Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.4 Bogotá oct./dic. 2013
Endoscopic Ultrasound Guided Biliopancreatic Diversion: Case Description and Literature Review
Elías Alfonso Forero Piñeros, MD. (1), Doménico Galasso, MD. (2), Erwan Bories, MD. (3), Marc Giovannini, MD. (4)
(1) Head of Digestive Endoscopy and Gastroenterology at the Central Police Hospital in Bogotá, Colombia.
(2) Gastroenterologist in the Digestive Endoscopy Unit of Surgical Operations in the Policlinico Universitario Agostino Gemelli of the Università Cattolica del Sacro Cuore in Rome, Italy.
(3) Gastroenterologist at L'Institut Paoli-Calmettes in Marseille, France.
(4) Head of Therapeutic Endoscopy at L'Institut Paoli-Calmettes in Marseille, France.
Received: 25-10-12 Accepted: 27-08-13
Abstract
In this paper we present the first reported case of endoscopic ultrasound-guided hepatic-gastrostomy, performed on a patient with a history of bariatric surgery (gastric banding). We review the patient's clinical history and the technology and accessories used. This case report is supplemented with a detailed and updated review of the medical literature regarding endoscopic ultrasound-guided biliary-pancreatic diversions. These procedures are rapidly developing in a way that is increasing the therapeutic armory for patients who require biliary or pancreatic derivations but who do not meet the requirements for endoscopic retrograde cholangiopancreatography (ERCP), and who are not candidates for, or who reject, the option of percutaneous biliary bypass.
These procedures include the hepatic gastrostomy guided by endoscopic ultrasound, biliary-pancreatic rendezvous guided by endoscopic ultrasound, endoscopic ultrasound-guided choledochoduodenostomy, endoscopic ultrasonography-guided cholecystogastrostomy and endoscopic ultrasound-guided pancreatic gastrostomy. This article provides a technical description of each of these procedures and the accessories required.
Finally, we present patient management following the guide of the most experienced pioneers of these techniques in the world. These procedures already have a well-recognized place in the therapeutic armory for patients who require this kind of diversion.
Keywords
Endoscopic ultrasound, hepaticogastrostomy, choledochoduodenostomy, rendez-vous, pancreaticogastrostomy.
CLINICAL CASE
Endoscopic ultrasound guided hepatogastrostomy in a patient previously treated with gastric banding.
The clinical history of this man showed obesity: at the time of hospitalization the patient's body mass index was 37.9, his height was 1.78 m, and his weight was 120 kg. The patient had obstructive sleep apnea syndrome and insulin-dependent diabetes and had undergoing laparoscopic gastric banding in 2006. He had been smoking 20 cigarettes a day for 35 years, and had 30% right internal carotid stenosis, asthma and moderate chronic obstructive pulmonary bronchoconstriction.
After initial onset of fatigue, nausea, diarrhea and cholestasis (without jaundice) which were associated with a 6 kg weight loss. Tests for increased liver function tumor markers were conducted with the following results: CA 19-9, 8.816 U / L, CEA 11.3 ng/ml, and alpha-fetoprotein 5.4 g/l. The 53 -year-old man was subjected to further tests (Table 1) including magnetic resonance imaging on May 7, 2012 which showed a 35mm nodular mass in the hepatic hilum. This led to a determination that the intrahepatic bile ducts were dilated.
The patient was referred to the Paoli Calmette Institute for further research including evaluation by endoscopic ultrasonography (EUS) and fine needle aspiration (FNA).
Computed axial tomography (CAT) performed at our hospital on May 31, 2012 found that the lesion appeared to extend into the left branch of the portal vein, the left hepatic artery and into the posterior lateral bile ducts. Hilar lymphadenopathies, celiac lymphadenopathies and lymphadenopathies in the vena cava and aorta were also detected.
The left branch of the neoplastic thrombus in the portal vein was punctured with an endoscopic ultrasound (EUS) guided cytological G 25 gauge needle (EchoTip, Cook Ireland Ltd., Limerick , Ireland) on June 12, 2012. Immunohistochemistry of the sample showed that it was intensely positive for CK7 and Muc1 and moderately positive for CK20 which led to a diagnosis of cholangiocarcinoma.
On June 26, 2012, while the patient was undergoing neoadjuvant chemotherapy, jaundice developed (total bilirubin 420 mmol/l, normal value 5.0 to 17.0 mmol/l ). An endoscopic retrograde cholangiopancreatography (ERCP) was performed and a 10cm bare self-expanding metal stent (Flex Wall biliary RX uncovered, Boston Scientific: Galway, Ireland) was placed to drain the right lobe. After the technically challenging drainage of the left lobe during ERCP failed, an 8.5 Fr catheter (Cook Ireland Ltd., Limerick, Ireland) was used to percutaneously drain the right lobe on June 28, 2012 draining
Three attempts (June 28, July 3 and July 19, 2012) were to place a percutaneous biliary stent to drain the left hepatic lobe in the common bile duct through the tumor failed. Instead, an external percutaneous 8.5 Fr stent (Cook Ireland Ltd., Limerick, Ireland) was placed. It drained 600 cm3/day of clear bile.
A CAT scan on July 2, 2012 detected a further increase in tumor volume of up to 40 mm in size, bile ducts were less dilated and the metal stent and external biliary drainage were correctly placed. The lymphadenopathy was stable but a small volume of ascites had appeared.
Since the third attempt to place a percutaneous biliary metallic stent left had failed, and the external drain was draining 300-600 cm3/day of clear bile, a EUS-guided liver gastrostomy was proposed to the patient as a final solution. The patient accepted.
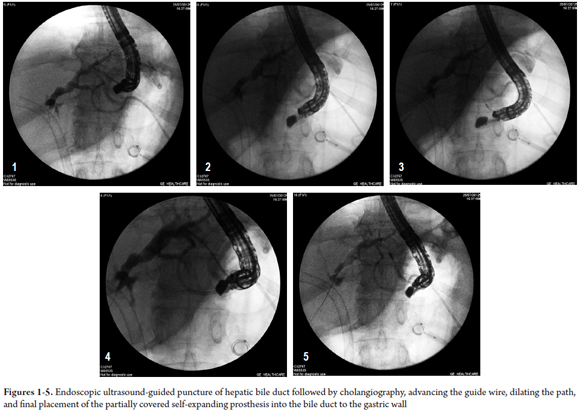
A review of the images from the CAT scan showed a possible window in the cardia for EUS-guided hepatogastrostomy. The external biliary drainage was closed on July 22, and on July 25, 2012 the EUS-guided hepatogastrostomy was successfully conducted.
Procedure
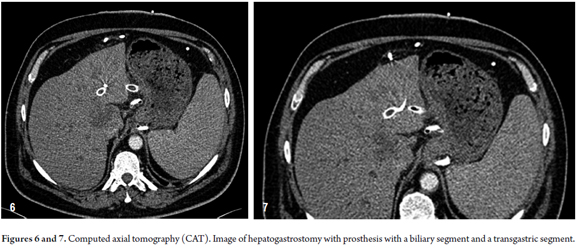
EUS examination of the left hepatic lobe using a therapeutic linear echoendoscope (EG- 3870 UTK, Pentax Corporation, Tokyo, Japan) and an ultrasonic processor (HI VISION Avius, Hitachi Medical Corporation: Tokyo, Japan) detected dilated left intrahepatic bile ducts and external biliary drainage. The patient was already receiving antibiotics (vancomycin and amikacin) because of positive blood cultures but had no fever for 12 hours prior to the procedure.
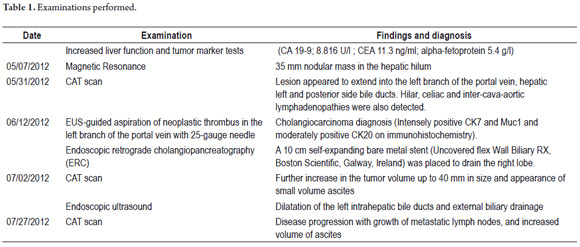
A liver bile duct drain was inserted through a puncture through the wall of the cardia just above the gastric band using an endoscopic ultrasound guided standard 19-gauge FNA needle (EchoTip, Cook Ireland Ltd., Limerick, Ireland). After injecting dye and performance of left hepatic cholangiography, a 450 cm long and 0.035 inches guide wire (Straight Tip Jagwire, Boston Scientific: Alajuela, Costa Rica) was introduced into the left hepatic bile duct.
A 6 Fr cystotome was advanced (Cook Ireland Ltd., Limerick, Ireland) along the guide wire in order to generate a biliary-gastric fistula through which the metallic stent could be inserted. The Endocut mode was used to achieve this goal.
Once the cystotome's tip was inside the bile duct, the guide wire was replaced with a stiffer 0.035-inch and 460 cm long tip (Snap -Tip, Endoskopie MTW: Wesel, Germany). A half covered 10 cm metallic stent was introduced (GIOBOR ' Niti-S half-covered biliary stent, Tae Woong Medical, Gyeonggi-do, South Korea) through the fistula that had been created.
This particular type of self-expanding metallic stent has three radiopaque markers: one in the middle and one at each end. They allow accurate positioning of both the covered and the uncovered portions of the stent.
The uncovered portion of the stent was placed completely inside the bile duct and the covered portion was placed through the fistula in order to avoid any known complications (biliary leaks, liver abscesses, stent migration, etc.).
Follow-up
There were no complications during the procedure or in the following two weeks.
A CAT scan on July 27, 2012 showed progression of the disease with growth of metastatic lymph nodes and increased volume of ascites. Both the biliary metal stent and the transgastric metal stent were in place. The external percutaneous drain, which had remained closed for the entire period, was removed and the patient was referred to oncologists for chemotherapy (gentamicin and cisplatin). The figures 1, 2, 3, 4, 5, 6,7,8 y 9 show the main steps in the procedure and the subsequent CAT scan image.
INTRODUCTION
EUS guided biliary drainage is an option for treating obstructive jaundice when ERCP drainage fails. EUS guided biliary drainage has been made possible by the development of ultrasound endoscopes and accessories which have created an additional alternative for percutaneous biliary drainage and an alternative to surgery. Although ERCP is still the gold standard of treatment for biliary drainage, it is not infallible. For example, to successful diversion of pancreatic cancer patients is only effective in 80% to 85% of cases which leaves a large group of patients that require alternative treatment. Cannulation of the large papilla fails in 3% to 12 % of cases even when precuts and infundibulotomy are used. (1)
Limitations of percutaneous biliary drainage include the requirement that intrahepatic bile ducts be enlarged and a 25% to 30 % complication rate. Complications include cholangitis, biliary leakage, fistula formation, bilioma, peritonitis, empyema, peritoneal bleeding and stent occlusion. Biliary bypass surgery has a 17% to 50% morbidity rate and 2% to 5% mortality rate. In some series the mortality rate has increased up to 10% to 15%. (2)
Although Wiersema described endoscopic ultrasound-guided cholangiopancreatography in 1996, (3) these techniques have been developed in referral centers with ERCP expert groups only in the last ten years. There is now a general guide to safe performance of most of these procedures. Various names have been proposed for grouping these procedures. They include endosonographic cholangiopancreatography, and the most generally used term, endoscopic ultrasound-guided biliary diversions. Other principal terms in use include EUS guided biliary or pancreatic rendezvous, EUS guided hepatogastrostomy and EUS guided choledochoduodenostomy. Other less frequently used EUS methods are EUS guided cholecystoduodenostomy and EUS guided pancreatogastrostomy. (4, 5, 6, 7, 8)
EUS guided procedures may be the first choice in cases for which ERCP is impossible and the second line choice when ERCP fails. Some of the reasons why ERCP fails to resolve biliary or pancreatic obstruction obstructions include peripapillary diverticula, anatomic variations of the biliopancreatic junction, tumor growth within a diversion stent, digestive compression by a tumor, obstruction of the gastric outlet and surgically altered gastrointestinal anatomy such as in gastric bypass reconstruction or Roux-en-Y anastomosis.
In some patients with altered gastrointestinal anatomy, it is possible to clear an obstruction by facilitating access to the bile duct by whole double balloon copies. Recently isolated cases of clearance of biliary obstruction combining ultrasound-guided rendezvous with double balloon enteroscopy have been reported. These procedures have included placement of a self-expanding metallic stent into a liver-jejunal anastomosis. (9, 10)
Although these EUS guided procedures have been primarily indicated for patients with malignant diseases, there have also been several reports of their use for clearance of biliopancreatic obstructions in patients with benign diseases with a similar clinical success. These have included choledocholithiasis, pancreatolithiasis, benign stenosis and biliary fistulas. In general, only an ultrasound-guided rendezvous is performed for patients with benign diseases. Experts generally avoid hepatogastrostomy or choledochoduodenostomy in patients with benign or potentially resectable malignant diseases even if the initial rendezvous attempt failed. In addition, increased incidences of adverse events have been reported for these procedures in patients with benign diseases than for patients with malignant diseases. (11)
The approach used by experts in high volume ERCP services, who also frequently perform these EUS guided procedures, is as follows:
- Following precut and/or an attempt to perform infundibulotomy, a rendezvous procedure is attempted on all patients for whom ERCP has failed and who have papilla accessible to a duodenoscope. If it is successful, the procedure is completed with standard ERCP. If the rendezvous cannot be completed, the patient is referred for percutaneous or surgical diversion. In cases for which these alternatives are not possible or not accepted by the patient, we proceed to biliodigestive diversion (choledochal duodenostomy or hepatogastrostomy), although this is avoided for patients with benign or potentially resectable malignant diseases.
- Depending on availability and experience, balloon enteroscopy is used to perform a biliary procedure for patients whose papillae are not accessible to a duodenoscope. If this is not possible or not available, the patient it is referred for percutaneous or surgical bypass. In cases in which these alternatives are not feasible, or are not accepted by the patient, we proceed to hepatogastrostomy with the same considerations discussed for malignancy. A combination of EUS rendezvous and balloon enteroscopy can be performed for some patients.
- For selected patients it is possible to combine endoscopic ultrasound-guided diversion procedures with usual antegrade diversion procedures such as balloon dilation and biliary structure stent placement. This is done along an EUS guided diversion path. Examples of such cases include placement of a self-expanding prostheses in the common hepatic duct to resolve an obstruction, resolution of the pressure gradient, reduction of the possibility of persistent leaks or fistulas at the site of the hepatogastrostomy puncture.
MATERIALS AND EQUIPMENT REQUIRED
1. Channel therapeutic linear array echoendoscope (bigger than 3.2 mm) which allows the advance of an 8.5 Fr prosthesis, or one larger than 3.7 mm, and which allows passage of accessories up to 10 Fr diameter.
2. Puncturing needles for endoscopic ultrasound. Usually 19G needles are used because they allow passage of 0.035 guide wires. 22 and 23 G needles only allow passage of 0.018 guides, even though they may occasionally be required to puncture from the duodenum which requires greater needle flexibility. Recently "access" needles (Wilson Cook) have been created to facilitate puncturing and to guide passage. The cutting part is inside the stiletto which is taken away before the guide wire is passed through. This prevents the guide wire from being stripped or cut and some segment of it remaining as a loose foreign body within the bile duct which would also prevent completion of the diversion procedure.
3. Accessories to dilate the pathway following puncture: A 6 Fr cystotome (Endoflex Company) is the most commonly used.
4. Biliary stents. These may be self-expanding plastic or metal (self-expanding metal stent SEMS). Although there are small variations depending on the type of diversion, in recent years the trend has been to use self-expanding metal stents for the following reasons:
SEMS maximum expansion effectively seals the puncture tract/dilation which can, in theory, prevent bile leakage.
Their large diameter provides better long-term permeability which can decrease the need for stent checkups.
Stent dysfunction because of tumor ingrowth or by coagulation is easier to handle in SEMS than in plastic stents: a new SEMS is placed inside the occluded stent. In contrast, changing an occluded plastic transmural stent usually requires guided replacement since removal has the risk of tract disruption with subsequent risk of passing the guide in the attempt to step back to the peritoneum. This practically means having to repeat the whole EUS guided diversion process from the beginning.
The advantages of SEMS must be weighed against the fact that their integration is a bit more difficult than insertion by ERCP and careful attention must be paid to details in proper placement to prevent the risk of shortening above the stent which has the risk of subsequent bile peritonitis. The first publication about this procedure reported the creation of a fistula with a plastic stent which was replaced with a SEMS. Currently, this is no longer done. Today, an internally covered self-expanding stent can be placed to prevent both migration, and - once it is inside the passage through the digestive wall - a fully covered stent is placed to prevent leakage. Giovannini and Bories recently designed a self-expanding metallic stent for hepatogastrostomies which is half uncovered and half covered. The uncovered half remains in the hepatic parenchyma and provides fixation while the covered half passes through the gastric wall and prevents bile leakage. (1) Fully covered stents used without other additional support have been shown to increase the likelihood of complications and migration, albeit these reports have been in small series 25% migration compared to 7 % average migration have been reported. (12) Recently, the fatal migration of a stent into the peritoneum was reported. (13) Also, fully covered stents do not totally prevent clogging by tumor ingrowth.
The following describes each of the EUS guided biliopancreatic diversion procedures currently in use and development.
EUS GUIDED RENDEZVOUS
EUS guided rendezvous consists of advancing a guide wire through the papilla by EUS guided aspiration of any linear bile or pancreatic duct. Once the guide is located in the duodenum, the endoscope is exchanged for a duodenoscope. Then the guide wire is caught with a loop or clip and ERCP is performed in the conventional manner.
The steps of an ultrasound biliary rendezvous are:
- Prior to the procedure, administer prophylactic antibiotics and rule out any contraindications.
- Guided by ultrasound vision, puncture the left hepatic biliary system (segment III) with a 19 G needle under linearly from the cardia. Less often this is done from the distal esophagus, in which case liver segment II is punctured.
- A 0.035-inch hydrophilic guide wire (Cook Tracer or Jagwire, Metro Boston) is passed through the biliary tract and out through the papilla into the duodenum.
- The endoscope is gently removed leaving the guide wire in place.
- A duodenoscope is inserted parallel to the guide wire in the third portion of the duodenum.
- The guide wire is captured with a snare and passed through the working channel of the duodenoscope; accessories needed are mounted on the guide wire to complete the bile duct diversion procedure (sphincterotomy, stone removal, dilation, placement stent, etc.).
One variant is to perform the rendezvous by puncturing the distal common bile duct from the second portion of the duodenum with rectified equipment which tends to direct the puncture toward the papilla. Following puncture, an angled guide wire is passed through the papilla. The rest of the procedure is the same as described above. If the puncture cannot be done through the second portion of the duodenum, it should be attempted through the antrum even though there are lower chances of success. (14, 15)
Another variant is the pancreatic duct rendezvous. Almost every report of this variant shows that the pancreatic duct is punctured from the stomach followed by passage of the guide wire into the second portion of the duodenum where it is recovered. Then the procedure is completed in the conventional manner. This positive approach has theoretically has less complications than other EUS guided drainage, plus it allows traditional ERCP instrumentation. If it cannot be completed in cases in which the guide wire cannot go through the stenosis or papilla, then the best option is to proceed with hepatogastrostomy or choledochoduodenostomy. (16) The downside of this approach is that 20% of attempts fail including those by experienced practitioners.
The largest review of reports of this procedure includes 45 patients. (16) It reported punctures from the duodenum in 19 patients, from the stomach in 18 patients and from the esophagus in 1 patient. Technical success was achieved in 36/45 (80%) of the procedures. Complications occurred in 2/45 (4%) and included one case of pneumoperitoneum and one case of bile leakage. There were no deaths. Resolution of obstructions by placement of transpapillary stents was achieved in 32 of 45 patients in whom it was attempted (65%). Another publication (9) has shown that biliary rendezvous was successful in 75% of the patients in whom it was attempted and resolved 86 % of the blockages. In that study pancreatic rendezvous was successful in only 56 % of cases. For some authors this is the preferred rescue method when traditional biliary cannulation fails. (17,18) In one comparison of this technique used with the puncture being made from the second portion of the duodenum with precut technique found that it was effective at achieving cannulation in 98.3 % of cases while the precut technique was only successful in 90.3% (P = 0.03). (14) In another series of 40 biliary rendezvous procedures, access was gained through the duodenum or antrum in 31 patients (77.5 %) and through the transgastric intrahepatic route in the other 9 patients (22.5 %). (15) This series reported complications in 5 patients (13%). They included mild to moderate pancreatitis, abdominal pain and pneumoperitoneum without mortality.
EUS GUIDED CHOLEDOCHODUODENOSTOMY
EUS guided choledochoduodenostomy creates a fistula or neopapilla which connects the common bile duct and the duodenum to allow biliary drainage. The procedure's steps are as follows:
- Administer prophylactic antibiotics and rule out any contraindications prior to performance of procedure.
- EUS guided transduodenal puncture of the bile duct with a 19G needle (if possible) or 22 G needle. The long axis of the extrapancreatic bile duct should be found with the endoscope lengthened (not shortened). In this position the endoscope should be adjusted to direct the needle towards the hepatic hilum. After achieving puncture, bile should be aspirated to check location and to decompress the bile duct. Then inject dye into the bile duct for cholangiography.
- Insert a 450 cm long 0.035 inch guide wire (Metro or Jagwire).
- Dilate the choledocoduodenal fistula with a 4 to 9 Fr Soehendra® biliary dilatation catheter or a 6 Fr cystotome. Some publications have reported use of the precut needle mounted on the guide wire for dilation, but this strategy has shown higher rates of complications. Some authors recommend that neither needle cautery nor a scalpel should be used to gain access because when drainage is unsuccessful there is a high immediate risk of luminal perforation, portal vein injury and ductal leakage. The use of a Needle Knife for fistula dilation in biliary diversions is the only statistically proven predictor for adverse post-procedure events. This should be avoided whenever possible, especially if the cutting direction is tangential to the wall. (11, 12, 19)
- Insert a biliary stent (plastic Tanembaumde 7-10 Fr or covered self-expanding metallic stent) and position it through the choledochoduodenostomy with its proximal end in the extrahepatic bile duct. The portion of the stent outside the duodenal wall should be at least 15 to 20 mm in length to prevent displacement.
One advantage of this procedure is that the image of the common bile duct is easier to see with the linear echoendoscope than the images of intrahepatic bile ducts. This eventually facilitates puncture and decreases risks. Nevertheless, many expert authors currently disagree with this idea, and it remains controversial. Another advantage is that the retroperitoneal position of the bile duct makes it an attractive access site in patients with ascites. This procedure is useful for patients with distal biliary obstructions and is good for hilar obstructions. As the diversion is usually far from the tumor it has less possibility of obstruction of the prosthesis from tumor ingrowth.
Limitations of this technique include the requirement for good visualization of the bile duct which is not always possible especially in patients with gastric or duodenal surgical impairment from a gastrectomy, a bypass, or some other procedure. Sometimes due to the equipment's position making a puncture with a 19G needle is not possible. In these cases the use of thinner needles is required which in turn requires the use of thinner guide wires which makes instrumentation and stent advancement more difficult. They must be replaced with additional guide wires which are thicker and firmer. In addition, this technique's full implementation should be avoided in patients with benign or potentially resectable malignant diseases.
A variant of this procedure which could be useful in patients with duodenal bulb neoplastic infiltration is to puncture from the gastric antrum. (20)
The choledochoduodenostomy was first reported by Giovannini. Thirteen studies with 33 patients showed an overall technical success rate of 92% to 95 % and clinical success in all patients. The overall complication rate was 19 %. Complications, in order of frequency, included pneumoperitoneum and focal bile peritonitis. The average time that stents remain permeable was reported to be 152 days. (11) Small series that have compared this procedure with the alternative of percutaneous drainage have found similar rates of success without differences in complications, costs or patient quality of life. (21, 22)
Another complication is stent migration. Early balloon dilation of SEMS stents and placement of SEMS inside a double pigtail plastic stent have been proposed to prevent migration. Complications can be divided into early complications occurring within 30 days of the procedure and late complications which occur more than 30 days (some say more than two weeks) after the procedure when the fistula is mature. Early complications include pneumoperitoneum and fugue which are treated with non-oral antibiotics, and occasionally with surgery. Late complications include stent migration and occlusion. Migration requires repeating biliary drainage. Occlusion of SEMS requires insertion of a new stent while occlusion of plastic stents requires replacement of the stent over a guide wire which has a higher risk of loss of access and passage of the guide wire into the peritoneum. Replacement over a guide wire involves passing a catheter along the biliary passage and through the occluded plastic stent. Then a snare is passed over the guide wire to remove the occluded stent through the duodenoscope channel. It is then replaced with a new stent. The guide wire is left in place the entire time.
When a stent migrates to the peritoneum, emergency surgery is required. A fatal stent migration to the peritoneum has already been mentioned. Follow up treatment should be individualized according to the patient and the technique used.
Among the proposed variants of this procedure is the choledocoantrumostomy of which six cases have already been reported to date. All were technically successful. The use of the axios stent (Lumena) has also been proposed. This is a self-expanding metal stent designed to facilitate palliative drainage of pancreatic pseudocysts in some individual patients. To date there are no reports of its use in choledocoduodenostomies. Other published variations consist of choledocoduodenostomies with fully covered self-expanding metal stents that were temporarily withdrawn when the fistula was mature (From 1 to 4 weeks after stent placement) to allow entry of a conventional endoscope (8.8 mm diameter, 2.8 mm operative channel) into the fistula. It is then used to perform photodynamic therapy of a bleeding tumor in the common bile duct of a patient or to perform argon plasma coagulation of a mucinous papillary tumor in a biliary duct. This has been repeated several times. (23) Choledocoduodenostomies have also been performed in patients who had uncovered duodenal self-expanding metal stents in situ. The procedure was performed through the side holes of the stent. (24,25)
EUS GUIDED HEPATOGASTROSTOMY (EUS-CHOLANGIO-DRAINAGE)
An EUS guided hepatogastrostomy creates a fistula between the left intrahepatic bile duct and the gastric wall. The procedure's steps are as follows:
- Administer prophylactic antibiotics (begin 3 to 5 days before procedure) and rule out any contraindications prior to performance of procedure.
- Use a linear echoendoscope to view the dilated left hepatic duct of segment III.
- Use the tip of the endoscope to position an inflated balloon in the middle or upper part of the lesser curvature of the stomach. Use a combination of fluoroscopic and EUS guidance to puncture the bile duct transgastrically with a 19-gauge needle. Aspirate bile to check the puncture and decompress the system. Then inject contrast medium for cholangiography of the obstruction site. Puncture from the upper gastric corpus with the needle directed to the hepatic hilum.
- Run a 0.020 inch guide wire (Terumo) through the puncture. Exchange the needle over this guide wire for a 6.5 Fr diathermic sheath knife (Endoflex set from Cysto-Gastro ) or a 6 Fr cystotome (Endoflex). Use cutting current to increase the diameter of the canal between the stomach and the left hepatic duct.
- Through this channel replace the 0.020 Terumo guide wire with a 0.035 guide wire (Metro or Jagwire). Remove the cystotome. Pass a covered self-expanding metal stent (8-10 cm range) over the guide wire through the gastric wall. Use a short, completely covered, self-expanding metallic stent (6 cm) to prevent development of bile leakage. One alternative for preventing leakage is to place a 6 to 7 Fr nasobiliary drain and leave it in for 48 hours under continuous vacuum. Another alternative which is currently under evaluation is use of the self-expanding metal stent designed by Giovannini and Bories. This stent is half uncovered and half covered. The covered half runs through the gastric wall. Other techniques are early balloon dilatation and placement of a double pigtail plastic stent inside the metal stent to prevent migration. (26)
- EUS-cholangio-drainage, first reported by Burmester (27) in 2003, uses the same principles as pancreatic pseudocyst drainage guided by endoscopic ultrasound.
- This procedure has additional theoretical advantages over percutaneous transhepatic drainage. They include the fact that the biliary tract is punctured with the guidance of with real-time ultrasound using color Doppler information which limits the possibility of vascular injury. The absence of ascites in the field of intervention further decreases the likelihood of complications while the absence of external drainage improves the quality of life of patients. Shah, Kahaleh, Giovannini and Bories, who have performed the largest numbers of these procedures as well as the largest numbers of choledochoduodenostomies, believe that the extrahepatic approach of choledochoduodenostomy is more difficult and has a much higher chance of complications than the intrahepatic approach of hepatogastrostomy. (28 and personal communication) Hepatogastrostomy may further facilitate repeated access to the bile duct without having to repeat the puncture. It also provides access and resolution of biliary obstructions in patients with duodenal self-expanding metal stents who experience recurrence of biliary obstructions. A hepatogastrostomy requires an endoscope that is straighter than those required by other EUS guided diversions. This favors transmission of the pushing force for insertion of the stent and makes it easier to penetrate small intrahepatic duct which is surrounded by the liver rather than the fibrotic wall of the common bile duct. Another reported advantage of this procedure is that it can be used in patients with hilar or distal biliary obstructions, although this requires a dilated left intrahepatic biliary tract. Another advantage is damage to the biliary tract is less likely which makes leakage less likely. Reported limitations of this technique include the following:
- The gastric wall is not perfectly opposite to the left lobe of the liver which allows some movement between the puncture of the gastric wall and that of the intrahepatic bile duct. This can lead to failure of the procedure.
- If the approach is through the esophagus there is a theoretical risk of mediastinitis.
- The puncture becomes difficult in patients with liver cirrhosis.
- There is also, as in choledochoduodenostomy, a risk of portal vein damage.
- It requires the use of small-caliber stents or small diameter self-expanding metal stents with release capability. (29)
- It is not recommended in patients with abundant ascites and severe coagulopathy.
- It is not useful for draining an obstructed right biliary route.
EUS guided hepatogastrostomy was first reported by Burmester (27) in 2003. So far, in more than 13 publications, over 120 cases of ultrasound-guided biliary diversions have been reported. Although slightly different accessories and stents have been used in different studies, the overall technical success rate is close to 98 % with rates of complications ranging between 14% and 36 % (twice that of ERCP). Among the most common complications are pneumoperitoneum, chyloperitoneum, infections in the stent, late stent migration, ileus (probably secondary to the use of morphine during anesthesia), cholangitis and bilioma. One death from peritonitis was reported in one of the first patients in which the technique was used. This large series included 51 hepatogastrostomies in which technical success was reported for 49 and clinical success in 46. Forty two were typical transgastric procedures, but 9 were performed through other access routes. Five were done through the jejunum in patients who had had total gastrectomies, and 4 were done through the esophagus with access over the cardia in patients in whom the duct selected for puncture was high in segment II. This series only reported complications in 20% of hepatogastrostomies. Another series of patients from a single institution (16) reported technical success in 27 out of 28 procedures (96%) and resolution of jaundice in 26 cases (96 %). Early complications were reported in 14% of cases. Giovannini, in a series of 36 hepatogastrostomies at his institution, reported 5 complications: 3 stent migrations, 1 hepatic abscess and 1 case of chyloperitoneum which was fatal. All complications, except for the case of chyloperitoneum (which occurred at the beginning of the procedure possibly due to stent contraction) were managed conservatively.
Most centers practicing both hepatogastrostomies and EUS guided choledocoduodenostomies show a remarkable preference for hepatogastrostomies. In some places the ratio is 8-1. Average duration of stent permeability reported after hepatogastrostomy is 132 days. Reported follow-up periods ranged of up to 184 days. (11, 30, 31)
Apart from jejunal and esophageal access, one variation reported for this method is photodynamic treatment of malignant bile duct stenosis in which an endoscope was introduced through the fistula created by the hepatogastrostomy. (23) It should also be mentioned that the hepatogastrostomy can be used to manage hilar and distal stenosis. This has been described in patients with multiple clogged prosthesis who cannot be retreated by ERCP and who are not candidates for, or reject, percutaneous drainage. There have also been descriptions of stent placement in a hepatojejunal anastomosis through a hepatogastrostomy. (10) In terms of handling and quality of life for patients with incurable malignant hepatic diseases that require definitive internal diversions, it is better to proceed with internal diversions such as hepatogastrostomy rather than definitive percutaneous diversion. (32)
EUS GUIDED CHOLECYSTODUODENOSTOMY
EUS guided cholecystoduodenostomy is the internal diversion of the gallbladder in cases of acute cholecystitis for patients who are not candidates for surgery. The procedure's steps are as follows:
- The gallbladder is viewed from the duodenal bulb or from the gastric antrum with the endoscope in the extended position.
- Using EUS guidance, a precut needle, cystotome or ultrasound 19 G needle is inserted into the vesicle.
- The stiletto is removed, bile is aspirated for decompression and dye is injected into the gallbladder for cholecystography.
- A 0.035 guide wire (Metro or Jagwire) is passed through the opening.
- The segment is dilated with Soehendra biliary stents or a balloon dilator.
- A 5-10 Fr plastic stent or self-expanding metallic stent is placed into the segment.
A series of 14 patients (19) who underwent this procedure has been reported with 100% technical and clinical success with no serious adverse events. Complications were found in 7 %, one of which was a case of pneumoperitoneum which was resolved with conservative management. Although there is very little experience with this procedure, limitations reported include gallbladder mobility which occasionally makes stent placement difficult and that the technique requires that potentially useful accessories have a small diameter. This technique clearly needs more study.
EUS GUIDED PANCREATOGASTROSTOMY
EUS guided pancreatogastrostomy creates a fistula between the main pancreatic duct and the gastric wall. The procedure's steps are as follows:
- Administer prophylactic antibiotics (begin 3 to 5 days before procedure) and rule out any contraindications prior to performance of procedure.
- Use a linear echoendoscope to view the main pancreatic duct which should be dilated.
- Take the tip of the endoscope with an inflated balloon, and under combined fluoroscopic and EUS guidance, transgastrically puncture the pancreatic duct with a 19G needle. Aspiration of pancreatic juice can be used to verify puncture and to decompress the system. Inject contrast medium for pancreatography of the obstructed site. Although it is theoretically possible to puncture from the duodenum, it is more difficult. It should be noted that all published procedures have been punctured transgastrically.
- Exchange the needle over a 0.020 inch guide wire (Terumo) for a 6.5- Fr diathermic sheath knife (Endoflex Cysto-Gastro set) or a 6 Fr cystotome (Endoflex). Introduce the knife using cutting current to increase the diameter of the canal between the stomach and the main pancreatic duct.
- Exchange 0.020 Terumo guide wire for a 0.035 guide wire (Metro or Jagwire). Remove the cystotome and pass a 7 Fr plastic stent over the guide wire.
- In cases where it is possible to advance the guide wire through the stricture and the papilla, the endoscope can be replaced by a duodenoscope and the ERCP procedure can be completed in the conventional manner.
The largest series reported 25 EUS guided pancreatographies, 10 pancreatogastrostomies and 100 % technical success. (9) Although the data is limited, it has been reported that pancreatic procedures are more difficult and have less technical and clinical success (pain improvement in this case) than EUS guided biliary diversions. Pancreatitis, including severe pancreatitis, has been reported as a major complication. Certainly, more studies of this procedure are also needed.
DISCUSSION
The development of EUS guided biliary and pancreatic diversion procedures has opened up new possibilities for patients that often have obvious advantages for quality of life, lowered costs and avoidance of surgery. Nevertheless, morbidity rates are still high. This requires that these procedures be performed by experienced teams that know how to correctly use needles, accessories, guide wires, dilators and stents. In order to increase success and reduce complications, the practitioner must always be able to subtly vary the positions of the echoendoscope and its orientation and be able to use techniques for anchoring stents. Also, accessories used in these procedures are still being developed. All variants of biliary bypassed and all access routes (endoscopic, percutaneous and surgical endosonography) should be considered complementary. It is very useful for endoscopists who are expert in ERCP and endosonographic diversion procedures to learn percutaneous technique so that they can maximize the possibility of resolving the patient's problem when a procedure fails or when ERCP is not possible. (33)
Extra care regarding technicalities must be taken. The most serious complications occur when inadequate biliary drainage after puncturing a high pressure duct system results in peritoneal leakage or cholangitis, and when inadequate pancreatic drainage results in pancreatitis or formation of pseudocysts. Plastic stents can cause cholangitis due to migration or early obstruction while shortening of self-expanding metal stents can lead to biloma or biliary peritonitis which requires percutaneous drainage or repetition of EUS guided diversion. One of two deaths reported among the cases referred to was related to severe peritonitis caused by shortening of a stent and the other by peritoneal migration of the stent. Other reported complications include subcapsular liver hematomas and perforations. Most complications manifest as transient abdominal pain, with or without pneumoperitoneum, which responds to conservative measures. When a choice can be made, experts recommend hepatogastrostomy over choledochoduodenostomy because hepatogastrostomies are easier to perform and have fewer chances of complications. (34, 35)
The largest series reported show that these EUS guided procedures end up being required in approximately 3.5 % of the cases that were initially referred for ERCP. In a series of 2,566 patients, 95 required EUS guided treatments, and only 3 patients underwent percutaneous bypass. The overall success rates for EUS guided treatment are 86 % for biliary procedures and 75 % for pancreatic procedures. (9) The average time reported for these procedures was 97 minutes with a range of 36 to 210 minutes.
There is a need for further studies and larger series with longer term follow-ups. These studies should specifically define the role of each of these procedures for patients with malignant or benign diseases. (36, 37, 38)
CONCLUSSIONS
EUS guided biliopancreatic drainage is an important new alternative for resolving obstruction of ductal systems when ERCP fails or cannot be done. Although there is a need for further studies to clarify indications for these procedures, and even though additional accessories that increase efficiency and reduce morbidity and mortality need to be developed, it is now generally accepted that EUS guided rendezvous procedures can be tried for patients with pancreatic and biliary obstructions or for whom failed ERCP has failed. If the guide wire cannot pass through the papilla but manages to pass through into the stenosis, direct treatment of the stenosis can be tried through the fistula created by the hepatogastrostomy or by choledochoduodenostomy. If this is not possible, a definitive diversion with stents (especially self-expanding metal stents) can be done through hepatogastrostomy or choledochoduodenostomy. These procedures show the greatest advantages over percutaneous definitive diversion and surgery in patients with unresectable malignant diseases. (2)
REFERENCES
1. Giovannini M, Bories E. EUS-Guided Biliary Drainage. Gastroenterol Res Pract. 2012;2012:348719. [ Links ]
2. Savides TJ, Varadarajulu S, Palazzo L, et al. Working Group document: evaluation of EUS-Guided hepaticogastrostomy. Gastrointest Endosc. 2009;69:S3-7. [ Links ]
3. Wiersema MJ, Sandusky D, Carr R, et al. Endosonography-guided cholangiopancreatography. Gastrointest Endosc 1996;43:102-6. [ Links ]
4. Artifon EL, Ferreira F, Sakai P, et al. Endoscopic ultrasound-guided biliary drainage. Korean J Radiol. 2012;13:S74-82. [ Links ]
5. Tarantino I, Barresi L, Triana M, et al. Endoscopic ultrasound guided biliary drainage. World J Gastrointest Endosc. 2012;4:306-11. [ Links ]
6. Attasaranya S, Netinasunton N, Jongboonyanuparp T, et al. The spectrum of endoscopic ultrasound intervention in biliary diseases: A single Centers experience in 31 cases. Gastroenterol Res Pract. 2012;2012:680753. [ Links ]
7. Chavalitdhramrong D, Draganov P. Endoscopic ultrasound-guided biliary drainage. World J Gastroenterol. 2012;18:491-7. [ Links ]
8. Artifon EL, Ferreira F, Pinhata J, et al. EUS-guided biliary drainage: a review article. JOP 2012;13:7-17. [ Links ]
9. Shah JN, Marson F, Weilert F, et al. Single-operator, single sesión EUS-guided anterograde cholangiopancreatography in failed ERCP or inaccesible papilla. Gastrointest Endosc. 2012;75:56-64. [ Links ]
10. Artifon EL, Safatle-Ribeiro AV, Ferreira FC, et al. EUS-guided anterograde transhepatic placement of a self-expandable metal stent in hepático-jejunal anastomosis. JOP. 2011;12:610-3. [ Links ]
11. Park DH, Jang JW, Lee SS, et al. EUS-guided biliary drainage with transluminal stenting after failed ERCP: predictors of adverse events and long-term results. Gastrointest Endosc. 2011;74:1276-84. [ Links ]
12. Kim TH, Hun Kim S, Lee SO, et al. Endoscopic ultrasound-guided biliary drainage with placement of a fully covered metal stent for malignant biliary obstruction. World J Gastroenterol. 2012;18:2526-32. [ Links ]
13. Martins FP, Rossini LG, Ferrari AP, et al. Migration of a covered metallic stent following endoscopic ultrasound-guided hepaticogastrostomy: fatal complication. Endoscopy. 2010;42(Suppl 2):E126-7. [ Links ]
14. Dhir V, Bhandari S, Bapat M, et al. Comparison of EUS-guided rendezvous and precut papillotomy techniques for biliary access. Gastrointest Endosc. 2012;75:354-9. [ Links ]
15. Iwashita T, Lee JG, Shinoura S, et al. Endoscopic ultrasound-guided rendezvous for biliary access after failed cannulation. Endoscopy. 2012;44:60-5. [ Links ]
16. Yamao K, Hara K, Bhatia V, et al. EUS-guided biliary drainage. Gut Liver. 2010;(Suppl 1):S67-75. [ Links ]
17. Iwashita T, Lee J. Endoscopic ultrasonography-guided biliary drainage: rendezvous technique. Gastrointest Endosc Clin N Am. 2012;22:249-58. [ Links ]
18. Yoon WJ, Brugge WR. EUS-guided biliary rendezvous: EUS to the rescue. Gastrointest Endosc. 2012;75:360-1. [ Links ]
19. Itoi T, Sofuni A, Itokawa F, et al. Endoscopic ultrasonography-guided biliary drainage. J Hepatobiliary Pancreat Sci. 2010;17:611-6. [ Links ]
20. Artifon EL, Okawa L, Takada J, et al. EUS-guided choledochoantrostomy: an alternative for biliary drainage in unresectable pancreatic cáncer with duodenal invasión. Gastrointest Endosc. 2011;73:1317-20. [ Links ]
21. Artifon EL et al. Biliary drainage in patients with unresectable malignant obstruction where ERCP fails: endoscopic ultrasonography-guided choledochoduodenostomy versus percutaneous drainage. J Clin Gastroenterol. 2012;46:768-74. [ Links ]
22. Yamao K, Hara K, Mizuno N, et al. Endoscopic ultrasound-guided Choledochoduodenostomy for malignant lower biliary tract obstruction. Gastrointest Endosc Clin N Am. 2012;22:259-69. [ Links ]
23. Eum J, Park do H, Ryu CH, et al. EUS-guided biliary drainage with a fully covered metal stent as a novel route for natural orifice transluminal endoscopic biliary interventions: a pilot study. Gastrointest Endosc. 2010;72:1279-84. [ Links ]
24. Khashab MA, Fujii LL, Baron TH, et al. EUS-guided biliary drainage for patients with malignant biliary obstruction with an indwelling duodenal stent. Gastrointest Endosc. 2012;76:209-13. [ Links ]
25. Ramirez-Luna MA, Téllez-Ávila FI, Giovannini M, et al. Endoscopic ultrasound-guided biliodigestive drainage is a good alternative in patients with unresectable cáncer. Endoscopy. 2011;43:826-30. [ Links ]
26. Perez-Miranda M, de la Serna C, Vila J, et al. Endosonography-guided cholangiopancreatography as a salvage drainage procedure for obstructed biliary and pancreatic ducts. World J Gastrointest Endosc. 2010;2:212-22. [ Links ]
27. Burmester E, Niehaus J, Leineweber T, et al. EUS-cholangio-drainage of the bile duct: report of 4 cases. Gastrointest Endosc. 2003;57:246-51. [ Links ]
28. Kahaleh M, Hernandez AJ, Tokar J, et al. Interventional EUS-guided cholangiography: evaluation of a technique in evolution. Gastrointest Endosc. 2006;64:52-9. [ Links ]
29. Park DH, Song TJ, Eum J, et al. EUS-guided hepaticogastrostomy with a fully covered metal stent as the biliary diversión technique for an occluded biliary metal stent after a failed ERCP. Gastrointest Endosc. 2010;71:413-9. [ Links ]
30. Park DH. Endoscopic ultrasonography-guided hepaticogastrostomy. Gastrointest Endosc Clin N Am. 2012;22:271-80. [ Links ]
31. Sharaiha RZ, Kalloo AN, Khashab MA. EUS-guided hepatoesophagostomy for transesophageal biliary drainage. Gastrointest Endosc. 2012;76:227-8. [ Links ]
32. Giovannini M, Moutardier V, Pesenti C, et al. Endoscopic ultrasound-guided bilioduodenal anastomosis: a new technique for biliary drainage. Endoscopy. 2001;33:898-900. [ Links ]
33. Yamao K, Bhatia V, Mizuno N, et al. EUS-guided choledochoduodenostomy for palliative biliary drainage in patients with malignant biliary obstruction: results of longterm follow-up. Endoscopy. 2008;40:340-2. [ Links ]
34. Bories E, Pesenti C, Caillol F, et al. Transgastric endoscopic ultrasonography-guided biliary drainage: results of a pilot study. Endoscopy. 2007;39:287-91. [ Links ]
35. Lee KH, Lee JK. Interventional endoscopic ultrasonography: present and future. Clin Endosc. 2011;44:6-12. [ Links ]
36. Katanuma A, Maguchi H, Osanai M, et al. Endoscopic ultrasound-guided biliary drainage performed for refractory bile duct stenosis due to chronic pancreatitis: a case report. Dig Endosc. 2012;24(Suppl 1):34-7. [ Links ]
37. Artifon EL, Pinhata Otoch J, Yábar A, et al. Endoscopy ultrasonography-guided biliary drainage in the surgical-endoscopy era. Rev Gastroenterol Peru. 2011;31:365-75. [ Links ]











 texto en
texto en