Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.1 Bogotá ene./mar. 2014
Endoscopic argon plasma therapy for neuroendocrine type I gastric tumors
Rodrigo Castaño, MD,1 Claudia Vargas MD. (1), Juan Camilo Pérez Cadavid MD. (1), Juan Pablo Dueñas MD. (1), Mario Hernán Ruiz MD. (1), Jorge A. Almenares MD. (1), Luis Miguel Ruiz MD. (1)
(1) Antioquia Group for the study of neuroendocrine tumors and GIST (GIST - NET GAE) in Medellin, Colombia.
Received: 12-08-13 Accepted: 19-12-13
Abstract
Background and Purpose: Since data regarding type I gastric carcinoid tumors and their evolution in prospective studies are scarce, unanimity has not been reached regarding treatment and follow-up. Our purpose is to describe the use of argon plasma coagulation (APC) for the eradication of multiple type I gastric carcinoid tumors and to observe behavior over time following treatment. Methods: Over a period of seven years from July 2005 to June 2012, fourteen patients with neuroendocrine gastric tumors were treated endoscopically at two tertiary medical centers. Argon plasma was applied to eradicate multiple gastric carcinoid tumors. Then, patients underwent follow-up endoscopic examinations. Results: None of the patients presented specific symptoms or signs related to the presence of chronic atrophic gastritis, and all diagnoses were incidental findings during endoscopy. Biopsies obtained endoscopically prior to intervention showed polyps that were type I intramucosal neuroendocrine tumors. The median diameter was 3.8 mm, with a range from 2mm to 10mm. Seven cases (50%) were associated severe atrophy while multifocal atrophy was found in 29% of the cases. H. pylori were found in seven patients (50%) during an average follow-up of 46 months. Follow-up periods ranged from 17 to 84 months. Patient survival was 100%. Even though ten patients (71%) suffered recurrences, all lesions were eradicated. Gastrin, chromogranin A, mitotic index, and Ki 67 were all evaluated, and patients were also tested for autoimmune diseases. No bleeding or perforations occurred during procedures. Conclusions: The use of endoscopic argon plasma to completely eradicate type I gastric neuroendocrine tumors is safe and easy.
Keywords
Type I gastric neuroendocrine tumors, endoscopy, argon plasma.
INTRODUCTION
The general incidence of neuroendocrine tumors (NETs) has grown over the last 30 years (1). Gastric carcinoid tumors, or as they are now called gastric neuroendocrine tumors (G-NETs), arise from the enterochromaffin cells (EC) which produce histamine although they may occasionally have a phenotype suggesting another origin. These include EC which produce somatostatin and cells that produce ghrelin (3). The EC are classified as gastrin dependent or independent although both express the CCK2 gastrin receptor (4). G-NETs are rare lesions which account for 7% of all NETs and 1% of all gastric tumors. The 2010 histological classification of these tumors by the World Health Organization (Table 1) is as follows:
a. Well-differentiated low grade and intermediate grade NETs (G1, G2)
b. Poorly differentiated NETs (G3, G4).
Gastrin dependent G-NETs are related to conditions that induce hypergastrinemia such as chronic atrophic gastritis (Type I, 70-80%) and gastrinomas with Zollinger Ellison Syndrome (Type II, 5-6%). Type III tumors (20%) and Type IV tumors (<5%) are independent of gastrin, can be present with normal levels of gastrin, and do not respond to gastrin as a promoter of proliferation (6, 7). Type II tumors are associated with mutations of the MEN-1 gene. Gastrin independent tumors have not been investigated enough and their pathogenesis is controversial (8, 9).
The Type I G-NETs are generally asymptomatic, while type II may lead to peptic ulcer disease because of hyperacidity. Types III and IV which are undifferentiated present large and ulcerated tumors (6). Often Type I G-NETs show no specific symptoms associated with chronic atrophic gastritis and diagnosis is an incidental finding during upper endoscopy. They are well-differentiated NETs with low Ki67 indices. Multiple Type I tumors often occur in the proximal stomach. They usually look like small Polyps (<2 cm) although up to 25% of Type I G-NETs are not macroscopically visible during endoscopy. They become only apparent in histological study. In such cases they are called intramucosal carcinoid tumors (10).
Previous studies of Type I G-NETs have been retrospective and have included limited numbers of patients of all types. They describe an indolent course with few reports of regional or metastatic compromise (11-15).
Management of Type I G-NETs has not yet been established, but several alternatives have been suggested. Gastrectomy has been proposed for multiple and/or larger lesions and for patients who relapse after endoscopic resection (16, 17). Somatostatin analogs have been proposed because they reduce tumor growth in vitro and in vivo (18-20). One management technique that has already been evaluated is endoscopic resection with subsequent endoscopic follow. Nevertheless, most of the small number of studies that evaluate this therapeutic option have small samples of patients with different management options and little information about recurrence (13, 15, 17, 21).
The first reported experiences of treatment of NET with argon plasma have shown good results treating bronchial NETs (22-25). NET management with argon plasma has been described in case reports, but there are very few reports, with small numbers of patients, of the use of argon plasma therapy for NETs in the gastrointestinal tract (26, 27). In 2005, two third level medical centers in Medellin began using argon plasma to treat Type I G-NETs with prospective follow-up of these patients. The main objectives of this study are to evaluate the clinical prognosis, including survival and tumor progression, of a consecutive series of patients with Type I G-NET managed with argon plasma. The secondary objectives are to evaluate the recurrence rate and risks of endoscopic removal.
METHODS
Patients and study design
Patients diagnosed with Type I G-NET between July 2005 and June 2012 who were managed endoscopically were included in this study and followed prospectively with endoscopy.
Upon entry into the study various variables of patient information were recorded in an Excel form developed for this purpose and for use as the source database. Information recorded included patients' sexes, ages, family histories of peptic ulcer disease, presence of thyroiditis and/or diabetes mellitus Type I, laboratory tests, degree of atrophy, state of EC, and H pylori infections.
Diagnoses of chronic atrophic gastritis were based on histological confirmation from multiple biopsies taken from the gastric antrum and corpus. Gastritis was evaluated according to the updated Sydney System (28). Gastric atrophy in the corpus was defined by the focal intestinal metaplasia or complete replacement of oxyntic glands by intestinal metaplasia. This variable was graded on a scale from absence of atrophy (grade 0), to mild atrophy (grade 1), to moderate atrophy (grade 2) to severe atrophy (grade 3). Antral atrophy was defined as focal intestinal metaplasia or complete disappearance of or antral glands and their replacement by metaplastic intestinal epithelium.
To define the stage of the EC, the classification system developed by Solcia was used (29). Proliferative cells with a diameter of less than 150 microns were considered to be hyperplastic, but could have different patterns including normal, simple line pattern, micronodular and adenomatous. Dysplasia was diagnosed when proliferation was greater than 150 microns but less than 500 microns. Proliferation greater than 500 microns was defined as a Type 1 G-NET.
Diagnosis of pernicious anemia was based on the presence of macrocytic anemia (hemoglobin <13 g/dl in men and <12 g/dl in women with CV> 100 fl), low levels of vitamin B12 (<197 pg/ml), IM B12 responsive and the presence of atrophic gastritis in the corpus (30).
A diagnosis of autoimmune thyroiditis was based on the presence of thyroid autoantibodies and ultrasound suggestive of thyroiditis, independent of thyroid function (31).
Complete blood tests and tests for serum gastrin, chromogranin A, ferritin and Vitamin B12 were performed. The presence of H pylori was established by biopsies of samples taken endoscopically from the antrum. Once the presence of H pylori was diagnosed, the patient was treated with standard triple therapy of omeprazole, clarithromycin and amoxicillin. Eradication was monitored by breath test one month after completion of therapy. The multiple endocrine neoplasia syndrome was ruled out on the basis of studying symptoms, personal history, family history, and laboratory tests.
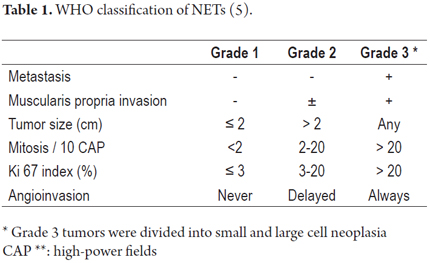
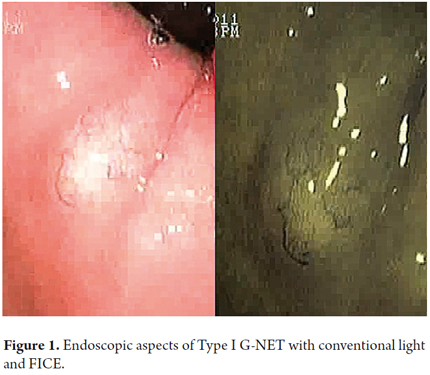
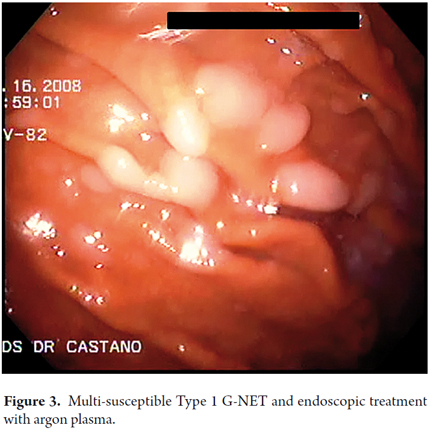
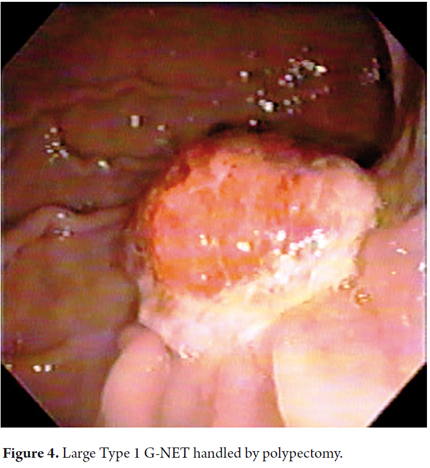
When viewed endoscopically, Type I G-NETs characteristically appear to be small polypoid lesions less than 1 cm in diameter with subepithelial lesions. They present normal or increased vascularity and yellowing mucosa. When depression, erosion or ulceration is observed the possibility of carcinoma is suggested (Figures 1, 2, 3 and 4).
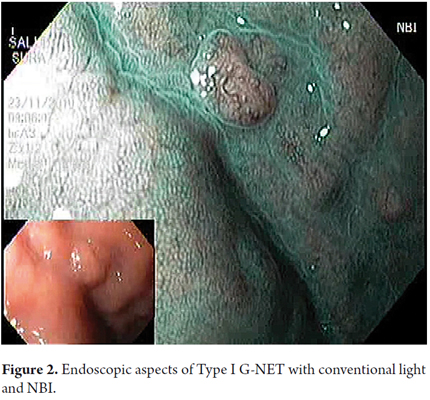
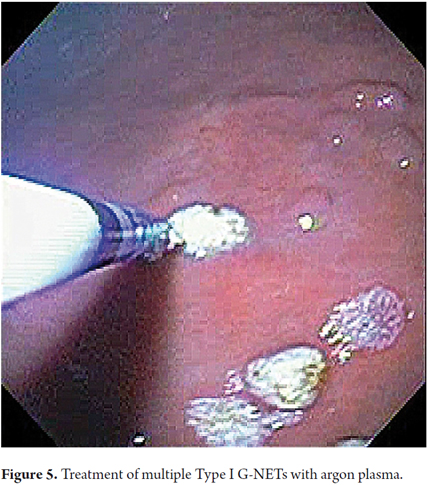
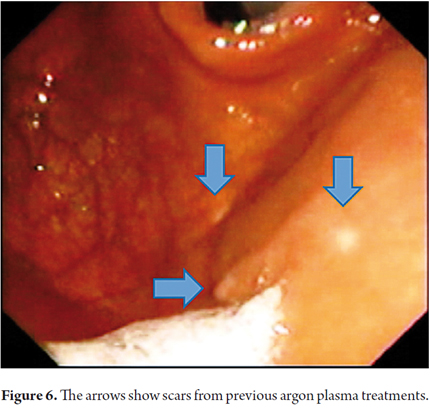
Therapeutic endoscopic procedures were performed under sedation with meperidine and midazolam. All visible lesions were treated with argon plasma with predetermined parameters of 50 W power, a flow rate of 1 lt/min, a distance of 2 to 4 mm from the catheter, and pulses of 1 to 2 seconds of duration. Contact with the tissue was avoided. Following procedures the area was cleaned to remove debris, saliva and/or mucus (Figures 5 and 6).
Biopsy samples were also taken from the corpus and antrum with oval, fenestrated and standard forceps. None of the patients was studied with endoscopic ultrasound. Patients were monitored for between 4 and 6 months following initial treatment During this follow up biopsies were taken from the antrum and corpus to try to identify intramucosal endocrine neoplasia (32). In cases of endoscopic removal of lesions samples were preserved in 10% buffered formalin, processed in 5 micron thick sections, and stained with hematoxylin and eosin for routine evaluation. No specific staining for H pylori or for detection of intestinal metaplasia was performed.
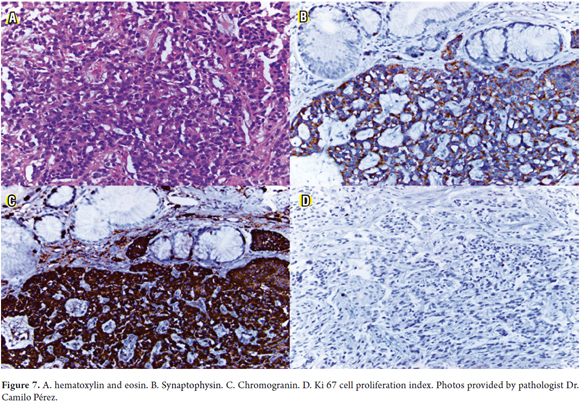
Histological studies used hematoxylin and eosin and various other stains that are specific for these neoplasias such as synaptophysin, chromogranin and cell proliferation index Ki 67 (Figure 7).
Follow-up time was measured the moment of endoscopic intervention in which the ablation of lesions with argon plasma was performed until the last follow up examination. Recurrence time was defined as the interval between therapy with argon plasma and detection of a new lesion during an endoscopic examination. Endoscopic complications were recorded at the time of surgery and during the follow-up period.
Data analysis and statistical evaluation
Statistical analysis was performed using IBM SPSS software version 18. Data were expressed as medians (interquartile range 25-75) Analysis of recurrence-free time was performed using the Kaplan-Meier analysis. Univariate risk factor analyses were made with the proportional risk Cox model. The p was considered significant when it was <0.05.
RESULTS
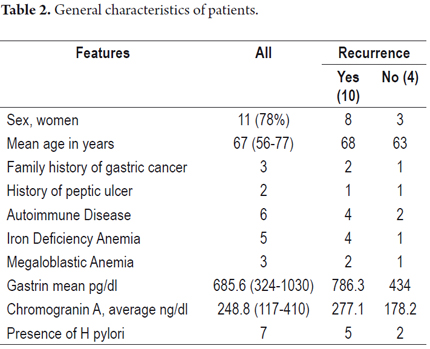
Fourteen patients with type I G-NETs were treated endoscopically with argon plasma. There were eleven women (78%), the average age was 67 years, and the average gastrin level was 685 pg/dl (Table 2).
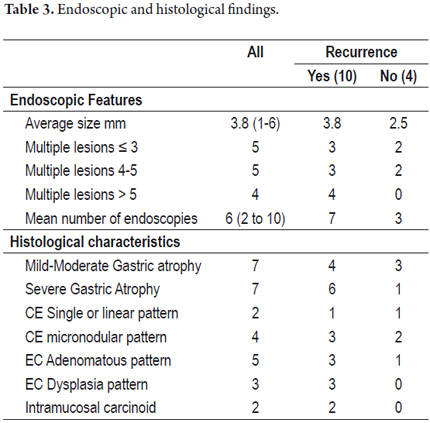
At diagnosis average lesion size was 3.8 mm. All patients had multiple lesions, and in two cases intramucosal carcinoids were present (Table 3).
All patients were treated endoscopically with argon plasma. There were no therapy-related complications and no need for hospitalization of any patients.
Fifty percent of these patients had mild to moderate atrophy, and 50% had severe atrophy. Four patients (29%) also had antral atrophy which configures multifocal atrophy.
Mean follow-up time was 46 months (18 to 84 months). The number of endoscopies performed during follow-up ranged from 2 to 10, and the average number was six. No tumor-related deaths occurred. During follow-up at least one imaging study was performed on patients (CT scan or abdominal MRI) but they did not show evidence of local, regional or distal compromise.
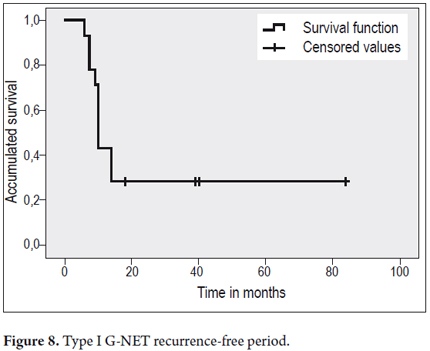
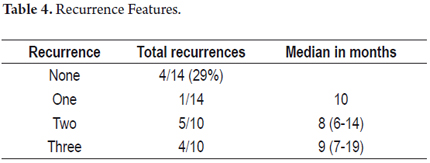
After initial therapy with argon plasma recurrences were detected during follow-up in 10 of 14 patients (71%), but four patients presented no additional lesions. The first recurrence occurred 6 months after treatment on average. The one year recurrence rate was 42% (Figure 8). Nine patients experienced multiple recurrences (Table 4).
When evaluating recurrence, we found that severe atrophy and anemia (75%) are risk factors for recurrence (85.7%).
DISCUSSION
This prospective multicenter study based on endoscopic monitoring of patients following treatment of Type 1 G-NETs with argon plasma ablation shows that this procedure is safe and effective. The study had a 100% survival rate without metastases or complications related to intervention plus it made early detection of recurrences of neuroendocrine neoplasms possible during follow-up.
This study shows that this neoplasm has a high rate of recurrence, and several patients had more than one recurrence. This possibility had has already been suggested by the study of Merola of a group of 32 patients managed with endoscopic resection of lesions with prospective follow-up (21).
Data from other retrospective and multicenter studies show that patients with Type 1 G-NETs arrive with multiple lesions in the gastric corpus and fundus and that behavior can be more aggressive with a higher rate of proliferation during follow up (13, 15, 21, 33-35).
To date it has been difficult to evaluate the biological behavior of Type I G-NETs and to standardize treatment because studies have been based on small numbers of patients and cases, have been retrospective and multicenter, have included all types of gastric neuroendocrine neoplasms, and have analyzing tumors with different prognoses together (13,15, 33-37).
Some authors suggest that surgery is the only alternative capable of preventing progression of tumors and resolving hypergastrinemia (16, 17). Proposals for management of Type I G-NETs include various surgical procedures depending upon the number and sizes of lesions and upon whether or not polyps are recurrent. Limiting treatment to conservative management has been proposed for cases of small Type I G-NETs (16, 38). Promising results have been reported in the literature for the use of somatostatin analogs in patients with multiple Type I G-NETs who have no signs of invasion (18-20, 33, 39, 40).
Another option is a conservative approach combined with endoscopic resection of lesions. Nevertheless, there are no series with long-term follow-up that demonstrate its effectiveness (41-43). Since Type I G-NETs develop slowly, other authors have proposed expectant management limiting resection to large polyps with signs of dissemination (12, 44). Nevertheless, since patients with atrophic gastritis body are at risk of developing carcinoma, resection of any raised lesion resection is mandatory. The annual incidence of adenocarcinoma in these patients is 0.25% in cases with intestinal metaplasia, 0.6% in cases of mild to moderate dysplasia, and 6% for severe dysplasia (45, 46).
The main objective of this study was to determine the clinical prognoses of Type I G-NET patients who were managed with endoscopic argon plasma ablation. The survival rate was 100% after a median of nearly 5 years of follow-up without evidence of local or distal metastatic compromise. These findings suggest that conservative endoscopic management is effective and safe. No patients had complications related to endoscopy, and none were hospitalized. Conversely, the increased morbidity and mortality and the unfavorable impact on the quality of life of undergoing gastrectomies must also be taken into account.
Surgery remains for patients with malignant lesions (carcinoma or poorly differentiated neuroendocrine tumors) if detected during evolution. In this study, no carcinoma-like lesions were found. Close monitoring allows early detection of local and regional compromise and avoids adjuvant therapy.
These tumors can also be detected as intramucosal neoplasias. Since many of them are not visible, it cannot be determined if they are recurrences or if they were present in previous endoscopies. In these patients, mapping with gastric biopsies during follow-up endoscopies increases the possibility of finding intramucosal neuroendocrine tumors (32, 46).
It has been suggested that Type I G-NETs are related to moderate to severe gastric atrophy in the gastric corpus, but they have also been found in cases of mild atrophy in the corpus (15-19). In our series, 50% of the patients presented mild to moderate gastric atrophy. This histological pattern is applicable to EC and to the mere presence of simple linear hyperplasia associated with Type I G-NETs. This is consistent with reports showing a small percentage of Type I G-NETs with simple linear hyperplasia (34).
The second objective was to evaluate the recurrence rate. In this series of cases the recurrence rate was 71% after initial resection. However, as previously suggested Type I G-NETs are often small multiple lesions which may even have intramucosal carcinomas. For this reason it is not possible to pinpoint how many really result from recurrence and how many were simply not seen during the initial endoscopy. Endoscopic examinations were performed between 6 and 12 months after the initial procedure depending upon whether or not recurrence had occurred. For patients without recurrences, follow-up was done every 12 months. This could be extended to every 2 to 4 years depending on the occurrence of gastric atrophy (21).
When tumors recur after the first intervention, risk factors must be identified. E endoscopic follow-up should be strictly reserved for these patients. Anemia (75%) and severe atrophy (8 5.7%) were present more often in patients who suffered recurrences than in those who did not.
The main limitations of this study are the small number of patients and the relatively short follow-up period. Due to the rarity of this condition, prospective and multicenter studies are required to solve these problems. Another limiting factor is the concept recurrence since there is a possibility that multiple residual lesions will remain after initial therapy. However, a six months follow-up period appears to be effective in controlling the growth of EC without affecting the probability of recurrence.
CONCLUSIONS
This study shows a high rate of recurrence of these lesions but with a very low risk of malignancy. It also shows that endoscopic management is safe and effective with 100% survival and that endoscopic follow-up allows early detection of recurrence. More studies are needed to determine the risk factors for recurrence of Type I G-NET.
REFERENCES
1. Ter-Minassian M, Chan JA, Hooshmand SM, et al. Clinical presentation, recurrence, and survival in patients with neuroendocrine tumors: results from a prospective institutional database. Endocrine-related cancer 2013;20(2):187-96. [ Links ]
2. Modlin IM, Oberg K, Chung DC, et al. Gastroentero-pancreatic neuroendocrine tumours. The lancet oncology 2008;9:61-72. [ Links ]
3. Latta E, Rotondo F, Leiter LA, Horvath E, Kovacs K. Ghrelin- and serotonin-producing gastric carcinoid. J Gastrointest Cancer 2012;43:319-23. [ Links ]
4. Rindi G, Luinetti O, Cornaggia M, Capella C, Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology 1993;104:994-1006. [ Links ]
5. Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas 2010;39:707-12. [ Links ]
6. Vargas CC, Castaño R. Tumores neuroendocrinos gastroenteropancreáticos. Rev Col Gastroenterol 2010;24:165-76. [ Links ]
7. Scherubl H, Cadiot G, Jensen RT, Rosch T, Stolzel U, Kloppel G. Neuroendocrine tumors of the stomach (gastric carcinoids) are on the rise: small tumors, small problems? Endoscopy 2010;42:664-71. [ Links ]
8. Nikou GC, Angelopoulos TP. Current concepts on gastric carcinoid tumors. Gastroenterology research and practice 2012;2012:287825. [ Links ]
9. Kidd M, Gustafsson BI. Management of gastric carcinoids (neuroendocrine neoplasms). Current gastroenterology reports 2012;14:467-72. [ Links ]
10. Delle Fave G, Capurso G, Milione M, Panzuto F. Endocrine tumours of the stomach. Best practice & research Clinical gastroenterology 2005;19:659-73. [ Links ]
11. Landry CS, Brock G, Scoggins CR, McMasters KM, Martin RC, 2nd. A proposed staging system for gastric carcinoid tumors based on an analysis of 1,543 patients. Ann Surg Oncol 2009;16:51-60. [ Links ]
12. Hosokawa O, Kaizaki Y, Hattori M, et al. Long-term follow up of patients with multiple gastric carcinoids associated with type A gastritis. Gastric cancer : official journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association 2005;8:42-6. [ Links ]
13. Jordan PH, Jr., Barroso A, Sweeney J. Gastric carcinoids in patients with hypergastrinemia. Journal of the American College of Surgeons 2004;199:552-5. [ Links ]
14. Gladdy RA, Strong VE, Coit D, et al. Defining surgical indications for type I gastric carcinoid tumor. Ann Surg Oncol 2009;16:3154-60. [ Links ]
15. Borch K, Ahren B, Ahlman H, Falkmer S, Granerus G, Grimelius L. Gastric carcinoids: biologic behavior and prognosis after differentiated treatment in relation to type. Annals of surgery 2005;242:64-73. [ Links ]
16. Modlin IM, Lye KD, Kidd M. Carcinoid tumors of the stomach. Surgical oncology 2003;12:153-72. [ Links ]
17. Dakin GF, Warner RR, Pomp A, Salky B, Inabnet WB. Presentation, treatment, and outcome of type 1 gastric carcinoid tumors. Journal of surgical oncology 2006;93:368-72. [ Links ]
18. Manfredi S, Pagenault M, de Lajarte-Thirouard AS, Bretagne JF. Type 1 and 2 gastric carcinoid tumors: long-term follow-up of the efficacy of treatment with a slow-release somatostatin analogue. Eur J Gastroenterol Hepatol 2007;19:1021-5. [ Links ]
19. Campana D, Nori F, Pezzilli R, et al. Gastric endocrine tumors type I: treatment with long-acting somatostatin analogs. Endocrine-related cancer 2008;15:337-42. [ Links ]
20. Grozinsky-Glasberg S, Kaltsas G, Gur C, et al. Long-acting somatostatin analogues are an effective treatment for type 1 gastric carcinoid tumours. European journal of endocrinology / European Federation of Endocrine Societies 2008;159:475-82. [ Links ]
21. Merola E, Sbrozzi-Vanni A, Panzuto F, et al. Type I gastric carcinoids: a prospective study on endoscopic management and recurrence rate. Neuroendocrinology 2012;95:207-13. [ Links ]
22. Cetinkaya E, Aras G, Sokucu SN, Ozgul A, Altin S. Treatment of endoluminal typical carcinoid tumor with bronchoscopic techniques. Tuberkuloz ve toraks 2009;57:427-30. [ Links ]
23. Karasulu L, Altin S, Dalar L, Sokucu S, Simsek N. [Two typical carcinoid cases treated by endobronchial approach]. Tuberkuloz ve toraks 2009;57:212-7. [ Links ]
24. Jabbardarjani H, Masjedi M, Herth F. Successful treatment of endobronchial carcinoid using argon plasma coagulation. Journal of bronchology & interventional pulmonology 2009;16:196-8. [ Links ]
25. Orino K, Kawai H, Ogawa J. Bronchoscopic treatment with argon plasma coagulation for recurrent typical carcinoids: report of a case. Anticancer Res 2004;24:4073-7. [ Links ]
26. Multiple gastric carcinoids type I. (en línea) GastroSource: (accesado 5 Feb 2013) Disponible en: http://www.gastrosource.com/Patient-Cases/Dr.Kruse/ [ Links ]
27. Green HL. Treatment of Duodenal Carcinoid with Argon Plasma Coagulation. Proc UCLA Healthcare 2003;7:37-8. [ Links ]
28. Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996;20:1161-81. [ Links ]
29. Solcia E, Bordi C, Creutzfeldt W, et al. Histopathological classification of nonantral gastric endocrine growths in man. Digestion 1998;41:185-200. [ Links ]
30. Lahner E, Caruana P, D'Ambra G, et al. First endoscopic-histologic follow-up in patients with body-predominant atrophic gastritis: when should it be done? Gastrointestinal endoscopy 2001;53:443-8. [ Links ]
31. Lahner E, Centanni M, Agnello G, et al. Occurrence and risk factors for autoimmune thyroid disease in patients with atrophic body gastritis. The American journal of medicine 2008;121:136-41. [ Links ]
32. Annibale B, Azzoni C, Corleto VD, et al. Atrophic body gastritis patients with enterochromaffin-like cell dysplasia are at increased risk for the development of type I gastric carcinoid. Eur J Gastroenterol Hepatol 2001;13:1449-56. [ Links ]
33. Granberg D, Wilander E, Stridsberg M, Granerus G, Skogseid B, Oberg K. Clinical symptoms, hormone profiles, treatment, and prognosis in patients with gastric carcinoids. Gut 1998;43:223-8. [ Links ]
34. Yu JY, Wang LP, Meng YH, Hu M, Wang JL, Bordi C. Classification of gastric neuroendocrine tumors and its clinicopathologic significance. World J Gastroenterol 1998;4:158-61. [ Links ]
35. Rindi G, Bordi C, Rappel S, La Rosa S, Stolte M, Solcia E. Gastric carcinoids and neuroendocrine carcinomas: pathogenesis, pathology, and behavior. World journal of surgery 1996;20:168-72. [ Links ]
36. Modlin IM, Lye KD, Kidd M. A 50-year analysis of 562 gastric carcinoids: small tumor or larger problem? Am J Gastroenterol 2004;99:23-32. [ Links ]
37. Rindi G, Azzoni C, La Rosa S, et al. ECL cell tumor and poorly differentiated endocrine carcinoma of the stomach: prognostic evaluation by pathological analysis. Gastroenterology 1999;116:532-42. [ Links ]
38. Ruszniewski P, Delle Fave G, Cadiot G, et al. Well - differentiated gastric tumors/carcinomas. Neuroendocrinology 2006;84:158-64. [ Links ]
39. Ferraro G, Annibale B, Marignani M, et al. Effectiveness of octreotide in controlling fasting hypergastrinemia and related enterochromaffin-like cell growth. The Journal of clinical endocrinology and metabolism 1996;81:677-83. [ Links ]
40. Fykse V, Sandvik AK, Qvigstad G, Falkmer SE, Syversen U, Waldum HL. Treatment of ECL cell carcinoids with octreotide LAR. Scandinavian journal of gastroenterology 2004;39:621-8. [ Links ]
41. Ichikawa J, Tanabe S, Koizumi W, et al. Endoscopic mucosal resection in the management of gastric carcinoid tumors. Endoscopy 2003;35:203-6. [ Links ]
42. Kokkola A, Sjoblom SM, Haapiainen R, Sipponen P, Puolakkainen P, Jarvinen H. The risk of gastric carcinoma and carcinoid tumours in patients with pernicious anaemia. A prospective follow-up study. Scandinavian journal of gastroenterology 1998;33:88-92. [ Links ]
43. Rappel S, Altendorf-Hofmann A, Stolte M. Prognosis of gastric carcinoid tumours. Digestion 1995;56:455-62. [ Links ]
44. Johnson FE, Smith JB, Janney CM, Ramrakhiani S. Multiple gastric carcinoids. Journal of the American College of Surgeons 2003;196:984-5. [ Links ]
45. de Vries AC, van Grieken NC, Looman CW, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology 2008;134:945-52. [ Links ]
46. Bordi C, Azzoni C, Ferraro G, et al. Sampling strategies for analysis of enterochromaffin-like cell changes in Zollinger-Ellison syndrome. Am J Clin Pathol 2000;114:419-25. [ Links ]











 texto en
texto en