Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.28 no.4 Bogotá Oct./Dec. 2013
Hepatic hydrothorax: case report and literature review
Luis Guillermo Toro Rendón, MD. (1), Elizabeth María Correa Gutiérrez, MD. (1), Óscar Iván Mcewen, MD. (2), Juan Ignacio Marín Zuluaga, MD. (3), Sergio Álvarez Vallejo, MD. (4), Octavio Germán Muñoz, MD. (3), Óscar Mauricio Santos, MD. (3), Juan Carlos Restrepo, MD. (5)
(1) Internist and Hepatology Fellow at the Universidad de Antioquia in Medellín, Colombia.
(2) Internal Medicine Resident at the Universidad Pontificia Bolivariana in Medellín, Colombia.
(3) Hepatology and Liver Transplant Unit at the Hospital Pablo Tobón Uribe in Medellín, Colombia.
(4) Interventional Radiologist and Head of the Radiology Department at the Hospital Pablo Tobón Uribe in Medellín, Colombia.
(5) Hepatology and Liver Transplant Unit at the Hospital Pablo Tobón Uribe and Professor in the Gastrohepatology Group at the Universidad de Antioquia in Medellín, Colombia.
Received: 15-07-13 Accepted: 27-08-13
Abstract
Hepatic hydrothorax is a rare complication that occurs in patients with liver cirrhosis. We report the case of a patient with NASH cirrhosis and evidence of portal hypertension who was admitted to the emergency department with coughing and chest pain. Transudative pleural effusions (according to Light's criteria) were found in association with ascites, but no cardiac cause, pleural effusion or pulmonary effusion could be found. Treatment with diuretics was begun, but was suspended because the patient developed significant renal dysfunction. Fluid was drained with a thoracostomy but additional loss of fluid led to further deterioration of renal function. It was decided to insert a transjugular portosystemic shunt (TIPS) to significantly decrease portal pressure and to progressively decrease ascitic fluid and pleural effusion. A subsequent review of the patient and radiological follow-up found no recurrence of symptoms, pleural effusion or ascites.
Keywords
Hepatic hydrothorax, pleural effusion, cirrhosis, transjugular intrahepatic portosystemic shunt (TIPS).
INTRODUCTION
Patients with advanced cirrhosis and portal hypertension have abnormal regulation of extracellular fluid volume. In many cases this results in accumulation of liquid in the abdominal cavity which causes ascites, and in other cases it results in accumulation of liquid in the lower extremities causing edema. In 5% to 10% of these patients, fluid accumulates in the pleural space. (1,2) Four large series of 1,155 cirrhotic patients with ascites found that the incidence of this complication is 6%. Patients with hydrothorax usually have advanced hepatic disease, and most require transplantation. (4) Below, we report on a case of a hepatic hydrothorax patient who was successfully treated with a transjugular intrahepatic portosystemic shunt (TIPS). Although this pathology is infrequent, it can mean serious problems for management of portal hypertension patient.
CASE DESCRIPTION
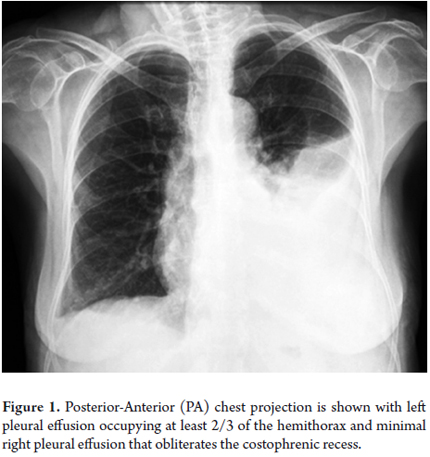
The patient was a 72-year-old woman with a history of hepatic cirrhosis due to nonalcoholic steatohepatitis (NASH) CHILD B (9 points). She had complications associated with portal hypertension including upper gastrointestinal tract hemorrhaging from grade II-III esophageal varices, hepatic encephalopathy, ascites without spontaneous bacterial peritonitis and partial portal thrombosis. She also had controlled diabetes mellitus type 2. She was admitted to the hospital following a week of coughing with transparent expectoration and chest pain with pleural characteristics. Left pleural effusion was found (See Figure 1). Diagnostic thoracentesis obtained transudate fluid with a liquid pleural protein to serum ratio of 0.24 and a pleural lactic dehydrogenase in liquid to serum ratio of 0.3.
Since the patient's echocardiography was normal and ascites was present, she was diagnosed as having symptomatic hepatic hydrothorax. 1,800 mL was evacuated using thoracentesis and medical management for hydrothorax was initiated. Sodium intake was restricted and gradual diuretic therapy begun, but the patient responded poorly and developed adverse effects including kidney damage and hyperkalemia. Treatment was suspended. Hydrothorax recurred within 48 hours together with serious symptoms of restricted respiration. Despite evacuating liquid through thoracentesis again, there was another recurrence of hydrothorax. An evaluation via thorax surgery was requested. A chest tube was implanted to accomplish chemical pleurodesis but tube output fluctuated between 1.5 and 2.0 liters per day making the procedure unsuccessful. Two management possibilities remained: open surgery to perform fenestration-closure and subsequent pleurodesis, or - bearing in mind the portal partial thrombosis - implantation of a TIPS.
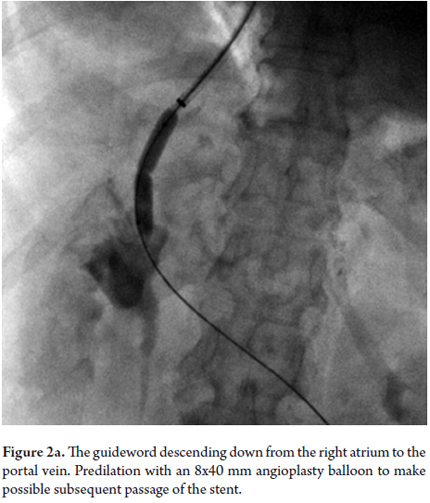
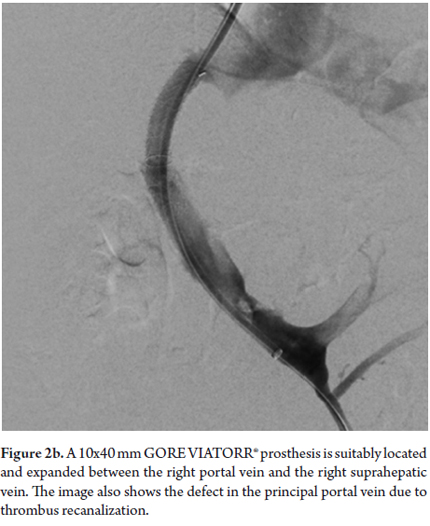
The patient was referred to the decision staff-making. Interventional radiology found TIPS implantation would be viable bearing in mind portal thrombosis. The TIPS was successfully implanted, overcame the portal thrombosis, and resulted in her hepatic venous pressure gradient falling from 23 mmHg before the procedure to 8mmHg after the procedure (See Figures 2a and 2b). The liquid draining from the thoracotomy gradually decreased, and the the tube was removed. Forty-eight hours later a chest x-ray (See Figure 3) showed that the hydrothorax was clearing up. Doppler indicated TIPS permeability. The patient was given encephalopathy prophylaxis with lactulose and rifaximin. Finally, six days after the procedure, the patient was discharged. To date, there has been no recurrence of hydrothorax, no encephalopathy, no TIPS dysfunction and no thrombosis.
DISCUSSION
Hepatic hydrothorax is defined as a pleural effusion in portal hypertension patients which is usually greater than 500 mL but which is not associated with primary heart, lung or pleural disease. The combination of portal hypertension and absence of cirrhosis is the sine qua non of hepatic hydrothorax. It is important to emphasize that more than 80% of patients with portal hypertension have cirrhosis. (3, 5) Moreover, the development of hydrothorax is not associated with a particular cause of cirrhosis, although in many of these patients its origin is alcoholic. (6)
Unlike patients with ascites who are able to tolerate large volumes of liquid (5-10 liters) with no symptoms or with only mild symptoms, hepatic hydrothorax patients can experience severe symptoms such as dyspnea, coughing or hypoxia in the presence of low volumes (1-2 liters) of pleural effusion. This is due to thoracic cavity structural characteristics.
PHYSIOPATHOLOGY
Hypoalbuminemia, increased flow and increased pressure in the thoracic duct and the azygos vein are common events in cirrhotic patients with portal hypertension. Although these events may partially explain the development of hydrothorax, the most likely cause is the transfer of large volumes of fluid from the abdomen to the pleural space through a pressure gradient via congenital or acquired diaphragm fenestrations. (4) Normally, there is a fine balance between the formation and absorption of pleural fluid which when altered leads to pleural effusion. The first description of a diaphragm defect as a causal link of hydrothorax goes back to 1955. (7) These defects are typically less than 1 cm long, sometimes microscopic, and are located in the tendinous portion of the diaphragm. (8) Negative intrapleural pressure produced by inhaling combined with positive intra-abdominal pressure favors the flow of fluid from the abdominal cavity to the pleural space. Some patients present mild or even undetectable ascites, but once the capacity of the pleural space to absorb more liquid is exceeded, hydrothorax appears. This theory is supported by studies that use human albumin marked with Tc-99m which have demonstrated unidirectional flows of this marker from the abdominal cavity to the pleural cavity. (9) Furthermore, autopsy studies of patients with hydrothorax confirm the presence of diaphragm defects (0.03 1.2 m). (10)
Hepatic hydrothorax most frequently develops on the right side (79.5% of cases). Only 17.5% occur on the left side and only 3% of cases are bilateral. (3) Even though the right-side tendency is not yet completely understood, it could be explained by the fact that the right side of the diaphragm is more susceptible to abnormalities occurring during the development of the embryo (11) as well as the close anatomic connection of the exposed surface of the liver with the diaphragm and the fact that the left diaphragm is thicker and has more muscular tissue.
CLINICAL PRESENTATION AND DIAGNOSIS
A diagnosis of hepatic hydrothorax must be suspected in a patient with cirrhosis and confirmed portal hypertension when that patient has a unilateral (generally right-sided) pleural effusion. In most cases, the predominant clinical manifestations are cirrhosis and ascites. However, a variety of respiratory symptoms such as dyspnea, nonproductive coughing, pleural-like chest pain and fatigue due to hypoxemia can also occur. Severity of such symptoms depends on the volume of pleural fluid accumulated. Although ventilator failure is seldom caused by acute hydrothorax tension, it may occur for other reasons in some patients and then be found as an incidental finding in chest x-rays. Hence, an association with pleural effusion has been documented. (12) The size of these pleural effusions generally tend to be small or moderate, and only 6% of patients have an effusion occupying more than a half of a hemithorax.
All patients with significant pleural effusions should undergo diagnostic thoracentesis to confirm hepatic hydrothorax and to exclude alternative diagnoses. A complete study of the pleural fluid should including cytochemistry, a cell count, pH, Gram stains, vial blood cultures, serum and pleural fluid proteins, albumin, LDH and bilirubin. Other tests of the pleural effusion should be done out according to clinic suspicion. They include triglycerides, Ziehl-Neelsen, adenosine diaminase, PCR, culture for mycobacterium detection and cytology and are done to exclude chylothorax, tuberculosis and malignancy. (1)
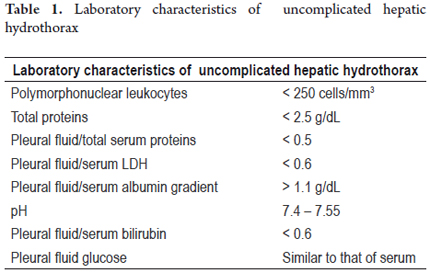
Uncomplicated hepatic hydrothorax is a transudate whose characteristics are similar to those of ascites fluid. Its albumin gradient is greater than 1.1 as is that of ascites fluid secondary to portal hypertension (Table 1). Further studies must be done if the fluid is an exudate and if it develops on the left side. (3)
Spontaneous bacterial empyema
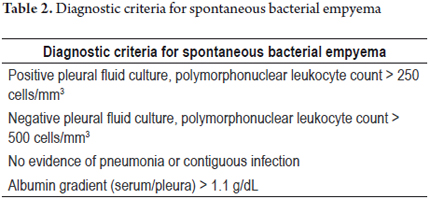
Spontaneous bacterial empyema (SBE) represents a complication of hepatic hydrothorax; it is defined as the spontaneous infection of the pleural fluid in a patient without pneumonia. (13) Diagnostic criteria are shown in table 2.
Although the pathogenesis of spontaneous bacterial empyema is unclear, it can occur as a result of direct extension of a bacterial infection from the peritoneal cavity. Nonetheless, Xiol X et al. have reported that 45% of these events were not associated with spontaneous bacterial peritonitis. (13) Spontaneous bacterial empyema can come about even without the presence of ascites. In such cases, a transitory bacteremia that infects the pleural space may be the underlying pathogenic mechanism. (14) Risk factors for its development include a high Child-Pugh score, protein levels below 1mg/dL, low C3 concentrations in pleural fluid and simultaneous spontaneous bacterial peritonitis (SBP).
Like the development of spontaneous bacterial peritonitis (SBP), spontaneous bacterial empyema is also an indication for transplantation.
The incidence of SBE of 15% to 20% of hospitalized cirrhotic patients is similar to that reported for SBP. (3) The microorganisms which most frequently cause empyema are enterobacteria - usually E. coli and K. pneumonia, and species of streptococcus and enterococcus. (15) Any patient with hepatic hydrothorax that presents fever, pleuritic pain, and/or encephalopathy must be evaluated for spontaneous bacterial empyema. Abba et al. have reported that the mortality rate for spontaneous bacterial empyema can be up to 20%.
Treatment of this pathology is based on application of third generation cephalosporins. Use of albumin to prevent hepatorenal syndrome development, a standard treatment for patients with high-risk SBP, has not been studied in patients with spontaneous bacterial empyema. These patients should receive prophylaxis with norfloxacin, ciprofloxacin and trimethoprim for life or until transplantation is performed. (3)
HEPATIC HYDROTHORAX MANAGEMENT
Patients with hepatic hydrothorax usually have an advanced stage of disease with important complications from portal hypertension. They are potential candidates for liver transplantation which is the definitive way to manage hepatic hydrothorax.
Therapy of patients who are not candidates for transplantation as well as for patients awaiting transplantation should be aimed at alleviating symptoms, preventing infections and avoiding lung complications. As in management of ascites, the main goal of reaching a negative sodium balance can be achieved through dietary and pharmacological intervention. (16) Reduction of sodium intake to 2gr/day (88mEg/day) (17) results in elimination of ascites in 10% to 20% of patients, but for most patients usage of diuretics is required to achieve a negative sodium balance. A combination of a long acting diuretic such as spironolactone with a loop diuretic such as furosemide can attain sodium excretion of at least 120mEg/day. Treatment is initiated with a 40mg/day dose of furosemide and 100mg/day dose of spironolactone. If there is no response and compliance with diet and medications has been checked, the dosage can be doubled daily over 5 days up to a maximum dose of 160mg/day of furosemide and 400mg/day of spironolactone. The goal is to reach a weight loss of 0.5kg/day in patients with no edema, and 1kg/day in patients with peripheral edema. (18, 19) For these patients who are considered to be diuretic-refractory patients, other therapeutic interventions should be performed.
Therapeutic thoracentesis is relatively safe, and immediately ameliorates respiratory symptoms. The frequency of occurrence of pneumothorax, the principal risk with this procedure, (5) increases from 7.7% for first-time therapeutic thoracentesis, to 34.7% for the fourth procedure. (20) No more than 2 liters should be drained unless the patient is being close monitored because of the risk of pulmonary edema due to associated re-expansion and hypotension. Nevertheless, if underlying ascites is not treated, hydrothorax tends to re-accumulate.
Platelet counts greater than 50,000 and prothrombin times less than twice the upper limit are safe for the procedure, and prophylactic transfusions are not required. (21)
Chest tubes, often used for management of hepatic hydrothorax, can increase morbidity and mortality in cirrhotic patients because they become difficult to remove and because they correct neither the base hepatic disease nor portal hypertension. Their use should be reserved for highly select cases. A study by Lawrence U et al. of 59 cirrhotic patients who required chest tube implantation found that tube was removed successfully in 66% of cases without requiring further procedures. It also found that failure of chest tube removal in one-third of the patients was related to increased mortality. (22)
It should be noted that presence of bacterial empyema is not an indication for a chest tube unless there is obvious evidence of pus in a thoracentesis.
In presence of refractory hydrothorax, primary repair of diaphragm defects has been attempted by means of thoracotomy or video-assisted thoracoscopy and placement of a TIPS. Cerfolio RJ et al. have published a retrospective study of 41 cirrhotic patients with refractory hepatic hydrothorax who underwent video-assisted thoracoscopy and talc pleurodesis to control hydrothorax symptoms in which they found diaphragm defects in only two patients. (23) When a defect cannot be identified using thoracoscopy, pleurodesis can be performed, but the risk of infection of this procedure is greater than 33%. This is probably due to the fact that fluid forms so quickly that adhesion of parietal and visceral pleura surfaces is prevented. (24) The study found that after intra-operatory application of talc, 7 patients (17%) required a second talc application through a tube. An overall success rate of 80% was achieved (33 of 41 patients), and only 4 patients (9.7%) suffered subsequent symptomatic fluid accumulation. (23)
Transjugular intrahepatic portosystemic shunts were introduced in 1988 for interventional treatment to decompress portal hypertension in order to treat variceal bleeding. (25) TIPS It is current accepted that the implantation of a TIPS is indicated when medical therapy to prevent recurrences of variceal bleeding fails, when there is variceal bleeding which is refractory to medical management, and when there is ascites which is refractory to medical management. TIPS has also been used to manage other complications of portal hypertension including hepatic hydrothorax, hepatorenal syndrome and portal vein thrombosis. Nonetheless, these are not universally accepted indications for placement of a TIPS, and placement of a TIPS in these cases has not been assessed in rigorous randomized trials. (26) In spite of this, there are many series that demonstrate response rates of 70% to 80% for management of hepatic hydrothorax. (3) Dhanasekaran et al. reported on a series of 73 patients who had TIPS placed for management of hepatic hydrothorax. They found clinical responses of 79% after a month and 75% after six months. Average patient survival time was 517 days. The 30, 60 and 90 day survival rates were 81%, 78% and 72%, respectively. The 1, 3 and 5 year survival rates were 48%, 26% and 15% respectively. Pre-TIPS creatinine levels were significantly associated with 30 day mortality. Patients with MELD scores of less than 15 survived longer after TIPS and had the best clinical response to placement. (27)
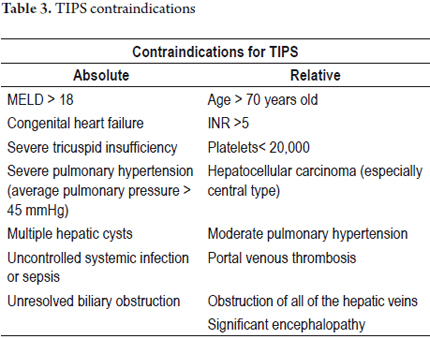
TIPS are less invasive and are related to a lower morbidity and mortality rates than surgical portosystemic shunts. Contraindications for TIPS are listed in Table 3. TIPS placement is generally guided by interventional radiology using an aseptic technique and is done under conscious sedation. A tube is guided through the right internal jugular to the hepatic vein and a connection is made to the intrahepatic portion of the portal vein. The hepatic vein is dilated and a metallic stent is implanted to creating an anastomosis between the portal vein and the hepatic veins which results in decompression of the hepatic vascular bed. Unless clinically significant encephalopathy exists, the goal of TIPS placement is to lower the hepatic venous pressure gradient to less than 12 mmHg. (26) TIPS placement reduces the portosystemic pressure gradient in 90% of cases. (28)
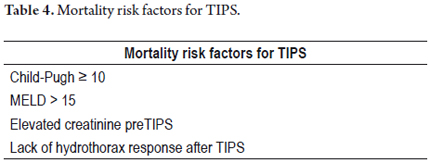
Principal complications after TIPS implantation are stent occlusion (25%), encephalopathy (31%), and hepatic subcapsular perforation with significant intraperitoneal hemorrhaging. Clinical hemobilia is rarely observed after the procedure, although in up to 20% of patients the stent is placed too far into the inferior vena cava (even into the right atrium at the cranial end) or is placed too far from the principal portal vein at the caudal end. Occasionally stents can migrate during or after implantation due to tube and balloon manipulation, kidney function deterioration (10%) and hepatorenal syndrome. (28, 29) Mortality rates after TIPS for refractory hydrothorax have been reported at between 25% and 40%. Deaths are usually caused by complications from terminal hepatic disease (30) which requires that this kind of procedure be done in medical centers with high possibilities of transplantation service. Mortality risk factors for TIPS implantation are listed in Table 4.
Although there are no guidelines for TIPS follow-up monitoring, it is generally accepted that Doppler echography should be done to assess bypass permeability every 3 months in the initial post-procedure period, and every 6 months thereafter.
In the case presented, the patient was considered for TIPS implantation because she did not have any absolute contraindication for this procedure (see Table 3). Although she was 72-year-old, the patient was in good functional condition. Her portal thrombosis was a technical contraindication that could be overcome. Two series of TIPS management of portal vein thrombosis in patients with variceal bleeding and refractory ascites have reported success rates up to 97%. (31, 32) For this reason, portal thrombosis is considered to be only a relative contraindication for TIPS implantation. This is especially true since a successful procedure benefits both management of variceal bleeding and ascites.
It is important to note that management of hepatic hydrothorax with a chest tube may increase morbidity and mortality. Patients who do not respond to medical management and who are potentially candidates for liver transplantation should be registered on the transplantation waiting list.
REFERENCES
1. Cárdenas A, Kelleher T, Chopra S. Review article: hepatic hydrothorax. Aliment Pharmacol Ther. 2004;20:271-9. [ Links ]
2. Strauss RM, Boyer TD. Hepatic hydrothorax. Sem Liver Dis. 1997;17:227-32. [ Links ]
3. Krok KL, Cárdenas A. Hepatic hydrothorax. Semin Resp Crit Med. 2012;33:3-10. [ Links ]
4. Gur C, Ilan Y, Shibolet O. Hepatic hydrothorax-pathophysiology, diagnosis and treatment-review of the literature. Liver Int. 2004;24:281-4. [ Links ]
5. Kinasewitz GT, Keddissi JI. Hepatic hydrothorax. Curr Opin Pulm Med. 2003;9:261-5. [ Links ]
6. Alberts WM, Salem AJ, Solomon DA, et al. Hepatic hydrothorax. Cause and management. Arch Int Med. 1991;151:2383-8. [ Links ]
7. Emerson PA, Davies JH. Hydrothorax complicating ascites. Lancet. 1955;268:487-8. [ Links ]
8. Huang PM, Chang YL, Yang CY, et al. The morphology of diaphragmatic defects in hepatic hydrothorax: thoracoscopic finding. J Thor Cardiov Surg. 2005;130:141-5. [ Links ]
9. Benet A, Vidal F, Toda R, et al. Diagnosis of hepatic hydrothorax in the absence of ascites by intraperitoneal injection of 99m-Tc-Fluor colloid. Postgrad Med J. 1992;68:153. [ Links ]
10. Chen A, Ho YS, Tu YC, et al. Diaphragmatic defect as a cause of massive hydrothorax in cirrhosis of liver. J Clin Gastroenterol. 1988;10:663-6. [ Links ]
11. Lazaridis KN, Frank JW, Krowka MJ, et al. Hepatic hydrothorax: pathogenesis, diagnosis, and management. Am J Med. 1999;107:262-7. [ Links ]
12. Roussos A, Philippou N, Mantzaris GJ, et al. Hepatic hydrothorax: pathophysiology diagnosis and management. J Gastroenterol Hepatol. 2007;22:1388-93. [ Links ]
13. Xiol X, Castellvi JM, Guardiola J, et al. Spontaneous bacterial empyema in cirrhotic patients: a prospective study. Hepatology. 1996;23:719-23. [ Links ]
14. Abba AA, Laajam MA, Zargar SA. Spontaneous neutrocytic hepatic hydrothorax without ascites. Respirat Med. 1996;90:631-4. [ Links ]
15. Sese E, Xiol X, Castellote J, et al. Low complement levels and opsonic activity in hepatic hydrothorax: its relationship with spontaneous bacterial empyema. J Clin Gastroenterol. 2003;36:75-7. [ Links ]
16. Garcia-Tsao G. Current management of the complications of cirrhosis and portal hypertension: variceal hemorrhage, ascites, and spontaneous bacterial peritonitis. Gastroenterology. 2001;120:726-48. [ Links ]
17. Gentilini P, Casini-Raggi V, Di Fiore G, et al. Albumin improves the response to diuretics in patients with cirrhosis and ascites: results of a randomized, controlled trial. J Hepatol. 1999;30:639-45. [ Links ]
18. Gines P, Cárdenas A, Arroyo V, et al. Management of cirrhosis and ascites. New Eng J Med. 2004;350:1646-54. [ Links ]
19. Runyon BA. Management of adult patients with ascites due to cirrhosis. Hepatology. 2004;39:841-56. [ Links ]
20. Castellote J, Xiol X, Cortes-Beut R, et al. Complications of thoracentesis in cirrhotic patients with pleural effusion. Rev Esp Enf Digest. 2001;93:566-75. [ Links ]
21. Xiol X, Castellote J, Cortes-Beut R, et al. Usefulness and complications of thoracentesis in cirrhotic patients. Am J Med. 2001;111:67-9. [ Links ]
22. Liu LU, Haddadin HA, Bodian CA, et al. Outcome analysis of cirrhotic patients undergoing chest tube placement. Chest. 2004;126:142-8. [ Links ]
23. Cerfolio RJ, Bryant AS. Efficacy of video-assisted thoracoscopic surgery with talc pleurodesis for porous diaphragm syndrome in patients with refractory hepatic hydrothorax. Ann Thor Surg. 2006;82:457-9. [ Links ]
24. Milanez de Campos JR, Filho LO, de Campos Werebe E, et al. Thoracoscopy and talc poudrage in the management of hepatic hydrothorax. Chest. 2000;118:13-7. [ Links ]
25. Rossle M, Gerbes AL. TIPS for the treatment of refractory ascites, hepatorenal syndrome and hepatic hydrothorax: a critical update. Gut. 2010;59:988-1000. [ Links ]
26. Harjit KB, Sanyal AJ. Transjugular intrahepatic portosystemic shunt: an overview. Clin Liver Dis. 2012;1:173-6. [ Links ]
27. Dhanasekaran R, West JK, Gonzales PC, et al. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol. 2010;105:635-41. [ Links ]
28. Fidelman N, Kwan SW, LaBerge JM, et al. The transjugular intrahepatic portosystemic shunt: an update. AJR. 2012;199:746-55. [ Links ]
29. Spencer EB, Cohen DT, Darcy MD. Safety and efficacy of transjugular intrahepatic portosystemic shunt creation for the treatment of hepatic hydrothorax. J Vasc Int Radiol. 2002;13:385-90. [ Links ]
30. Gordon FD, Anastopoulos HT, Crenshaw W, et al. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25:1366-9. [ Links ]
31. Luca A, Miraglia R, Caruso S, et al. Short- and long-term effects of the transjugular intrahepatic portosystemic shunt on portal vein thrombosis in patients with cirrhosis. Gut. 2011;60:846-52. [ Links ]
32. Han G, Qi X, He C, et al. Transjugular intrahepatic portosystemic shunt for portal vein thrombosis with symptomatic portal hypertension in liver cirrhosis. J Hepatol. 2011;54:78-88. [ Links ]











 text in
text in