months before diagnosis. The average number of fecal depositions per day is six. (14)
Endoscopic findings
Although the mucosae of most patients with CC appear normal, findings such as altered mucosal vascular patterns and nodularity have been reported. Also, there have been cases of perforation which occurred during colonoscopy but were reported 1 to 5 days after the procedure was performed. (17, 28)
As in LC, alterations occurring in CC may be distributed irregularly, but are most common in the proximal colon. (17) However, since CC does not compromise only the right colon, (19) it is recommended that multiple biopsies of the mucosa be taken, including from the right colon, the transverse colon, the descending colon and the rectosigmoid colon. It is preferable to send them to the laboratory in separate containers. (19)
Histological findings
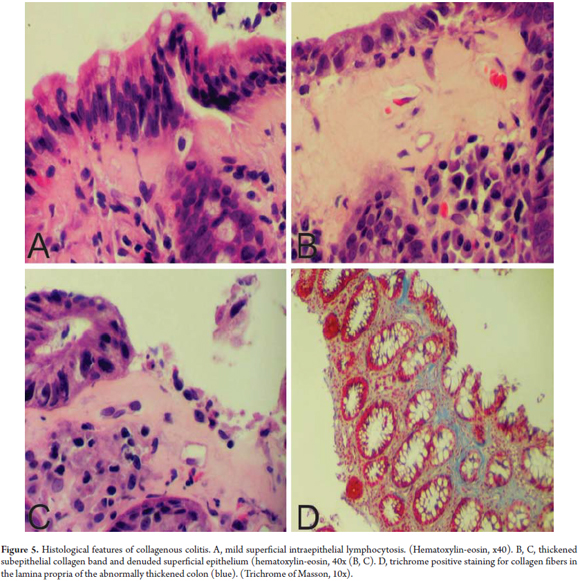
By definition, CC is characterized by thickening of the subepithelial collagen band to a thickness of more than 10 microns. (18) However, since measuring this band is time consuming and impractical, in addition to a description of increased thickness of the band, the key for diagnosis of CC is finding abnormal distribution of this band extending inside the lamina propria trapping capillaries and fibroblasts (Figure 4). (20) HE staining does not always make the distribution pattern of collagen obvious. In these cases, Masson trichrome staining is quite helpful. The presence of an abnormal collagen band by itself is not sufficient to diagnose CC. Other characteristics should be present. These include increased mononuclear infiltrate in the lamina propria (in some cases with abundant eosinophils), increased IEL levels (smaller increases than in LC) and damage to the superficial epithelium. In CC the superficial epithelium is usually separated from the basal membrane which exposes the abnormal collagen band (Figure 5). (20)
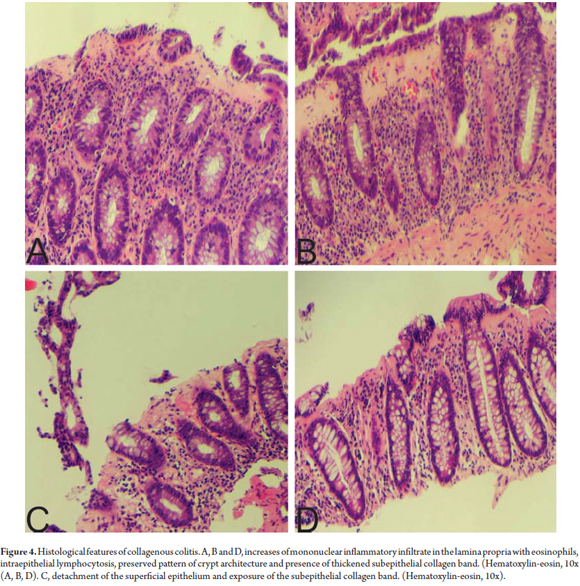
At this point it might be helpful to note that assessment of the subepithelial collagen band must be performed in a well-oriented biopsy because tangential cuts of the colonic mucosa may give the false impression of thickened mucosa.
As in LC, histological variants with no apparent prognostic significance have been described in cases of CC. These include pseudomembranous CC and CC with giant cells. Pseudomembranous CC is very rare. It is characterized by fibrinopurulent pseudomembranes protruding from the mucosa in the context of typical CC. (29) CC with giant cells is typical CC with subepithelial CD68 + giant cells. (24)
Differential Diagnosis
Other conditions that may appear with thickened subepithelial collagen bands include chronic ischemia, mucosal prolapse, diabetes mellitus and hyperplastic polyps. However, all of these lack accompanying inflammatory infiltrate. (30) CC may appear with histological features similar to those found in IBD, such as distortion of the crypt architectural, cryptitis, Paneth cell metaplasia and isolated foci of superficial erosion of the mucosa. These appear on a mucosal base which is otherwise typical of CC. (25) Differentiating between CC and IBD is simple given that the crypt architecture is preserved in CC. However, CC and IBD are not mutually exclusive, and cases have been reported in which CC has progressed to ulcerative colitis while others have transitioned from ulcerative colitis to CC. (4, 5)
CLINICAL COURSE OF LC AND CC
Between 20% and 48% of patients with LC have spontaneous clinical remissions, but have relapses after a 14 month follow-up. This suggests chronic and intermittent clinical development for at least for half of the patients. (31) CC has a very similar pattern with spontaneous clinical remissions in 25% of patients accompanied by improvements in histopathological findings. (31) Results of clinical remission of CC in 85.7% of patients treated through oral administration of budesonide have been described although relapses have been observed in up to 60% of them after oral treatment is stopped. (31) Thus, clinical course of CC is similar to that of the LC, chronic and intermittent.
CONCLUSIONS
MC is a clinical and histopathological term that groups together LC and CC. It is used to describe a medical condition characterized by chronic watery diarrhea with colonic mucosa that appears to be normal when viewed endoscopically, but with histopathological findings consisting of increased mononuclear inflammatory infiltrate in the lamina propria, intraepithelial lymphocytosis, damage to the superficial epithelium which does not alter the architectural pattern of the crypts, and the presence of a thickened collagen band in the CC. Although the etiology is still uncertain, both LC and the CC have associations with autoimmune diseases and are more common in women. MCs incidence and prevalence have been increasing in developed countries, but there are still no exact figures for countries like Colombia. Nevertheless, pathologists must be able to recognize and diagnose these entities in order to provide suitable care for these patients.
Acknowledgements
The authors acknowledge the general support given by the pathology laboratory of Compensar and the Universidad de la Sabana in Colombia for the preparation of this manuscript.
REFERENCES
1. Read NW, Krejs GJ, Read MG, et al. Chronic diarrhea of unknown origin. Gastroenterology. 1980;78:264-71. [ Links ]
2. Fernández-Bañares F, Salas A, Forné M, et al. Incidence of collagenous and lymphocytic colitis: a 5-year population-based study. Am J Gastroenterol. 1999;94:418-23. [ Links ]
3. Olesen M, Eriksson S, Bohr J, et al. Lymphocytic colitis: a retrospective clinical study of 199 Swedish patients. Gut. 2004;53:536-41. [ Links ]
4. Giardiello FM, Jackson FW, Lazenby AJ. Metachronous occurrence of collagenous colitis and ulcerative colitis. Gut. 1991;32:447-9. [ Links ]
5. Pokorny CS, Kneale KL, Henderson CJ. Progression of collagenous colitis to ulcerative colitis. J Clin Gastroenterol. 2001;32:435-8. [ Links ]
6. Matteoni CA, Goldblum JR, Wang N, et al. Celiac disease is highly prevalent in lymphocytic colitis. J Clin Gastroenterol. 2001;32:225-7. [ Links ]
7. Riddell RH, Tanaka M, Mazzoleni G. Non-steroidal anti-inflammatory drugs as a possible cause of collagenous colitis: a case-control study. Gut. 1992;33:683-6. [ Links ]
8. Thomson RD, Lestina LS, Bensen SP, et al. Lansoprazole-associated microscopic colitis: a case series. Am J Gastroenterol. 2002;97:2908-13. [ Links ]
9. Beaugerie L, Patey N, Brousse N. Ranitidine, diarrhea, and lymphocytic colitis. Gut. 1995;37:708-11. [ Links ]
10. Duncan H, Talbot I, Silk D. Collagenous colitis and cimetidine. Eur J Gastroenterol Hepatol. 1997;9:819-20. [ Links ]
11. Limsui D, Pardi DS, Smyrk TC, et al. Observer variability in the histologic diagnosis of microscopic colitis. Inflamm Bowel Dis. 2009;15:35-8. [ Links ]
12. Pardi DS, Loftus EV, Smyrk TC, et al. The epidemiology of microscopic colitis: a population based study in Olmsted County, Minnesota. Gut. 2007;56:504-8. [ Links ]
13. González N, Guerra L, Sanguinetti A, et al. Prevalence of microscopic colitis in a group of patients from Montevideo, Uruguay. Acta Gastroenterol Latinoam. 2010;40:216-20. [ Links ]
14. Kao KT, Pedraza B, McClune A, et al. Microscopic colitis: a large retrospective analysis from a health maintenance organization experience. World J Gastroenterol. 2009;15:3122-7. [ Links ]
15. Giardiello FM, Lazenby AJ, Bayless TM, et al. Lymphocytic (microscopic) colitis: clinicopathologic study of 18 patients and comparison to collagenous colitis. Dig Dis Sci. 1989;34:1730-8. [ Links ]
16. Fernández-Bañares F, Salas A, Esteve M, et al. Collagenous and lymphocytic colitis. Evaluation of clinical and histological features, response to treatment, and long-term follow-up. Am J Gastroenterol. 2003;98:340-7. [ Links ]
17. Koulaouzidis A, Saeed AA. Distinct colonoscopy findings of microscopic colitis: Not so microscopic after all? World J Gastroenterol. 2011;17:4157-65. [ Links ]
18. Mahajan D, Goldblum JR, Xiao SY, et al. Lymphocytic colitis and collagenous colitis: a review of clinicopathologic features and immunologic abnormalities. Adv Anat Pathol. 2012;19:28-38. [ Links ]
19. Yantis RK, Odze RD. Optimal approach to obtaining mucosal biopsies for assessment of inflammatory bowel disorders of the gastrointestinal tract. Am J Gastroenterol. 2009;104:774-83. [ Links ]
20. Montgomery EA, Voltaggio L. Biopsy interpretation of the gastrointestinal tract mucosa. Volume 1, Non-Neoplasia. Philadelphia, PA: Lippincott Williams & Wilkins; 2012. [ Links ]
21. Lazenby AJ, Yardley JH, Giardiello FM, et al. Lymphocytic ("microscopic") colitis: a comparative histopathologic study with particular reference to collagenous colitis. Hum Pathol. 1989;20:18-28. [ Links ]
22. Mohamed N, Marais M, Bezuidenhout J. Microscopic colitis as a missed cause of chronic diarrhea. World J Gastroenterol. 2011;17:1996-2002. [ Links ]
23. Goldstein NS, Bhanot P. Paucicellular and asymptomatic lymphocytic colitis: expanding the clinicopathologic spectrum of lymphocytic colitis. Am J Clin Pathol. 2004;122:405-11. [ Links ]
24. Brown IS, Lambie DLJ. Microscopic colitis with giant cells: a clinico-pathological review of 11 cases and comparison with microscopic colitis without giant cells. Pathology. 2008;40:671-5. [ Links ]
25. Goldstein NS, Gyorfi T. Focal lymphocytic colitis and collagenous colitis: patterns of Crohns colitis? Am J Surg Pathol. 1999;23:1075-81. [ Links ]
26. Ferry JA. Lymphoid tumors of the GI tract, hepatobiliary tract, and pancreas. En: Odze RD, Goldblum JR, Eds. Surgical pathology of the GI tract, liver, biliary tract, and pancreas. Philadelphia, PA: Saunders Elsevier; 2010, p. 701-32. [ Links ]
27. Gremse DA, Boudreaux CW, Manci EA. Collagenous colitis in children. Gastroenterology. 1993;104:906-9. [ Links ]
28. Allende DS, Taylor SL, Bronner MP. Colonic perforation as a complication of collagenous colitis in a series of 12 patients. Am J Gastroenterol. 2008;103:2598-604. [ Links ]
29. Yuan S, Reyes V, Bronner MP. Pseudomembranous collagenous colitis. Am J Surg Pathol. 2003;27:1375-9. [ Links ]
30. Unal A, Guven K, Yurci A, et al. Is increased colon subepithelial collagen layer thickness in diabetic patients related to collagen colitis? An immunohistochemical study. Pathol Res Pract. 2008;204:537-44. [ Links ]
31. Miehlke S, Madisch A, Karimi D, et al. Budesonide is effective in treating lymphocytic colitis: a randomized-doubleblinded-placebo controlled study. Gastroenterology. 2009;136:2092-100. [ Links ]











 texto em
texto em