Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.1 Bogotá ene./mar. 2014
Topological analysis of the interactome of Helicobacter pylori reveals topologically essential proteins: identification of therapeutic targets
Andrés Julián Gutiérrez E. Lic., MSc., (1), Martín Alonso Bayona R. MSc. (2)
(1) Degree in Biology, MS in Basic Medical Sciences, Teacher and Researcher in the Faculty of Medicine and Coordinator of the Molecular Biology Laboratory of the Basic Science and Applied Human Genetics Group at the University of Applied and Environmental Sciences in Bogotá, Colombia. email: andresgutierrez@colombia.com
(2) Bacteriologist, Master's in Microbiology, Teacher and Researcher in the Faculty of Medicine and Coordinator of the Basic Medical Area of the Basic Science and Applied Human Genetics Group at the University of Applied and Environmental Sciences in Bogotá, Colombia. email: mabayona@udca.edu.co
Funding
Grant from the office of the Vice Rector of internal research of the University of Applied and Environmental Sciences
Received: 08-07-13 Accepted: 19-12-13
Abstract
Helicobacter pylori, which have colonized half the world's population, have a prevalence of 70% to 90 % in developing countries and 25% to 50 % in industrialized countries. It is well established that the infection is associated with the development of chronic gastritis, gastric and duodenal ulcers and stomach cancer in humans. Helicobacter pylori were the first pathogenic bacteria whose interactome was deduced. Topologic analysis identified 702 proteins involved in 1,359 interactions coverage of 97.7 % with scale-free networks. In other words, the interactome contains topologically essential proteins, however, because these proteins and their physiological associations have not been described, we cannot identify potential therapeutic targets. To identify topologically essential proteins we reconstructed the interactome of H. pylori strain ATCC 26695 using BioNetBuilder and Cytoscape NetworkAnalyzer the cytoHubba web application. The reconstruction presented 896 proteins and 2416 interactions with a coverage of 96 % of the proteome adjusted to the distribution of the power law. In other words, the reconstruction presented a scale-free network. We used NetworkAnalyzer and the Hubba application to identify essential proteins according to the BC and K parameters. On that basis we constructed a subnetwork. Analysis of this subnetwork showed that the type IV secretion system interacts with metabolic subsystems of lipids, amino acids and nucleic acids by means of proteins for which this interaction has not been suggested. Moreover, these interactions can be explained by profiles of expression that are dependent on pH, adhesion and iron homeostasis. This allows us to complements both the basic biology of the strain as well as to postulate putative new therapeutic targets.
Keywords
Helicobacter pylori, interactome, graph theory, topological nodes, type IV secretion system, therapeutic target.
INTRODUCTION
Helicobacter pylori has colonized the stomachs of half of the world's population (1, 2). Its prevalence in adults ranges from 70% to 90% in developing countries and is between 25% and 50% in industrialized countries (3). It is known that infection is associated with chronic gastritis, gastric ulcers and stomach cancer mediated by adhesion of bacteria strains to host epithelial cells (1, 2, 4, 5, 6, 7). Nevertheless, not all of them not all produce infections (8).
An interaction network is composed of nodes and connections. The nodes may be distributed randomly or may follow a specific distribution. Free scale means that nodes are densely connected (11). The relationship between the topological properties (genes or proteins) of nodes and their functional essentiality is well established (42). In fact, there are bioinformatic programs, for example Hubba, for identifying these nodes from an interactome (33). The interactome of Helicobacter pylori strain ATCC 26695 had 1,200 interactions and covered 46% of the proteome (9). Subsequently, it was reported to be free scale with 702 proteins and 1,359 interactions with a coverage of 97.7% (10, 11, 12, 13). It was also possible to biochemically validate 76% of these interactions (14). To date the urease complex, the flagellar complex and the type IV secretion system complex have been characterized (14, 15, 16, 17, 18, 19, 20). Protein feedback interactions that could affect the activity of specific regulons have recently been suggested. These include the ferric uptake regulator (Fur), RopD and heat shock (21). Nevertheless, no study has determined the topological essentiality and physiological significance of interactome proteins that could lead to the identification of therapeutic targets.
In this study we have constructed a subsidiary network of topologically essential proteins that we propose as putative therapeutic targets. It was also possible to determine the topological centrality of the type IV secretion system and protein interactions linking it with metabolic subsystems. These interactions reveal physiological associations which can be explained in the context of gene expression under specific conditions of pH, adherence and iron homeostasis.
MATERIALS AND METHODS
The reconstruction of the network was developed in versions 2.8.2 and 3.0.1 of Cytoscape (22, 23). BioNetBuilder 2.0 was used to mine databases for interaction data on H. pylori strain ATCC 26695 (24). Databases used were BIND, KEGG, MINT, Intact and DIP 5.0 (25, 26, 27, 28, 29). All network nodes were named with Uniprot code, and repeated nodes and loops were removed (30).
Topological parameters evaluated included grade (k) which indicates the degree of connectedness of one node with other nodes; intermediation (BC) which indicates the number of times that a node is visited; and centrality (CC) which indicates which nodes are closest to the center of the network (31). The NetworkAnalyzer plugin was used for this evaluation (32). To determine the topological centers, nodes with the highest values for k and BC were compared with the results obtained from Hubba http://hub.iis.sinica.edu.tw/Hubba/ (33). Two subsidiary networks were constructed with these comparative data: integrated subsidiary network K, and subsidiary network DS following the Double Screen parameter (DS).
Perturbation was analyzed with the PerturbationAnalyzer plugin for Cytoscape (35). Then, subnet k nodes were mapped against the results of the analysis of perturbation. Only those mapped nodes that displayed values greater than 2 were selected (34). The integrated subset of the network (k) was subjected to interference analysis using the Interference 1.0 Cytoscape plugin for use as a control (36). Perturbations were performed assuming that the concentrations of all nodes had doubled. All nodes of Integrated subnet k and Subnet DS were mapped in UniProt (Universal Protein Resource), KEGG (Kyoto Encyclopedia of Genes and Genomes), and DEG (Database of Essential Genes) (26, 30, 37). BLAST (Basic Local Alignment Search Tool) was used to compare information against a human database and DrugBank (38, 39). All analyses were carried out on a bioinformatics Dell T7600 work station.
RESULTS
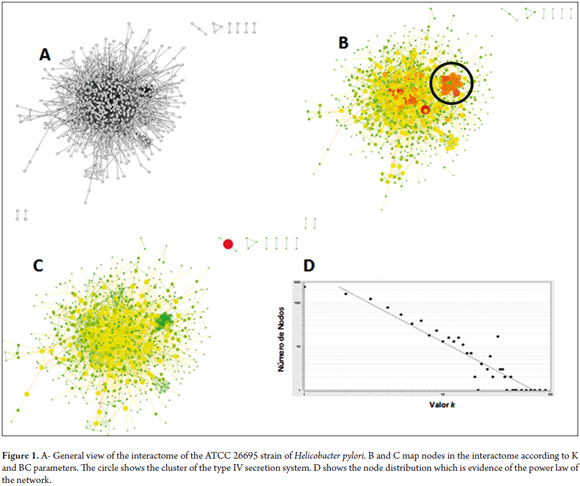
The network had 896 nodes and 2,416 interactions covering 96% of the proteome. The K parameter and the number of nodes correlated positively (0.901, R2 = 0.884) and its distribution was confirmed by the power law (Figure 1) (40). Of the 50 most common nodes according to K mapped in KEGG, 18% are hypothetical proteins; 25% are Cag8, Cag7, CagA, and Cag-Alpha of the type IV secretion system; and 8% are rpoBC, SpoT, PyrF and Ndk regulatory proteins. Other proteins detected included FlgB, protein A of the UvrABC complex, CobB, HcpB, AspB and Bcp of various metabolic subsystems. Finally, 8% of the proteins were from metabolic subsystems that synthesize fatty acids such as AccD, AcpP, FabE and proteins similar to lipase. CytoHubba results for the 50 most common nodes for K are similar, however the values of each node changes and there are two different nodes corresponding to glnA and feoB (Table 1, Figure 1B and Figure 1C).
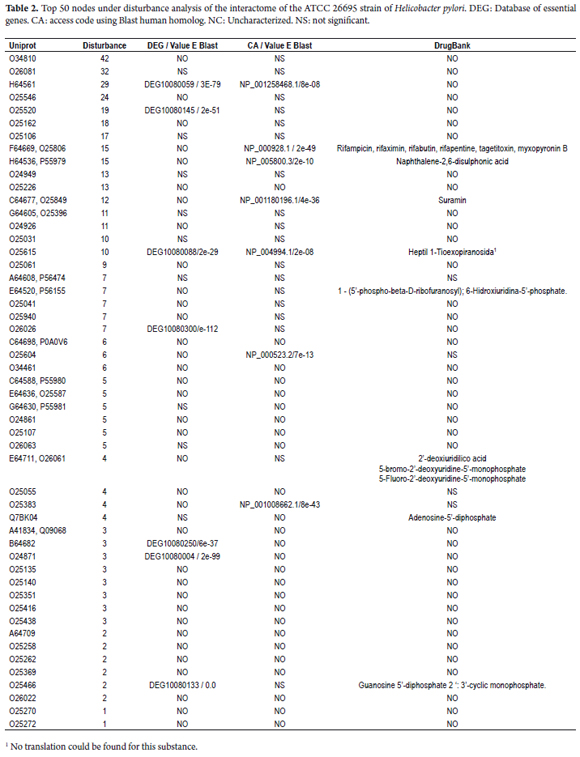
Of the 50 most common for the BC parameter, 30% are hypothetical proteins; 8% are regulatory proteins; 6% are proteins of the flagellar apparatus such as FlgB, PflA and FliS; 10% correspond to DNA repair, replication and RNA degradation systems including MutS2, TopA, rpoD, RNase J and protein A from the UvrABC system; 6% are from the synthetic lipid metabolism including AcpP, FabE, and proteins similar to lipase; 8% are amino acid metabolism proteins such as CobB, SdaA, AspB and Delta-1-pyrroline-5-carboxylate dehydrogenase; and the remaining 24% include HcpB feoB, selA, 6-carboxy-5 ,6,7,8-tetrahidropterin synthetase, TktA, CopA , the substrate binding protein of the ABC transporter, and urease complex secretion system proteins including CagA Cag8 Cag7 Cag Alpha and ureI channel. Mapping the perturbation analysis results against top 50 KB results shows that hcpB flgB, rpoBC, CagA, Cag7, Cag alpha, Cag3, SpoT, UvrABC, Cag16, Cag 8 AccD, AcpP, AspB, bcp, FabE, feoB, pyrF, queD, GppA and ureI (accompanied by hypothetical proteins) are the topologically essential proteins of the interactome of the ATCC 26695 strain of Helicobacter pylori. The interference analyses showed that the network breaks when any of these topological centers is eliminated (Table 2 and Figure 2C).
The cytoHubba DS parameter groups nodes into several clusters. Cluster 1 represents the metabolism of pyruvate and propanoate, fatty acid synthesis, and the central metabolism. Of these, nodes without human homologues are ppsA, FabH, carboxin and spermidine, FabE, Pta, porA, porB and porD and porG which is also essential in DEG (DEG10080201 (E = 5e-93)). Cluster 2 represents the metabolism of amino acids and nitrogen. Only two proteins do not have human homologues: panD and murl. Cluster 3 represents the metabolism of nucleic acids and specific amino acids. Among the proteins in this cluster that are without human homologues are SurE and Gpt. Cluster 4 represents systems of replication, repair, and recombination. Significant nodes according to DEG are the protein (dnaN DEG10080082 (E = 0.0)), the HP1247 protein (DEG: DEG10080237 (E = 0.0)), the ropZ protein (DEG:DEG10080134 (E = 2e-36)) and HoLB (DEG:DEG10080229 (E = 8e-94)). Finally, Cluster 5 represents the type IV secretion system, and there is evidence of interactions between the queD protein and Cluster 6 which represents the ATP synthase system. (Figure 2B).
DISCUSSION
Scale-free means that interactome nodes follow a power law distribution, are known to be essential, organize the network, and are evolutionarily conserved (40, 41, 42, 43, 44, 45, 46). In this study we identified topological nodes for the type IV secretion system (TIVSS), the flagellar machinery, the stress response system, for recombination, repair and transcription systems, for iron homeostasis, for protection against reactive oxygen species and for the urease system.
The K subset of the network shows that the type IV secretion system (TIVSS) is a topological central axis (See Figure 1 and Figure 2) for which the Cag7 Cag8, Cag3, Cag16, Cag18, Cag Alpha and CagA proteins are topological nodes. These interactions have been experimentally confirmed (18, 47). These nodes perform essential functions for the TIVSS molecular dynamics. Specifically, Cag18, Cag19 and Cag7 recognize integrins that promote the secretion of CagA (51, 52, 53) the translocation of which is mediated by Cag16 (54). Consequently, CagA can destroy the epithelial barrier and modify host signaling pathways (48). In addition, Cag3 is essential for the TIVSS assembly (49) and Cag Alfa facilitates substrate transport (50).
We suggest that interactions between CagA, Cag3, Cag8, Cag7 and Cag16 regulate TIVSS and may link physiologically with metal homeostasis, acclimation to acidic media, adhesion to host cells and flagellar assembly. These suggestions are based on expression data. For example, Cag16 and Cag8 are upregulated at pH 4.5 without urea (55), Cag3 expression is influenced by Fur and by adhesion (56, 21), and cagA is upregulated at pH 2.5 and induced by FlgS (57, 58). Also, expression of Cag3 and CagA is regulated by iron in the logarithmic growth phase (59). a situation which occurs with other CAG genes whose proteins were not identified here as essential nodes (21). This study shows that these protein interactions follow a physiological pattern that could imply the existence of TIVSS regulation forms that have not been studied and that these proteins could be considered as putative therapeutic targets.
Within the flagellar machinery two proteins identified as topological nodes have for the first time been proposed as putative therapeutic targets. First, the FliS protein is essential for flagellar assembly because it prevents polymerization of Flagellin (60). In fact, FliS mutants lose their flagella and become paralyzed (61, 62, 63). Second, the FlgB protein which is located between the peptidoglycan and the internal membrane is important in the formation of the duct for the flagellar motor (60). Physiologically, flgB expression increases in pH 4.5 without urea (55) as when it has adhered to the cell (56), but, contradictorily, it is not influenced by FlgS (57) which is supposed to be a master regulon. In fact, HspR, HrcA y Fur are the ones that positively influence the expression of flgB linking the protein to stress response and metal ion homeostasis. This makes it possible to generate new hypotheses for experimental work. On the other hand, the expression of fliS is regulated by sigma 28 and HrcA, making it a gene that responds to stress events. In other words, it is a type III flagellar regulation gene (21).
During starvation and at low pH, H. pylori produce ppGpp, a regulator which coordinates adaptation for survival (66). The topological nodes SpoT, GppA and NdK identified herein represent the complete enzymatic machinery for processing it metabolically (64). Adaptation occurs through SpoT under a limiting amino acid situation, during aerobic shock or when exposed to an acidic medium (65). SpoT mutant strains do not grow in a stationary phase but transform themselves into their coccoid forms. In addition, they cannot survive Turing stress conditions (65). SpoT and GppA expression are significantly repressed when the bacterium is in its most virulent growth phase (67, 58, 55). It has been experimentally determined that SpoT is an essential protein for the bacterial strain (Table 2). These proteins should be considered to be very novel therapeutic targets because they allow us to hypothesize ways of attacking environmental forms of the bacteria.
Another topological nodes is protein A of the UvrABC system. In E. coli and in H. pylori, this protein is the first to recognize DNA fragments that need to be repaired (70, 71). Mutant strains of this gene do not survive in ultraviolet light and recombine less often (72). Subsequently, a node that corresponds to the RpoBC protein has been identified within the transcriptional machinery. It is encoded by a fused gene that contains fully functional RNA polymerase β and β subunits (68).
Finally, we mention a number of topological nodes that are members of a series of various systems. HpcB protein is a member of a family of proteins that hydrolyze penicillin rings and cephalosporanic acid derivatives. Structurally HpcB is considered unique and has been proposed as a new family of penicillin-binding proteins (69). It has been known that iron is essential for homeostasis of Helicobacter pylori, but in fact, the most intricate transcriptional regulatory network is governed by Fur (21). Another node corresponds to FeoB, main iron transporter which is essential for growth and virulence (73). BCP is another protein that has been identified. It is a peroxiredoxin that protects the bacterium from reactive oxygen species. BCP mutant strains do not survive in the presence of superoxide and organic hydroperoxides. In addition, after three weeks of colonization they die (74). AccD and AccP proteins are necessary for H. pylori's microaerophilic metabolism (75). Finally, the UreI protein is a urea channel located in the inner membrane whose expression increases as pH decreases from pH 7.4 to 4.5 without urea (55, 58, 76). At pH 4.5 flgS controls its expression (57). We propose that the FabE QueD, AspB , CobB and pyrF nodes are topological centers and that the FabE and QueD proteins interact with Cag7, Cag12 and Cag8 in accordance with the Double Screen subsidiary network. This article has examined the proteins considered topological nodes for the interactome of the ATCC 26695 strain of Helicobacter pylori and proposes new therapeutic targets that have key roles in the physiology of the bacteria.
Acknowledgements
We thank the University of Applied and Environmental Sciences for financing this project and Doctor Carlos Barragan for his suggestions about writing the article.
REFERENCES
1. Loughlin M. Novel therapeutic targets in Helicobacter pylori. Expert OpinTher Targets. 2003;7:725-35. [ Links ]
2. Dunn B, Cohen H, Blaser M. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720-41. [ Links ]
3. Konstantinos P, Gerassimos J. Pathogenesis of Helicobacter Pylori Infection: Colonization, Virulence Factors of the Bacterium and Immune and Non-immune Host Response. HOSPITAL CHRONICLES. 2012;7(1):32-37. [ Links ]
4. Amieva M, Salama N, Tompkins L, Falkow, S. Helicobacter pylori enter and survive within multivesicular vacuoles of epithelial cells. Cell.Microbiol. 2002;4:677-90. [ Links ]
5. Hazell S, Lee A, Brady L, and Hennessy W. Campylobacter pyloridis and gastritis: association with intercellular spaces and adaptation to an environment of mucus as important factors in colonization of the gastric epithelium. J. Infect. Dis. 1986;153:658-63. [ Links ]
6. Kwok T,Backert S, Schwarz H, Berger J, Meyer T. Specific entry of Helicobacter pylori into cultured gastric epithelial cells via a zipperlike mechanism. Infect. Immun. 2002;70:2108-20. [ Links ]
7. Oh J, Karam S, Gordon J. Intracellular Helicobacter pylori in gastric epithelial progenitors. Proc. Natl. Acad. Sci. 2005;102:5186-91. [ Links ]
8. Mbulaiteye S, Hisada M, EL-omar E. Helicobacter pylori associated global gastric cancer burden. Front. Biosci. 2009;14:1490-1504. [ Links ]
9. Rain J, Selig L, De Reuse H, Battaglia V, Reverdy C, Simon S, Lenzen G, Petel F, Wojcik J, Schächter V, et al. The protein-protein interaction map of Helicobacter pylori. Nature. 2001; 409 (6817):211-15. [ Links ]
10. Daniel W, Xiaohua H. Topological Analysis and Sub-Network Mining of Protein-Protein Interactions. In: David T. Research and Trends in Data Mining Technologies and Applications. USA: IGI Global; 2007: p. 209-40. [ Links ]
11. Barabasi A, Albert R. Emergence of scaling in random networks. Science.1999;286: 509-12. [ Links ]
12. Newman M. The structure and function of complex networks.SIAM Review. 2003;45: 167-256. [ Links ]
13. Albert R, Jeong H, Barabasi A. Error and attack tolerance of complex networks. Nature. 2000;406: 378-82. [ Links ]
14. Terradot L, Durnell N, Li M, Ory J, Labigne A, Legrain P, Colland F, Waksman G. Biochemical characterization of protein complexes from the Helicobacter pylori protein interaction map: strategies for complex formation and evidence for novel interactions within type IV secretion systems. Mol Cell Proteomics. 2004;3(8):809-19. [ Links ]
15. Fong Y, Wong H, Chuck C, Chen Y, Sun H, Wong K. Assembly of preactivation complex for urease maturation in Helicobacter pylori: crystal structure of UreF-UreH protein complex. J Biol Chem. 2011;286(50):43241-9. [ Links ]
16. Voland P, Weeks D, Marcus E, Prinz C, Sachs G, Scott D. Interactions among the seven Helicobacter pylori proteins encoded by the urease gene cluster. Am J PhysiolGastrointest Liver Physiol. 2003;284(1):G96-G106. [ Links ]
17. Lam W, Woo E, Kotaka M, Tam W, Leung Y, Ling T, Au S. Molecular interaction of flagellar export chaperone FliS and cochaperone HP1076 in Helicobacter pylori. FASEB J. 2010;24(10):4020-32. [ Links ]
18. Busler V, Torres V, McClain M, Tirado O, Friedman D, Cover T. Protein-protein interactions among Helicobacter pylori cag proteins. J Bacteriol. 2006;188 (13):4787-800. [ Links ]
19. Kutter S, Buhrdorf R, Haas J, Schneider-Brachert W, Haas R, Fischer W. Protein subassemblies of the Helicobacter pylori Cag type IV secretion system revealed by localization and interaction studies.JBacteriol. 2008;190 (6):2161-71. [ Links ]
20. Jurik A, Hausser E, Kutter S, Pattis I, Prassl S, Weiss E, Fischer W. The coupling protein Cagbeta and its interaction partner CagZ are required for type IV secretion of the Helicobacter pyloriCagA protein. Infect Immun. 2010;78(12):5244-51. [ Links ]
21. Danielli A, Amore G,Scarlato V. Built shallow to maintain homeostasis and persistent infection: insight into the transcriptional regulatory network of the gastric human pathogen Helicobacter pylori. PLoS Pathog. 2010;6(6):e1000938. [ Links ]
22. Smoot M, Ono K, Ruscheinski J, Wang P, Ideker T. Cytoscape 2.8: new features for data integration and network visualization. Bioinformatics. 2011;27 (3):431-32. [ Links ]
23. Shannon P, Markiel A, Ozier O, Baliga N, Wang J, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13 (11):2498-2504. [ Links ]
24. Konieczka J, Drew K, Pine A, Belasco K, Davey S, Yatskievych T, et al. BioNetBuilder 2.0: bringing systems biology to chicken and other model organisms. BMC Genomics .2009; 10 Suppl 2:S6. doi: 10.1186/1471-2164-10-S2-S6. [ Links ]
25. Bader G, Betel D, Hogue C. BIND: the Biomolecular Interaction Network Database. Nucleic Acids Res. 2003;31(1):248-50. [ Links ]
26. Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40 (Database issue): D109-14. [ Links ]
27. Licata L, Briganti L, Peluso D, Perfetto L, Iannuccelli M, Galeota E, et al. MINT, the molecular interaction database: 2012 update. Nucleic Acids Res. 2012;40(Database issue): D857-61. [ Links ]
28. Kerrien S, Aranda B, Breuza L, Bridge A, Broackes-Carter F, Chen C, Duesbury M, et al. The IntAct molecular interactiondatabase in 2012. Nucleic Acids Res. 2012;40 (Database issue):D841-46. [ Links ]
29. Xenarios I, Salwínski L, Duan X, Higney P, Kim S, Eisenberg D. DIP, the Database of Interacting Proteins: a research tool for studying cellular networks of protein interactions. Nucleic Acids Res. 2002;30(1):303-05. [ Links ]
30. Dimmer E, Huntley R, Alam-FaruqueY, Sawford T, O'Donovan C, Martin M, Bely B, et al. The UniProt-GO Annotation database in 2011. Nucleic Acids Res. 2012;40 (Database issue):D565-70. [ Links ]
31. Raman K. Construction and analysis of protein-protein interaction networks. Autom Exp. 2010;2 (1):2. [ Links ]
32. Assenov Y, Ramírez F, Schelhorn S, Lengauer T, Albrecht M. Computing topological parameters of biological networks. Bioinformatics 2008;24(2):282-84. [ Links ]
33. Lin C-Y, Chin C-H, Wu H-H, Chen S-H, Ho C-W, Ko M-T. Hubba: hub objects analyzer--a framework of interactome hubs identification for network biology. Nucleic Acids Res. 2008;36(Web Server issue):W438-43. [ Links ]
34. Antal M, Böde C, Csermely P. Perturbation waves in proteins and protein networks: applications of percolation and game theories in signaling and drug design. Curr Protein Pept Sci. 2009;10 (2):161-72. [ Links ]
35. Li F, Li P, Xu W, Peng Y, Bo X, Wang S. PerturbationAnalyzer: a tool for investigating the effects of concentration perturbation on protein interaction networks. Bioinformatics. 2010;26 (2):275-77. [ Links ]
36. http://www.cbmc.it/~scardonig/interference/Interference.php, Center for Biomedical Computing [sede web]. Italia: http://www.cbmc.it [Actualizada enero del 2010; accesado 25 de Abril de 2013]. Disponible en: http://www.cbmc.it/~scardonig/interference/Interference.php [ Links ]
37. Zhang R, Lin Y. DEG 5.0, a database of essential genes in both prokaryotes and eukaryotes. Nucleic Acids Res 2009;37 (Database issue):D455-58. [ Links ]
38. Altschul S, Madden T, Schäffer A, Zhang J, Zhang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389-3402. [ Links ]
39. Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, et al. DrugBank 3.0: a comprehensive resource for omics' research on drugs.Nucleic Acids Res. 2011;39(Database issue):D1035-41. [ Links ]
40. Roy S. Systems biology beyond degree, hubs and scale-free networks: the case for multiple metrics in complex networks.Syst Synth Biol 2012;6(1-2):31-34. [ Links ]
41. Barabasi A, Albert R. Emergence of scaling in random networks. Science.1999;286:509-12. [ Links ]
42. Jeong H, Mason S, Barabasi A, Oltvai Z. Lethality and centrality in protein networks. Nature. 2001;411:41-42. [ Links ]
43. He X, Zhang J. Why do hubs tend to be essential in protein networks? PLoS Genet. 2006;2:e88. [ Links ]
44. Albert R, Jeong H, Barabasi A. Error and attack tolerance of complex networks. Nature. 2000;406: 378-82. [ Links ]
45. Han J, Bertin N, Hao T, Goldberg D, Berriz G, et al. Evidence for dynamically organized modularity in the yeast protein-protein interaction network. Nature. 2004;430: 88-93. [ Links ]
46. Wuchty S, Almaas E. Peeling the yeast protein network. Proteomics. 2005;5: 444-49. [ Links ]
47. Kutter S, Buhrdorf R, Haas J, Schneider-Brachert W, Haas R, Fischer W. Protein subassemblies of the Helicobacter pylori Cag type IV secretion system revealed by localization and interaction studies. J Bacteriol. 2008;190 (6):2161-71. [ Links ]
48. Wu J, Xu S, Zhu Y. Helicobacter pylori CagA: A Critical Destroyer of the Gastric Epithelial Barrier. Dig Dis Sci. 2013. [ Links ]
49. Pinto-Santini D, Salama N. Cag3 is a novel essential component of the Helicobacter pylori Cag type IV secretion system outer membrane subcomplex. J.Bacteriol. 2009;191(23):7343-52. [ Links ]
50. Savvides S, Yeo H, Beck M, Blaesing F, Lurz R, Lanka E, Buhrdorf R, Fischer W, Haas R, Waksman G. VirB11 ATPases are dynamic hexameric assemblies: new insights into bacterial type IV secretion. Embo J. 2003;22(9):1969-80. [ Links ]
51. Jimenez L, Kutter S, Sewald X, Ertl C, Weiss E. et al. Helicobacter pylori type IV secretion apparatus exploits b1 integrin in a novel RGDindependent manner. PLoSPathog. 2009;5: e1000684. [ Links ]
52. Pham K, Weiss E, Jiménez L, Breithaupt U, Haas R, Fischer W. CagI is an essential component of the Helicobacter pylori Cag type IV secretion system and forms a complex with CagL. PLoS One. 2012;7(4):e35341. [ Links ]
53. Tegtmeyer N, Wessler S, Backert S. Role of the cag-pathogenicity island encoded type IV secretion system in Helicobacter pylori pathogenesis. FEBS J 2011;278(8):1190-1202. [ Links ]
54. Ling F, Wang X, Dai D, Yu M, Chen C, Qian J, Liu C, Zhang Y, Ding J, Guan X, Shao S. The Helicobacter pylori Protein CagM is Located in the Transmembrane Channel That is Required for CagA Translocation. CurrMicrobiol. 2013;5. [ Links ]
55. Wen Y, Marcus E, Matrubutham U, Gleeson M, Scott D, Sachs G. Acid-adaptive genes of Helicobacter pylori. Infect Immun 2003;71(10):5921-39. [ Links ]
56. Kim N, Marcus E, Wen Y, Weeks D, Scott D, Jung H, et al. Genes of Helicobacter pylori regulated by attachment to AGS cells. Infect Immun. 2004;72 (4):2358-68. [ Links ]
57. Wen Y, Feng J, Scott D, Marcus E, Sachs G. The pH-responsive regulon of HP0244 (FlgS), the cytoplasmic histidine kinase of Helicobacter pylori.J Bacteriol. 2009;191(2):449-60. [ Links ]
58. Merrell D, Goodrich M, Otto G, Tompkins L, Falkow S. pH-regulated gene expression of the gastric pathogen Helicobacter pylori. Infect Immun. 2003;71(6):3529-39. [ Links ]
59. Merrell D, Thompson L, Kim C, Mitchell H, Tompkins L, Lee A, Falkow, S. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect Immun. 2003;71(11):6510-25. [ Links ]
60. Lertsethtakarn P, Ottemann K, Hendrixson D. Motility and chemotaxis in Campylobacter and Helicobacter. Annu Rev Microbiol. 2011;65:389-410. [ Links ]
61. Lam W, Woo E, Kotaka M, Tam W, Leung Y, Ling T, Au S. Molecular interaction of flagellar export chaperone FliS and cochaperone HP1076 in Helicobacter pylori Faseb J. 2010;24(10):4020-32. [ Links ]
62. Allan E, Dorrell N, Foynes S, Anyim M, Wren B. Mutational analysis of genes encoding the early flagellar components of Helicobacter pylori: evidence for transcriptional regulation of flgellinA biosynthesis. J. Bacteriol. 2000;182, 5274-77. [ Links ]
63. Zhang Z, Dorrell N, Wren B, Farthingt M. Helicobacter pylori adherence to gastric epithelial cells: a role for non-adhesin virulence genes. J Med Microbiol. 2002;51(6):495-502. [ Links ]
64. Wells D, Gaynor E. Helicobacter pylori initiates the stringent response upon nutrient and pH downshift. J. Bacteriol. 2006;188:3726-29. [ Links ]
65. Mouery K, Rader B, Gaynor E, Guillemin K. The stringent response is required for Helicobacter pylori survival of stationary phase, exposure to acid and aerobic shock. J.Bacteriol. 2006;188(15):5494-5500. [ Links ]
66. Zhou YN, Coleman WG Jr, Yang Z, Yang Y, Hodgson N, Chen F, et al. Regulation of cell growth during serum starvation and bacterial survival in macrophages by the bifunctional enzyme SpoT in Helicobacter pylori. J Bacteriol. 2008;190(24):8025-32. [ Links ]
67. Thompson L, Merrell D, Neilan B, Mitchell H, Lee A, Falkow S. Gene expression profiling of Helicobacter pylori reveals a growth-phase-dependent switch in virulence gene expression. Infect Immun. 2003;71(5):2643-55. [ Links ]
68. Raudonikiene A, Zakharova N, Su W, Jeong J, Bryden L, Hoffman P, et al. Helicobacter pylori with separate beta- and beta'-subunits of RNA polymerase is viable and can colonize conventional mice. MolMicrobiol. 1999;32(1):131-38. [ Links ]
69. Luthy L, Grutter MG, Mittl PR. The crystal structure of Helicobacter pylori cysteine-rich protein B reveals a novel fold for a penicillin-binding protein.J Biol Chem. 2002;277(12):10187-93. [ Links ]
70. Truglio J, Croteau DL, Van Houten B, Kisker C. Prokaryotic nucleotide excision repair: the UvrABC system. Chem Rev. 2006;106(2):233-52. [ Links ]
71. Moolenaar G, Monaco V, Van der Marel G, Van Boom J, Visse R, Goosen N. The effect of the DNA flanking the lesion on formation of the UvrBDNApreincision complex. Mechanism for the UvrA-mediated loading of UvrB onto a DNA damaged site. J.Biol Chem. 2000;275:8038-43. [ Links ]
72. Moccia C, Krebes J, Kulick S, Didelot X, Kraft C, Bahlawane C, Suerbaum S. The nucleotide excision repair (NER) system of Helicobacter pylori: role in mutation prevention and chromosomal import patterns after natural transformation. BMC Microbiol. 2012;12:67-9. [ Links ]
73. Velayudhan J, Hughes N, McColm A, Bagshaw J, Clayton C, Andrews S, Kelly, D. Iron acquisition and virulence in Helicobacter pylori: a major role for FeoB, a high-affinity ferrous iron transporter. MolMicrobiol. 2000;37(2):274-86. [ Links ]
74. Wang G, Olczak A, Walton J, Maier R. Contribution of the Helicobacter pylori thiol peroxidase bacterioferritin comigratory protein to oxidative stress resistance and host colonization. Infect Immun. 2005;73(1):378-84. [ Links ]
75. Burns B, Hazell S, Mendz G. Acetyl-CoA carboxylase activity in Helicobacter pylori and the requirement of increased CO2 for growth. Microbiology. 1995;141(12):3113-18. [ Links ]
76. Bury-Moné S, Skouloubris S, Labigne A, De Reuse H. The Helicobacter pylori UreI protein: role in adaptation to acidity and identification of residues essential for its activity and for acid activation. Mol Microbiol. 2001;42(4):1021-34. [ Links ]











 texto en
texto en