Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.4 Bogotá out./dez. 2013
Helicobacter hepaticus model of infection: the human hepatocellular carcinoma controversy
Yessica Agudelo Zapata, MD. (1), Rodrigo Castaño Llano, MD. (2), Mauricio Corredor, PhD. (3)
(1) Medical Doctor and General Surgeon in the Gastrohepatology Group at Universidad de Antioquia in Medellin, Colombia.
(2) Gastrointestinal Surgeon and Endoscopist in the Gastrohepatology Group at the Universidad de Antioquia in Medellin, Colombia.
(3) Institute Professor of Biology in the Faculty of Natural Sciences and the GEBIOMIC Group the Gastroenterology Group at the Universidad de Antioquia in Medellin, Colombia.
Received: 07-05-13 Accepted: 27-08-13
Abstract
The discovery of Helicobacter 30 years ago by Marshall and Warren completely changed thought about peptic and duodenal ulcers. The previous paradigm posited the impossibility of the survival of microorganisms in the stomach's low pH environment and that, if any microorganisms survived, they would stay in the duodenum or elsewhere in the intestine. Today the role of H. pylori in carcinogenesis is indisputable, but little is known about other emerging species of the genus Helicobacter in humans. Helicobacter hepaticus is one of these species that has been studied most, after H. pylori. We now know about their microbiological, genetic and pathogenic relationships with HCC in murine and human infections. This review aims to show the medical and scientific community the existence of new species of Helicobacter that have pathogenic potential in humans, thus encouraging research.
Keywords
Helicobacter hepaticus, Helicobacter pylori, Helicobacter spp., hepatocellular carcinoma.
INTRODUCTION
Culturing Helicobacter pylori and recognizing their clinical relevance has helped renew interest in bacteria from this genus associated with the gastrointestinal and hepatobiliary tracts in humans and other animals. Many of these bacteria have been now identified as new Helicobacter species, and this genus now consists of 32 validated species. (1) H. pylori was described more than a century ago, and it is now known that this bacterium has infected the human stomach for millennia and probably for millions of years. (2) Nevertheless, it was not taken into account in medicine until 1983 when Warren and Marshall rediscovered it. (3) The population genetics of H. pylori mimics that of humans and seems to reflect ancient human migrations. Therefore, humans probably acquired H. pylori early in their history. (4) Apart from humans, the only natural hosts of H. pylori appear to be primates, but in laboratory experiments this bacterium can infect animals including mice, dogs and gerbils which could lead to a proposal to use H. pylori as an evolutionary marker. (4)
H. pylori have been bly associated with gastritis, peptic ulcer, gastric cancer, and gastric lymphoma. (2) It is even considered a carcinogen by the International Agency for Cancer. (5) Nevertheless, idiopathic thrombocytopenic purpura (ITP), an entity among the extra-gastrointestinal diseases that have been studied over the last decade, has the most abundant evidence in the literature. It has even been shown that eradication of this bacterium increases platelet counts. (6)
In both children and adults H. pylori have also been weakly associated with other entities outside the gastrointestinal tract in places such as the liver and bile ducts. These include cirrhosis, hepatocellular carcinoma, primary sclerosing cholangitis and primary biliary cirrhosis. (7) Other diseases involved are iron deficiency anemia, chronic diarrhea, atherosclerosis, and impaired growth and development. All of these associations are controversial because they are only supported by case reports, small pilot studies or in vitro data. (8, 9, 10)
H. pylorus is not the only species of human Helicobacter. Helicobacter species can be divided into two groups: gastric and enterohepatic. Helicobacter hepaticus, another species of Helicobacter that has been widely studied, is found within the enterohepatic group. This relatively new bacterium has been implicated in hepatitis, hepatocellular carcinoma and biliary tumors in rodent models and recently, in human samples. This has generated controversy in the scientific community which has asked if H. hepaticus is a human pathogen, and if it is involved in hepatocellular carcinoma or in other diseases since there is clear evidence in mouse models. (11)
CONTROVERSY
Proof that this bacterium causes disease in humans, and may even be implicated in hepatocellular carcinoma (HCC), is not clear within the logic of science. Many researchers cannot accept the possibility of this hypothesis. (12) Arguing causality will be more difficult in this case than it was when Warren and Marshall demonstrated the importance of H. pylori for humanity because systematic study of these other bacteria will require proper insulation and methods of identification in both healthy and sick patients. (13) The controversy that has arisen over this issue has led to reviews and even a meta-analysis in 2008 about the relationship between HCC and Helicobacter species. The meta-analysis found a significant positive association between the bacteria and the risk of HCC. (14) Nevertheless, results should be interpreted with caution because there have been few studies. A conclusion that other reviews have arrived at is that better designed studies and prospective studies are needed to validate the hypothesis. (14, 15, 12)
During the last decade, studies have focused on the relationship between H. pylori and chronic Helicobacter spp. infections associated with several extra-gastric manifestations. These include ischemic heart disease, liver diseases and hematological disorders. Since eradication of gastric H. pylori is easy and relatively inexpensive, this would important for public health. (16, 17, 18)
In humans, the presence of bacterial DNA in the liver of HCC patients is also indicative of the likely presence of Helicobacter spp. in this organ. However, the difficulty of culturing the microorganism places the reality of these infections in doubt (Koch principle). (12) This can be explained by low bacterial levels and bacterial adaptation to a special environment as reported in the livers of mice. (17) Proper isolation and identification methods for these bacteria are required to find out whether this genus will influence the management of intestinal and systemic diseases as dramatically as H. pylori influenced the treatment of gastroduodenal diseases. (13)
Evidence has shown time and time again that Helicobacter, in addition to its correlation with viral hepatitis and hepatocellular carcinoma, seems to have an association with HCC. This strengthens the conclusion of the meta-analysis and reviews that there are not enough prospective and good-quality studies to validate the hypothesis. (17, 12)
FROM GASTRIC CANCER TO LIVER CANCER
Gastric cancer and liver cancer are diseases with different etiological agents and mechanisms of carcinogenesis, but what can the Helicobacter genus find to grow on in these media? And, how can it harm two very different types of cells?
Gastric cancer's origin is multifactorial within which H. pylori play an important role, although the bacterium's exact participation in carcinogenesis is still difficult to determine. Nevertheless, factors that indirectly and directly associate H. pylori with gastric cancer are known. The direct associations include the fact that when there is an H. pylori infection, the risk of gastric cancer is 2 to 3 times greater than otherwise, but if there are anti-CagA antibodies the risk increases 11 times. When combined with an alteration of the gene encoding the synthesis of interleukin-1B-511, the risk increases 87 times. (5) Indirect associations include the inflammatory response triggered in the infected stomach which produces molecular and morphological changes that can progress to cancer. (5)
Similarly, HCC is the first malignant primary neoplasm of the liver and the fifth leading cause of consultation because of malignant disease in the digestive tract. (19) Its etiology is closely linked to processes that lead to cirrhosis such as chronic hepatitis B and C infections. However, the mechanism by which the tumor develops is not yet well understood. (19) It is also known that when hepatocellular carcinoma develops in patients with no history of hepatitis or cirrhosis, it is a rapidly progressing disease with a high mortality rate. (19)
The history of the relationship between Helicobacter and liver cancer dates back to 1992 when the National Institute of Cancer in the United States found a high rate of liver tumors in mice infected with the bacteria. (20, 21)
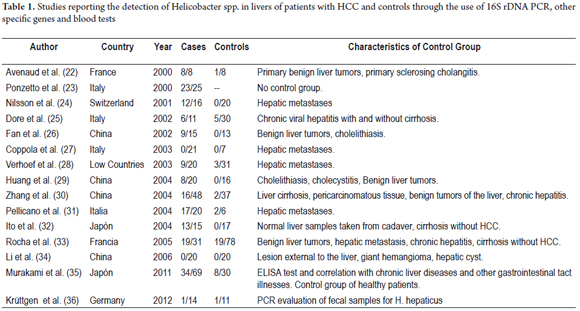
This finding led to a series of investigations which, by 2000, had found genetic material from Helicobacter spp. including H. pylori in tissue from human livers and HCC patients. From these findings, several researchers have reported Helicobacter DNA in HCC tissue samples (Table 1). (22-36) Nevertheless, the implications of infection by these bacteria have not been well studied because all studies use 16S rDNA PCR and, as already mentioned, cultures of liver species have not been achieved.
These bacteria have been associated with hepatotropic viruses B and C which supports the thesis that Helicobacter spp. may play a role in the evolution of liver damage in chronic viral hepatitis from cirrhosis to HCC. However, determinants of this evolution are not well understood. This evidence was reported by Rocha et al. and Dore et al. who tried to prove the association of the Helicobacter species with hepatitis C, cirrhosis and HCC. (33, 25) These two studies included a wide range of patients from which they examined tissue samples from patients with HCC, cirrhosis, and hepatitis C. Bacterial DNA was found in 4.2% (33) of the liver biopsies from controls and in 3.5% (25) of the liver biopsies of patients with chronic hepatitis C. However, the prevalence of Helicobacter species was higher in patients with HCV cirrhosis (68%) and in those with cirrhosis and hepatocellular carcinoma (61%) suggesting that these bacteria may have roles in causing progression to HCC. There have been also studies that show correlations between serological H. hepaticus, liver disease, and HBV and HCV infections and which conclude that Helicobacter infections may play a role in the development of liver disease and in particular may increase the risk of developing liver disease related to infections with hepatotropic viruses. (35, 37)
In the last year, a PCR study of stool samples from various patients with hepatitis B and C, and other liver and gastrointestinal tract diseases found no association that would provide support for a pathogenic role for H. hepaticus in the viral etiology of HCC. The authors say that the results do not exclude a role for H. hepaticus in cases of HCC caused by other carcinogens, such as aflatoxins. (36)
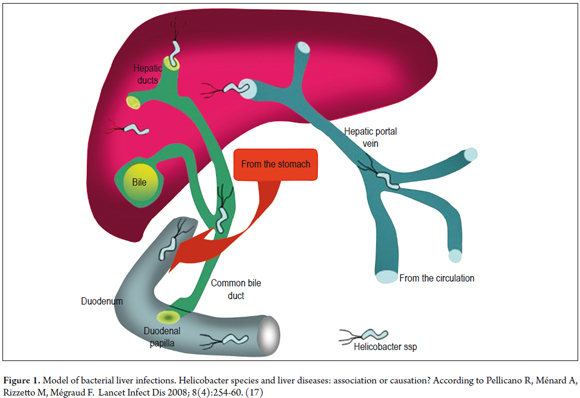
When studying the pathophysiology of two cancer processes as different as gastric cancer and liver cancer, it is valid to ask how the Helicobacter genus can infect so many different environments. When the bacterium infects the gastroduodenal mucosa, it alters the function and anatomy by inducing inflammation which favors the appearance of dysplasia. (38) To effect these changes in the gastroduodenal environment these bacteria have virulence factors such as adherence factor, urease, proteolytic enzymes, cytotoxic proteins, and vacuolating proteins. Finally, they have cellular stress proteins that allow them to survive in adverse situations. (38) With these characteristics of gastric infections in mind, it is necessary to illustrate the environment that the liver represents for bacteria. This habitat does not have such rugged conditions as the stomach does. Since the pH of bile ducts is slightly alkaline or neutral values, virulence factors such as urease are not needed for survival, hence the presence or absence of urease differentiates the gastric species from the enterohepatic species. (39) There are other aspects of the liver that differ from the stomach that represent a challenge for the bacteria when entering the hepatocyte or a suitable place within the liver to replicate. Infection models that explain the interaction between Helicobacter spp., H. pylori, H. hepaticus and liver tissue have been suggested. There are two routes, ascending through bile duct or entering through the portal vein (Illustrated in Figure 1). (17) The first route is more expeditious.
An in vitro investigation of the adhesion of H. pylori to hepatocytes has been used as a model for Helicobacter spp. (7) This bacterium is able to adhere to and then invade hepatocytes in vitro although this depends on virulence factors and their persistence in cell cultures. In turn, integrin β1 is related as a receptor for internalizing bacteria into hepatocytes. It has been found that H. pylori affect cell replication mainly by inducing apoptosis with a compensatory increase of DNA synthesis to balance the increase of lost cells. Persistent liver infections can increase both apoptosis and DNA synthesis, suggesting that persistence of Helicobacter plays an important role in the pathogenesis of liver diseases and changes the critical balance between cell proliferation and apoptosis. This seems to involve the pathogenesis of a variety of human diseases including cancer. (40)
Although persistence has been determined by molecular methods of Helicobacter spp. (including H. hepaticus and H. pylori) in hepatocytes, it has not been possible to culture cells directly in human livers. Nevertheless, this has been done in mice (41), but this bacterium grew in only 11.5% of cases, while 66.6% of mouse model cases tested positive by PCR. (41)
Two markers which have been studied are H19 and intestinal trefoil factor 3. The expressions of these markers increases in cells infected with progressive hepatocellular dysplasia:. (42) Toxins such as H. hepaticus which produces cytolethal distending toxin (CDT) with DNase activity and possibly promotes tumor development have been included as direct mechanisms that cause hepatocellular damage in vivo. (17, 43) Other virulence factors include VacA and Type 4 bacterial secretion systems. In general Helicobacter spp. is a b inducer of pro-inflammatory cytokines that contribute to development of malignant diseases through damaging DNA. (43)
H. HEPATICUS IN OTHER MOUSE AND HUMAN DISEASES
Although the habitat of H. hepaticus is in the liver and biliary tract, it has also been demonstrated to be present in the large and small intestines in mice and has been associated with irritable bowel syndrome, (21) colitis and typhlitis. These relationships are still poorly understood but seem to be important, and there is evidence from murine models that they support the hypothesis. (44, 45, 46)
Several studies have shown the presence of enterohepatic Helicobacter species in humans which have been associated with enteritis, hepatitis, and cholecystitis in immunosuppressed patients. (42) Others have found Helicobacter species associated with ulcerative colitis and concomitant liver disease, and these bacteria have been associated with liver diseases in children. (47) Other evidence shows that H. hepaticus can be associated with liver and biliary tract diseases in humans as reiterated in this document, and even with biliary tract cancer. (48, 49)
HISTORY OF HELICOBACTER HEPATICUS
The helicobacter hepaticus bacterium was discovered in 1994 by Fox et al. who isolated it from mice livers with chronic active hepatitis. The bacterium also colonizes the colonic mucosa in mice. (41) Based on an analysis of the sequence of 16S rRNA genes, this organism was classified as a novel Helicobacter, named hepaticus. It was recognized as an efficient colonizer of the gastrointestinal tract which, in mice, is potentially pathogenic in persistent hepatitis and liver cancer. This bacterium is the prototype of enterohepatic Helicobacter species which has recently been found to infect humans and cause disease. (41, 47, 48)
Morphological characteristics of the microorganism
Helicobacter hepaticus is a spiral-shaped bacterium with bipolar flagellum. Examination by dark field and phase microscopy reveals motile spiral gram negative bacteria, measuring from 1.5 to 5.0 µm in length and from 0.2 to 0.3 µm in width. The body varies in shape and size from curved to spiral with one or more coils. (41)
Physiologic and biochemical characteristics of the bacteria
Like other Helicobacter species such as H. muridarum and H. rappini, H. hepaticus has b urease activity. Although it does not live in an acid environment, studies have shown that H. hepaticus produces a urease similar to that produced by H. pylori. (50)
In addition to being urease positive, H. hepaticus is also oxidase and catalase positive. H. hepaticus strains consistently produce H2S using lead acetate, and they reduce nitrates to nitrites. These are microaerophilic bacteria which grow at 37 °C. All strains are resistant to cephalothin and nalidixic acid but sensitive to metronidazole. (41)
Bacterial genetics
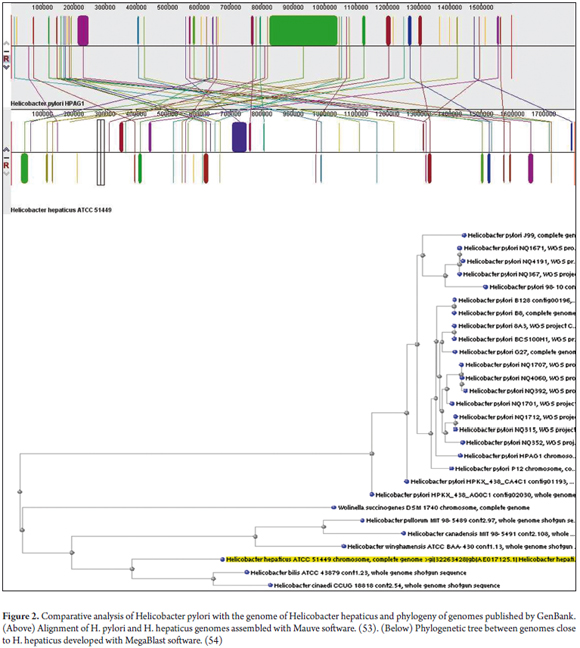
In 2003 the complete sequence of the H. hepaticus ATCC51449 bacterium was reported. (50) It has a circular chromosome of 1,799,146 bp encoding 1,875 proteins. A total of 938 are orthologous with H. pylori with which H. hepaticus ATCC51449 shares some virulence factors. At the least, these include adhesins, cytotoxins VacA and all the Cag pathogenicity islands. It also shows similarity with Campylobacter jejuni and exhibits similar pathogenic mechanisms (See Figure 2). (50)
It has become clear that H. hepaticus is an organism with pathogenic potential, although the absence of many of the virulence and colonization factors of H. pylori explains its inability to colonize the stomach. Its extensive physiological similarity with C. jejuni is correlated to enteric colonization. The reasons for this tropism for the hepatobiliary tract and in particular the reasons for its carcinogenic potential are not clearly understood, but the availability of genome sequencing provides the opportunity for a systematic exploration of tissue tropism mechanisms and carcinogenesis induced by H. hepaticus.
H. hepaticus has many factors in common with H. pylori. Persistent infection of a host leads progressively to chronic inflammation in either case, and in both cases this inflammation can progress to hepatocellular carcinoma. H. hepaticus does not colonize the stomach, but shares a habitat in the small intestine with C. jejuni, a bacterium that frequently causes diarrhea in humans. (21)
IMMUNOLOGY
It is known that H. pylori infections stimulate both innate and acquired immune responses. The initial step in this process is recognition of the microorganism by NOD1 (Nucleotide-binding oligomerization domain protein I). Meanwhile, H. pylori also stimulates the innate immune response against H. pylori which includes the release of antibacterial peptides and the infiltration of the mucosa by all types of immune effector cells. (51, 5) In addition to this primary response, an acquired local systemic cellular and humoral immune response is triggered which persists throughout life. The T cell response is essentially Th1 which is the "wrong" response since H. pylori is an extracellular germ. Like other similar microorganisms, H. pylori should trigger a Th2 response. The Th1 response produces gamma (IFN γ) interferon, tumor necrosis factor alpha (TNF-α), IL-12, and IL-18. (51) the Th2 response produces IL-4, IL-5, IL-10, fibroblast transforming growth factor β, mucosal immunoglobulin IgA and IgE, and it reduces the inflammation caused by Th1 in response to H. pylori. This affects progression from chronic gastritis to atrophy, dysplasia and cancer. (5)
Some experimental mouse models have shown that H. hepaticus activates Th17 cells which release cytokines associated with inflammation such as IL-17 and releases the nuclear factor kappa B associated with carcinogenesis. Nevertheless more studies are needed to determine whether this response is homologous with the immune response generated by H. pylori. (51, 36)
Interestingly, it has been proposed that this type of bacteria's relationship with the immune system could occur through innate immunity which would provide a possible explanatory model of bacterial intervention in autoimmune diseases. (52)
CONCLUSION
It is still difficult to assert or discard a relationship of H. hepaticus with hepatocellular carcinoma. It is even risky since several paradigms have shown how compromising it can be to make such an assertion without experimental evidence. H. pylori has been the most obvious example of this. In the 1980's, Prusiner had already spoken about the existence of prions, HIV had struck a blow against scientific advances in health, disease prevention and vaccination since mankind had overcome smallpox.
For this reason reports from Rocha et al., Dore et al. and Murakami et al. suggested an association of Helicobacter spp. in the evolution of chronic liver disease related to HCV and HBV in humans. (25, 33, 35) Nevertheless, the ability to grow Helicobacter spp. after isolation from liver has not yet been shown in any report which has generated enormous difficulty. Until now, trust has been based on PCR amplification which is a test that still leaves huge questions because it omits Koch propagation. Slowly, that trust has begun to be questioned since some microorganisms do not fully satisfy the principle of Koch. For example, the premise that the infectious agent must be isolated from the body in a pure culture isolated from the lesions caused by the disease is not true for Mycobacterium leprae. Contravention of the Koch postulate by this bacterium and others prevents classical conclusions and leaves the door open to new techniques such as PCR or deep sequencing using metagenomics becoming alternative diagnostic gold standards for elucidation of the human microbiome and virobioma. (12)
REFERENCES
1. Hayashi S, Shimomura H, Hirai Y. Latest advances in non-pylori Helicobacter species. Nippon Rinsho. 2009;67:2271-8. [ Links ]
2. Correa P. Impacto social de la infección por helicobacter pylori en Colombia. Rev Acad Nac Med Colomb. 1999;21:51. [ Links ]
3. Marshall B, Warren JR. Unidentified curved bacillus on gastric epithelium in active chronic gastritis. Lancet. 1983;1:1273-5. [ Links ]
4. Eppinger M, Baar C, Linz B, et al. Who ate whom? Adaptive helicobacter genomic changes that accompanied a host jump from early humans to large felines. Plos Gen. 2006;2:1097-110. [ Links ]
5. Otero W, Gómez M, Trespalacios AA. Helicobacter pylori: después de todo. Temas escogidos de gastroenterología. Asoc Colomb Gastroenterol. 2007:43-56. [ Links ]
6. Campuzano G. Proof of an Association between helicobacter pylori and idiopathic thrombocytopenic purpura in Latin America. Helicobacter. 2007;12:265-73. [ Links ]
7. Ito K, Yamaoka Y, Ota H, et al. Adherence, Internalization, and Persistence of Helicobacter pylori in Hepatocytes. Dig Dis Sci. 2008;53:2541-9. [ Links ]
8. Franceschi F. Helicobacter pylori and extragastric diseases. Clin Gastroenterol. 2007;21:325-34. [ Links ]
9. Moyaert H, Franceschi F, Roccarina D, et al. Extragastric manifestations of helicobacter pylori infection: other helicobacters. Helicobacter. 2008;13(Suppl. 1):47-57. [ Links ]
10. Pellicano R, Franceschi F, Saracco G, et al. Helicobacters and extragastric diseases. Helicobacter. 2008;14(Suppl. 1):58-68. [ Links ]
11. Ward J, Anver M, Haines D, et al. Chronic active hepatitis in mice caused by helicobacter hepaticus. Am J Pathol. 1994;145:959-68. [ Links ]
12. Velázquez E, Peix A, Gómez A. Microorganismos y cáncer: evidencias científicas y nuevas hipotésis. Cir Esp. 2011;89:136-44. [ Links ]
13. Orourke J, Grehan M, Lee A. Non-pylori helicobacter species in humans. Gut. 2001;49:601-6. [ Links ]
14. Xin Y, Chen A, Dong Q, et al. Association between the presence of H pylori in the liver and hepatocellular carcinoma: a meta-analysis. World Gastroenterol. 2008;14:307-12. [ Links ]
15. Wu X, Chen D. Helicobacter pylori and hepatocellular carcinoma: correlated or uncorrelated. J Gastroenterol Hepatol. 2006;21:345-7. [ Links ]
16. Tu Q, Okoli AS, Kovach Z, et al. Hepatocellular carcinoma: prevalence and molecular pathogenesis of Helicobacter spp. Comp Med. 2009;59:534-44. [ Links ]
17. Pellicano R, Ménard A, Rizzetto M, et al. Helicobacter species and liver diseases: association or causation? Lancet Infect Dis. 2008;8:254-60. [ Links ]
18. Otero W, Trespalacios A, Otero E. Helicobacter pylori: Tratamiento actual. Un importante reto en gastroenterología. Rev Colomb Gastroenterol. 2009;24:279-92. [ Links ]
19. Argüello P, Albis R, Escovar J, et al. Hepatocarcinoma: patología maligna de mal pronóstico. Rev Colomb Gastroenterol. 2003;18:153-7. [ Links ]
20. Takayama S, Takahashi H, Matsuo Y, et al. Effect of helicobacter bilis infection on human bile duct cancer cells. Dig Dis Sci. 2009;54:123-7. [ Links ]
21. Suerbaum S, Josenhans C, Sterzenbach T, et al. The complete genome sequence of the carcinogenic bacterium Helicobacter hepaticus. Proc Natl Acad Sci USA. 2003;100:7901-6. [ Links ]
22. Avenaud P, Marais A, Monteiro L, et al. Detection of helicobacter species in the liver of patients with and without primary liver carcinoma. Cancer. 2000;89:1431-9. [ Links ]
23. Ponzetto A, Pellicano R, Leone N, et al. Helicobacter infection and cirrhosis in hepatitis C virus carriage: is it an innocent bystander or a troublemaker? Med Hypotheses. 2000;54:275-7. [ Links ]
24. Nilsson HO, Mulchandani R, Tranberg KG, et al. Helicobacter species identified in liver from patients with cholangiocarcinoma and hepatocellular carcinoma. Gastroenterology. 2001;120:323-4. [ Links ]
25. Dore M, Realdi G, Mura D, et al. Helicobacter infection in patients with HCV-related chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Dig Dis Sci. 2002;47:1638-43. [ Links ]
26. Fan X, Peng X, Huang Y, et al. Helicobacter species ribosomal DNA recovered from the liver tissue of Chinese patients with primary hepatocellular carcinoma. Clin Infect Dis. 2002;35:1555-7. [ Links ]
27. Coppola N, de Stefano G, Marrocco C, et al. Helicobacter spp. and liver diseases. Infez Med. 2003;4:201-7. [ Links ]
28. Verhoef C, Pot R, de Man R, et al. Detection of identical Helicobacter DNA in the stomach and in the non-cirrhotic liver of patients with hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2003;15:1171-4. [ Links ]
29. Huang Y, Fan X, Wang Z, et al. Identification of Helicobacter species in human liver samples from patients with primary hepatocellular carcinoma. J Clin Pathol. 2004;57:1273-7. [ Links ]
30. Zhang SQ, Bao Y, Zu M. The correlation between Helicobacter infection and hepatocellular carcinoma. Zhongguo Zhongliu Linchuang. 2004;31:761-4. [Artículo en chino] [ Links ].
31. Pellicano R, Mazzaferro V, Grigioni W, et al. Helicobacter species sequences in liver samples from patients with and without hepatocellular carcinoma. World J Gastroenterol. 2004;10:598-601. [ Links ]
32. Ito K, Nakamura M, Toda G, et al. Potential role of Helicobacter pylori in hepatocarcinogenesis. Int J Mol Med. 2004;13:221-7. [ Links ]
33. Rocha M, Avenaud P, Menard A, et al. Association of Helicobacter species with hepatitis C cirrhosis with or without hepatocellular carcinoma. Gut. 2005;54:396-401. [ Links ]
34. Li N, Zhang SH, Xuan SY, et al. Study on Helicobacter infection in liver tissue from hepatocellular carcinoma. Zhonghua Liuxingbingxue Zazhi. 2006;27:894-6. [Artículo en chino] [ Links ].
35. Murakami K, Takahashi R, Ono M, et al. Serodiagnosis of Helicobacter hepaticus infection in patients with liver and gastrointestinal diseases: western blot analysis and ELISA using a highly specific monoclonal antibody for H. hepaticus antigen. J Gastroenterol. 2011;46:1120-6. [ Links ]
36. Kruettgen A, Horz H, Weber J, et al. Study on the association of Helicobacter species with viral hepatitis induced hepatocellular carcinoma. Gut Microbes. 2012;3:228-33. [ Links ]
37. Murakami K. Infection of Helicobacter species and liver disease. J Gastroenterol. 2012;47:724-5. [ Links ]
38. Sierra F. Helicobacter pylori y enfermedades gástricas. En: Franco F, Sierra F. Fundamentos de medicina, gas troenterología y hepatología. 5ta. ed. Medellín: CIB; 2004. p. 53-8. [ Links ]
39. Solnick J, Vandamme P. Taxonomy of the Helicobacter Genus. En: Mobley H, Mendz GL, Hazell SL, editores. Helicobacter pylori. Physiology and Genetics. Washington: ASM Press; 2001. Cap. 5. [ Links ]
40. Ito K, Yamaoka Y, Yoffe B, et al. Disturbance of apoptosis and DNA synthesis by Helicobacter pylori infection of hepatocytes. Dig Dis Sci. 2008;53:2532-40. [ Links ]
41. Fox J, Dewhirst F, Tully J, et al. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238-45. [ Links ]
42. Boutin S. Molecular pathogenesis of Helicobacter hepaticus induced liver disease, Massachusetts Institute of Technology [internet]. 2011 [citado 2012 oct. 15]. Disponible en: http://biblioteca.universia.net/html_bura/ficha/params/id/34665877.html [ Links ]
43. Liyanage N, Manthey K, Dassanayake R, et al. Helicobacter hepaticus cytolethal distending toxin causes cell death in intestinal epithelial cells via mitochondrial apoptotic pathway. Helicobacter. 2010;15:98-107. [ Links ]
44. Li X, Fox J, Whary M, et al. SCID/NCr mice naturally infected with helicobacter hepaticus develop progressive hepatitis, proliferative typhlitis, and colitis. Infect Immun. 1998;66:5477-84. [ Links ]
45. Fox J, Yan L, Shames B, et al. Persistent hepatitis and enterocolitis in germfree mice infected with helicobacter hepaticus. Infect Immun. 1996;64:3673-81. [ Links ]
46. Dieleman L, Arends A, Tonkonogy S, et al. Helicobacter hepaticus does not induce or potentiate colitis in interleukin-10-deficient mice. Infect Immun. 2000;68:5107-13. [ Links ]
47. Casswall T, Németh A, Nilsson I, et al. Helicobacter species DNA in liver and gastric tissues in children and adolescents with chronic liver disease. Scand J Gastroenterol. 2010;45:160-7. [ Links ]
48. Hamada T, Yokota K, Ayada K, et al. Detection of Helicobacter hepaticus in human bile samples of patients with biliary disease. Helicobacter. 2009;14:545-51. [ Links ]
49. Gopal N, Anil K, Vijay K. Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J Gastroenterol. 2010;16:5395-404. [ Links ]
50. Beckwith C, Mcgee D, Mobley H, et al. Expression, and catalytic activity of helicobacter hepaticus urease. Infect Immun. 2001;69:5914-20. [ Links ]
51. Darling AC, Mau B, Blattner FR, Mazmanian S, McBride S. Microbial health factor. The Scientist-Magazine of the Life Sciences [internet]. 2009 [citado 2012 oct. 15]. Disponible en: http://www.the-scientist.com/article/display/55864/#ixzz0mWl9gtq [ Links ]
52. Yanagisawa N, Haruta I, Kikuchi K, et al. Are dysregulated inflammatory responses to commensal bacteria involved in the pathogenesis of hepatobiliary-pancreatic autoimmune disease? an analysis using mice models of primary biliary cirrhosis and autoimmune pancreatitis. ISRN Gastroenterology. 2011:ID 513-4. [ Links ]
53. Altschul S, Gish W, Miller W, Myers E, et al. Basic local alignment search tool. J. Mol. Biol, 1990, 215:403-10. [ Links ]
54. Darling A, et al. Mauve: multiple alignment of conserved genomic sequence with rearrangements. Genome Res. 14, 1394-403. [ Links ]











 texto em
texto em