Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.1 Bogotá Jan./Mar. 2014
Pharmacokinetics: efficacy and safety of MMX mesalamine formulation for treating ulcerative colitis
Joaquín Hinojosa MD. (1), Víctor Navas MD. (2), Cristina Saro MD. (3)
(1) Gastroenterology Department of the Hospital de Manises in Valencia, Spain.
(2) Medical Department of Shire Pharmaceuticals Ibérica S.L. in Madrid, Spain.
(3) Gastroenterology at the Hospital of Cabuenes in Asturias, Spain.
Received: 17-10-13 Accepted: 19-12-13
Abstract
MMX® mesalazine - is an enteric formulation of 5-ASA with Multi Matrix technology for delayed and prolonged administration of high doses of mesalazine throughout the colon. Clinical trials have demonstrated its clinical and endoscopic efficacy for inducing remission. At doses of 2.4g to 4.8 g/day (2-4 tablets in a single daily dose) the rate of clinical remission from was 29.2%, and the rate of endoscopic remission was 41.2 % after 8 weeks of treatment. The clinical rate of remission for patients who received placebos was 9% and the endoscopic rate was 22.2 %. This efficacy of this formulation for inducing remission in left-sided colitis has been shown to be similar to topical treatment (60% clinical remission after 8 weeks of administration of MMX mesalazine compared to 50% with topical treatment. Although rates of remission induction therapy are similar to those obtained with conventional mesalazine trials, this trial of MMX mesalazine used the more stringent criteria of endoscopic improvement. 88.9% of patients treated with 2.4 g/day in a single dose without recurrence after 12 months. The safety profile is similar to those of standard mesalazines. The MMX formulation is a promising treatment for patients with UC because data efficiency and dosage convenience may improve compliance.
Keywords
Ulcerative colitis, mesalazine, remission induction, compliance, endoscopy.
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory disease of the colon and rectum. It is characterized by a diffuse superficial inflammation of the lining of the colon which usually starts above the anal margin and extends proximally and continuously (1, 2). Lesions are most commonly located in the rectum, sigmoid colon and the left colon, but can also affect the entire colon (3). The underlying etiology is unknown, but both genetic and environmental factors have been implicated (3).
UC's most frequent forms of presentation - proctitis, proctosigmoiditis, and left colitis - account for 60% to 80% of new cases (1). Typical symptoms include rectal bleeding and diarrhea which are often accompanied by other symptoms such as abdominal pain or tenesmus (2). UC is characterized by remissions and relapses. In a period of one year, 50% of UC patients present relapses (1). Furthermore, the disease persists for a long time in patients with extensive UC, and this prolonged evolution is a risk factor for developing colon cancer (1). The risk increases 0.5% to 1% per year in cases that last more than 8-10 years (2). This explains the crucial importance of maintenance therapy once remission is obtained.
The incidence of UC varies from 0.5 to 24.5 cases per 100,000 individuals per year worldwide (1). Although this incidence is higher in the developed countries of Europe and North America (2-12 new cases per 100,000 people per year) (2, 3), recent studies indicate a progressive increase parallel to industrialization in Eastern Europe, Latin America and Asia (1). It is estimated that prevalence is 70-200 cases per 100,000 in the United States (2). There are about 250,000-500,000 people with UC whose care is estimated to cost USD 192 million a year for hospitalization and USD 138 million a year for medication (4).
Following clinical practice guidelines (5), the first-line pharmacological choice for treatment and maintenance of remission for mild to moderate UC is mesalazine (5-aminosalicylic acid, 5-ASA) or its derivatives. They are generally administered as prodrugs (1). These treatments are administered orally, topically, or in combination. Their effectiveness has been clearly demonstrated (2, 6, 7).
Given that the disease affects only the large intestine, and taking into account the local effect, formulations for rectal administration have been developed in addition to the various oral formulations. Rectal administration aims to prevent gastric and ileal absorption so that greater quantities of the drug reach the colon (8-10). In long-term treatment, rectal presentations or multiple dose oral formulations may pose difficulties for compliance. These circumstances and the influence of other factors (patient age, socioeconomic status, etc.) (11) can have negative consequences for disease control (14).
MMX MESALAZINE PHARMACOKINETICS
The oral formulation of mesalazine, based on MultiMatrix technology (MMX®, registered trademark of Cosmo Technologies Ltd., Wicklow, Ireland and MMX Multi Matrix System®, registered trademark of Cosmo S.p.A., Milan, Italy) is designed to achieve sustained and prolonged drug release thus enabling administration of a high dose once a day (2-4 tablets, 1.2 g / tablet) (3).
MMX mesalazine, under the brand name Lialda® (registered trademark of Giuliani International Ltd., Dublin, Ireland) was approved for treatment of UC remission by the Food and Drug Administration (FDA) of the United States in January 2007. In most European countries it is marketed under the name Mezavant® (1) (registered trademark of Giuliani International Ltd., Dublin, Ireland) where it is used to induce remission of UC and for maintenance treatment. The Spanish Agency for Medicines and Health Products (AEMPS - Agencia Española de Medicamentos y Productos Sanitarios) stated that this product is a medical innovation of therapeutic interest in February 2009 (12).
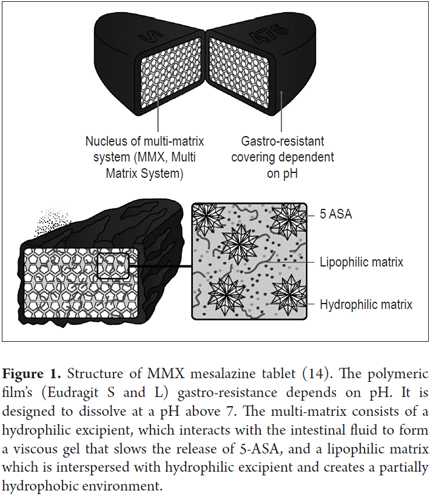
MMX-mesalazine is presented in an enteric coated tablet with a film containing two polymers (Eudragit S and L). There is a double polymeric matrix system inside containing drug microparticles embedded in the lipophilic matrix which when intermixed with hydrophilic matrix delay and prolong administration.
The film disintegrates only at pH ≥ 7 which typically occurs in the terminal ileum (Figure 1) (13). As the coating breaks down, the hydrophilic matrix interacts with the gastrointestinal contents, absorbs water, and swells to form a viscous gelatinous mass. This delays diffusion of the drug contained in the core to the luminal space of the colon. The lipophilic matrix delays infiltration of intestinal liquid into the tablet core which prolongs the release of the drug (1). Consequently the mesalazine is released more slowly and gradually and is distributed more evenly throughout the colon than in other oral preparations (3, 4).
The hypothesis regarding this release of medicine throughout the colon is supported by several in vitro and in vivo studies. Tenjarla et al. (14) used an in vitro model of the gastrointestinal system to simulate the physiological conditions of the human digestive tract when fasting and after food intake. Less than 1% of the medicine was released anterior to the colon in the simulated stomach and small intestine was while in the simulated colon it reached 78.0% postprandially and 68.5% under fasting conditions. Considerable amounts of medicine were released over a period of 8-18 hours (49.6 mg/hour under postprandial conditions, and 40.7 mg/h under conditions of fasting). In studies of 5-ASA transit and release in the gastrointestinal tract in healthy volunteers using scintigraphic techniques, Brunner et al. (15) observed that erosion of the tablet began after 6.9±1.1 hours in the ascending or transverse colon and that the radiotracer spread evenly throughout the colon to its distal portion. The average absorption was 19.9% in the small intestine and ileum and 80.1% in the colon. Maximum concentrations of 5-ASA in the blood occurred after 8-12 hours, coinciding with passage through the transverse and descending colon. In another study, also using scintigraphic techniques in healthy volunteers, Brunner et al. (16) evaluated gastrointestinal transit and release sites of 5-ASA formulations in tablets and granules (Salofalk®). With both formulations, the time the medication took to reach the ileocecal valve was shorter, and the maximum concentrations of 5-ASA in blood occurred after 3 or 4 hours had elapsed, coinciding with the passage of the drug through this region. In other words, this occurred much earlier than it did in the MMX mesalazine study. Radioactive labeling has been used to compare MMX mesalazine with the pH-dependent delayed-release Asacol® formulation. As measured by the marker, disintegration of the tablet started after an average of 4.75 hours with MMX mesalazine and after an average of 6.16 hours with Asacol®. However, complete disintegration was recorded at 17.37 hours with MMX mesalazine and at 7.27 with Asacol® (17-19). These findings indicate that MMX mesalazine releases 5-ASA in a delayed manner throughout the colon, while the pH-dependent slow release preparation releases the medicine mainly in the ascending colon.
Pharmacokinetic studies in healthy volunteers with multiple 2.4 and 4.8g doses of MMX mesalazine administered with food indicate that the total absorption of the administered dose is about 24% (20) which indicates that the formulation has a primarily local effect.
Safety and efficacy in inducing remission
Several clinical trials have been conducted to evaluate the efficacy and safety of MMX mesalazine (Table 1).
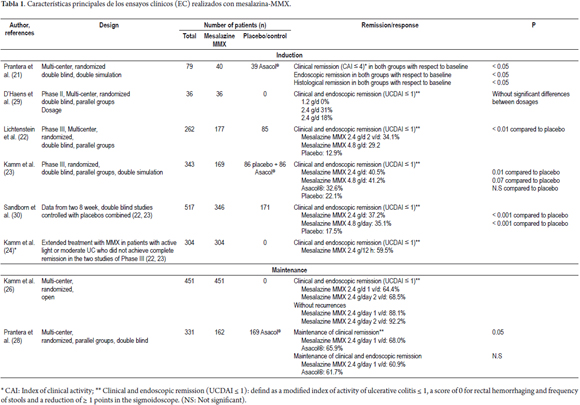
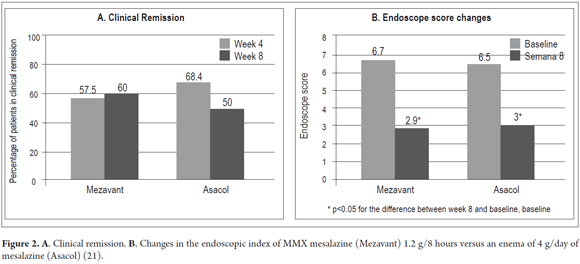
Prantera et al. (21) reported the results of a randomized double-blind, double-dummy study conducted with 79 patients who had mild to moderate left UC (clinical activity index, CAI ≥ 6). Doses of 1.2 g MMX mesalazine were administered three times a day. In addition, administration of a placebo enema was compared with an enema of 4g of 5-ASA (plus one placebo tablet) for 8 weeks. Patients with proctitis (<15 cm long) were excluded. The primary endpoint was clinical remission (CAI score ≤ 4) at 4 weeks. The proportion of patients who achieved clinical remission was similar for both treatments: with MMX mesalazine 57.5% achieved remission after four weeks and 60.0% achieved remission after eight weeks, whereas with 5-ASA enemas 68.4% achieved remission after four weeks and 50.0% achieved remission after eight weeks (Figure 2). Both treatments significantly reduced the CAI score after four weeks (4.3 and 3.9 points) and 8 weeks (5.7 and 4.9 points) (Table 1). Endoscopic remission was observed at 8 weeks in 45.0% of patients treated with MMX mesalazine, but only in 36.8% of those treated with enemas. Both groups showed statistically significant improvements in endoscopic index (EI ≤ 2) (Figure 2). In this study, the histological remission rate was 15.0% in the MMX mesalazine group versus 8% in the group treated with enemas. Treatment adherence was 97.0% and 87.5%, respectively. MMX mesalazine was well-tolerated. There were no serious or unexpected adverse events. At least one adverse event in 15.0% of the patients treated with MMX mesalazine and in 28.0% of those treated with enemas were recorded. There was a single case of treatment discontinuation due to adverse events in the group treated with enemas. In phase III, two eight-week, multicenter, randomized, parallel group, double-blind, double-dummy studies of MMX mesalazine which evaluated clinical and endoscopic remission were published. The first (22) used a control group which received placebos. From the sample of 280 patients with mild to moderate UC parallel groups were formed. For 8 weeks, one group received 1.2 g of MMX mesalazine twice a day (n = 93), another group received 4.8 g/day in a single dose (n = 94), and the control group received placebos (n = 93). The primary endpoint was the percentage of patients with clinical and endoscopic remission. A strict index was used to define remission: a modified UC-DAI score of less than or equal to 1. A score of 0 was assigned to cases of rectal bleeding and frequent stools, and the score was reduced by at least 1 point compared to the baseline of the sigmoidoscopy score. Patients whose mucosa showed any degree of friability received sigmoidoscopic scores greater than or equal to, so they were not considered to have reached the primary endpoint. Remission was achieved with a significantly higher frequency (p<0.01) among patients treated with 1.2 g MMX mesalazine twice a day or 4.8 g/day of MMX mesalazine in a single daily dose than in the placebo group (Table 1, Figure 3). Both MMX mesalazine dosages were generally well tolerated with no clinically significant observed differences in safety with placebos. 223 adverse events occurred in 129 patients; most of them of mild to moderate intensity. Only 7 patients experienced a total of 8 serious adverse events.
The second study (23) was a double-dummy, placebo-controlled trial with an active ingredient used for as comparison (delayed release mesalazine without MMX technology: Asacol®). The study included 343 patients with mild to moderate UC. MMX mesalazine dosages studied were 2.4 g/day (n=86) and 4.8 g/day both in single daily doses (n=85). 2.4g/day of Asacol® was administered in three daily doses of 0.8g (n=86). A placebo control group (n=86) was also used. The therapy was continued for 8 weeks. As in the previous study, the main purpose was to evaluate clinical and endoscopic remission. Although 22.1% of placebo patients achieved remission, significantly higher percentages of the other groups achieved remission. In the group that received 2.4 g/d of MMX mesalazine 40.5% achieved remission (OR 2.4, p = 0.01) and in the group that received 4.8 g/d, 41.2% achieved remission (OR 2.5, p = 0.007) (Figure 3). All active treatments were well tolerated, with no observable differences among groups. A total of 72 patients experienced 115 adverse events during the study, and 5 experienced a total of 8 serious adverse events. Only four patients discontinued the study due to adverse events (two in the placebo group, one from the 2.4 g group and one from the Asacol® group) (23).
In a pooled analysis of both phase III studies, serious adverse events were more common with placebos (6.1%) than with 2.4 or 4.8 g of MMX mesalazine (1.1% and 2.2%, respectively). The most frequent adverse events observed with active ingredients were headaches and flatulence (3).
Despite treatment efficacy (Table 1), it should be noted that many patients with UC do not achieve remission after 8 weeks of treatment with mesalazine and often receive corticosteroids or immunosuppressants. Kamm et al. (24) conducted an extended open multicenter study in which patients who had not achieved remission after 8 weeks of treatment with 2.4g/day MMX mesalazine, 4.8g/day MMX mesalazine, 2.4 g/day Asacol® or from placebos in both phase III trials were combined. All of these patients received the same treatment of 2.4 g/12 hours MMX mesalazine for 8 an week extension of the original studies. The main goal was clinical and endoscopic remission defined in the same way as in the original studies. 59.5% of 304 patients with mild to moderate UC in the study achieved complete remission after the additional treatment period. This percentage did not depend on the previously received treatment (mesalazine formulations or placebo). MMX mesalazine was well-tolerated 156 adverse events were observed in 72/312 patients (23.1%). There were 60 treatment-related adverse events in 27/312 patients (8.7%) and nine serious adverse events of which one (pancreatitis) was considered to be related to treatment.
Sandborn et al. (25) presented results of the acute extension phase study which evaluated the time until disappearance of symptoms, defined as the first day without rectal bleeding and with normal stool frequency. 181/304 patients achieved remission of symptoms. The median time until resolution of symptoms was 15 days.
These clinical trials which were methodologically rigorous and which included clinical and endoscopic assessment criteria confirm the efficacy and safety of MMX mesalazine as a treatment for inducing remission of UC in 8 weeks. Data from the open extension study indicates that continuous treatment may be useful to induce a response in patients who do not achieve remission during the initial treatment period.
Efficacy and safety in maintenance treatment
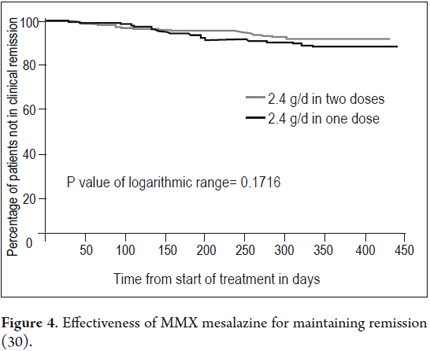
Maintenance treatment with MMX mesalazine in mild-to-moderate colitis has been investigated in a randomized open, multicenter trial involving 459 patients. Remission was strictly defined according to clinical and endoscopic criteria as described above. Patients had presented mild to moderate exacerbation of UC (30). All patients came from one of the two randomized studies of acute treatment for 8 weeks (n=246) or from the extension study of 8 additional weeks with 4.8g/day MMX mesalazine (n=213). Patients were randomized to receive maintenance therapy for 12 months with 2.4g/day MMX mesalazine in a single dose (two tablets of 1.2 g) or two doses (one tablet of 1.2 g twice a day). The primary endpoint was safety and tolerability, assessed on the basis of adverse events and time until withdrawal.
Treatment was generally well tolerated. A total of 174 of the 459 patients (37.9%) had 384 adverse events. Most were mild to moderate in severity. Twenty-two serious adverse events occurred (10 with the single dose and 12 with two daily doses) in 18 patients (3.9%). Most of these events consisted of digestive problems (9 patients). 76 adverse events in 47 patients (10.2%) were considered to be related to treatment, with no significant differences between groups. Treatment was discontinued due to adverse events in 21 patients (4.9%) treated with a daily dose and 10 (4.3%) with two daily doses (11). Differences between groups were not observed during the time until treatment was discontinued. In spite of the long period of treatment, compliance was very good: a total of 442 patients (96.3%) consumed 80% or more of prescribed medication, with no significant differences between the two dosage regimens (30). Efficacy of maintenance treatment, which was a secondary endpoint of this trial was also very positive (population for analysis of efficacy, n = 451) (30) After 12 months, 64.4% of patients treated with a daily dose (2.4 g / day) and 68.5% with two daily doses (1.2 g/12h) had clinical and endoscopic remission. At the same time, 88.9% and 93.2% of subjects in each of these groups were not withdrawn due to recurrence, which was defined as absence of needing to increase the MMX mesalazine dose or administer other treatments, including surgery (Figure 4). Hanauer et al. (31) conducted a pooled analysis of data concerning induction and maintenance of remission in patients from the two randomized, placebo-controlled phase III trials and from the previously mentioned maintenance study treated with MMX mesalazine in doses of 2.4g or 4.8g per day (excluding patients treated with placebo or with a formulation without MMX in randomized and placebo-controlled phase III trials). Recurrence during maintenance is also defined as the need for alternative medication or surgery due to an exacerbation of UC. Overall, 63.6% (220/346) of patients achieved remission after 8 or 16 weeks of treatment. 218 of these 220 patients were added to maintenance therapy for 12 months, and at the end of the study 89.9% of them (196/218) had not withdrawn due to recurrence. Altogether, 56.6% of patients with mild- moderate UC (196/346) who began treatment with MMX mesalazine achieved complete remission (clinical and endoscopic) without additional medication, remission was maintained for 12 months without exacerbations requiring alternative treatment. The adverse event profile at all used doses was similar to that observed in the placebo groups of the original studies.
There are few direct comparative data of other MMX mesalazine formulations of 5-ASA referring to maintenance treatment. Prantera et al. (32) published a multicenter, randomized, double-blind, parallel group, in 331 patients with left UC in maintenance therapy for 12 months with MMX mesalazine 2.4 g/day in a single dose study, or Asacol® 2.4 g/day in two divided doses. All patients had been in remission for at least one month before the test and had undergone ≤1 relapse in the previous year. Assessment of efficacy was based on the maintenance of clinical remission, and clinical and endoscopic remission. Patients received dairies to write down the specific symptoms of the disease, based on the first two paragraphs of the UC-DAI scale (stool frequency and rectal bleeding). Both treatments were similarly well tolerated, there were no significant differences in regard to clinical remission (68.0% vs with MMX mesalazine 65.9% with Asacol®) or endoscopic and clinical remission (60.9% vs. 61.7%).Maintenance of remission was also assessed from patient diaries, using as criteria stool frequency worsening and rectal bleeding increase (score ≥ 2, with a score ≥ 1 for rectal bleeding during a minimum of 2 weeks). After 12 months, remission occurred in 62.2% of patients treated with MMX mesalazine, compared with 51.5% treated with Asacol® (p=0.053).
Thus, although there are no clear evidence to support the superiority of a formulation of 5-ASA with respect to another, MMX mesalazine-represents an important advance in the treatment of UC with 5-ASA. Existing data set indicates that use of high concentrations of these tablets, with sustained-release throughout the colon (including the distal portion), allows more comfortable treatment, with less tablets in a single daily dose, which promotes compliance both in remission of acute phase and maintenance.
CONCLUSION
MMX mesalazine is a gastro-resistant formulation of delayed and extended release of 5-ASA, which enables uniform distribution of the medicine throughout the colon, thanks to its gastro-resistant coating and the double array. Clinical trials with solid methodological design with clinical and endoscopic evaluation have demonstrated the efficacy and safety of MMX mesalazine to achieve remission in patients with mild to moderate UC. One of the studies indicated that its effectiveness may be similar to the topical treatment for induction of remission in left colitis. Its role in the treatment of proctitis has not been defined. Furthermore, data obtained showed an acceptable safety and efficacy profile as a maintenance therapy for prevention of new exacerbations of the disease. Efficacy data and its daily administration in both remission and maintenance treatment, make MMX mesalazine a promising pharmacological option in ulcerative colitis.
REFERENCES
1. Schreiber S, Kamm MA, Lichtenstein GR. Mesalamine with MMX technology for the treatment of ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2008;2(3):299-314. [ Links ]
2. Hu MY, Peppercorn MA. MMX mesalamine: a novel high-dose, once-daily 5-aminosalicylate formulation for the treatment of ulcerative colitis. Expert Opin Pharmacother. 2008;9(6):1049-58. [ Links ]
3. McCormack, P.L., D.M. Robinson, C.M. Perry. Delayed-release Multi Matrix System (MMX) mesalazine: in ulcerative colitis. Drugs. 2007;67(17):2635-42. [ Links ]
4. Kale-Pradhan, P.B., R.S. Pradhan, S.M. Wilhelm. Multi-Matrix System Mesalamine: To Use or Not To Use. Ann Pharmacother. 2008;42(2):265-269. [ Links ]
5. Dignass, A., Lindsay J.O., Sturm A., Windsor A., Colombel J.F. Allez M. et al. Second European evidence-based Consensus on the diagnosis and management of ulcerative colitis: Current management. J Crohns Colitis. 2012;6(10):991-1030. [ Links ]
6. Marshall, J.K., M. Thabane, A.H. Steinhart, J.R. Newman, A. Anand, E.J. Irvine.Rectal 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2010(1):CD004115. [ Links ]
7. Sutherland, L.J.K. Macdonald.Oral 5-aminosalicylic acid for induction of remission in ulcerative colitis. Cochrane Database Syst Rev. 2006(2):CD000543. [ Links ]
8. Desreumaux, P.S. Ghosh.Review article: mode of action and delivery of 5-aminosalicylic acid - new evidence. Aliment Pharmacol Ther. 2006;24 Suppl1: 2-9. [ Links ]
9. Ng, S.C.M.A. Kamm.Review article: new drug formulations, chemical entities and therapeutic approaches for the management of ulcerative colitis. Aliment Pharmacol Ther. 2008;28(7):815-29. [ Links ]
10. Sandborn, W.J.Oral 5-ASA therapy in ulcerative colitis: what are the implications of the new formulations? J Clin Gastroenterol. 2008;42(4):338-44. [ Links ]
11. Shale, M.J.S.A. Riley.Studies of compliance with delayed-release mesalazine therapy in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2003;18(2):191-8. [ Links ]
12. Declaración de innovacion galénica de interés terapéutico, Agencia Española de Medicamentos y Productos Sanitarios, Editor. 2009: (Archivado por Shire Pharmaceuticals Ibérica). [ Links ]
13. Baker, D.E.MMX mesalamine. Rev Gastroenterol Disord. 2006;6(3):146-52. [ Links ]
14. Tenjarla, S., V. Romasanta, E. Zeijdner, R. Villa, L. Moro.Release of 5-aminosalicylate from an MMX mesalamine tablet during transit through a simulated gastrointestinal tract system. Adv Ther. 2007;24(4):826-40. [ Links ]
15. Brunner, M., R. Assandri, K. Kletter, M. Tschurlovits, M.E. Corrado, R. Villa, et al. Gastrointestinal transit and 5-ASA release from a new mesalazine extended-release formulation. Aliment Pharmacol Ther. 2003;17(3):395-402. [ Links ]
16. Brunner, M., R. Greinwald, K. Kletter, H. Kvaternik, M.E. Corrado, H.G. Eichler, et al. Gastrointestinal transit and release of 5-aminosalicylic acid from 153Sm-labelled mesalazine pellets vs. tablets in male healthy volunteers. Aliment Pharmacol Ther. 2003;17(9):1163-9. [ Links ]
17. Wray, H., R. Joseph, M. Palmen, D. Pierce. MMXTM Mesalamine and delayed-release mesalamine: A pharmacokinetic and scintigraphic comparison. in Canadian Digestive Diseases Week. 2009. Banff, Alberta, Canada. [ Links ]
18. Wray, H., R. Joseph, M. Palmen, D. Pierce.Combined pharmacokinetic and scintigraphic analyses for the comparison of 5-ASA release profiles from MMX mesalamine and another delayed-release mesalamine formulation. Inflammatory Bowel Diseases. 2008;14(S3):S19-S20 (Abstract P-0030). [ Links ]
19. Wray, H, R. Joseph, M. Palmen, D. Pierce.A pharmacokinetic and scintigraphic comparison of MMXTM mesalamine and delayed-release mesalamine. Am J Gastroenterol. 2008;103(s1): S433-S434 (abstract). [ Links ]
20. Prospecto: Informacion para el usuario. 2008 [consultada: 2011]; Disponible en: https://sinaem4.agemed.es/consaem/especialidad.do?metodo=verFichaWordPdf&codigo=70144&formato=pdf&formulario=PROSPECTOS. [ Links ]
21. Prantera, C., A. Viscido, L. Biancone, A. Francavilla, L. Giglio, M. Campieri.A new oral delivery system for 5-ASA: preliminary clinical findings for MMx. Inflamm Bowel Dis. 2005;11(5):421-7. [ Links ]
22. Lichtenstein, G.R., M.A. Kamm, P. Boddu, N. Gubergrits, A. Lyne, T. Butler, et al. Effect of once- or twice-daily MMX mesalamine (SPD476) for the induction of remission of mild to moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2007;5(1):95-102. [ Links ]
23. Kamm, M.A., W.J. Sandborn, M. Gassull, S. Schreiber, L. Jackowski, T. Butler, et al. Once-daily, high-concentration MMX mesalamine in active ulcerative colitis. Gastroenterology. 2007;132(1):66-75;quiz 432-3. [ Links ]
24. Kamm, M.A., G.R. Lichtenstein, W.J. Sandborn, S. Schreiber, K. Lees, K. Barrett, et al. Effect of extended MMX mesalamine therapy for acute, mild-to-moderate ulcerative colitis. Inflamm Bowel Dis. 2009;15(1):1-8. [ Links ]
25. Sandborn, W., M. Kamm, G. Lichtenstein, M. Sumner, R. Joseph. MMX mesalamine therapy for the induction of remission beyond 8 weeks: how long before symptoms resolution?. Am J Gastroenterol. 2008;103(s1):S435 (abstract). [ Links ]
26. Kamm, M.A., G.R. Lichtenstein, W.J. Sandborn, S. Schreiber, K. Lees, K. Barrett, et al. Randomised trial of once- or twice-daily MMX mesalazine for maintenance of remission in ulcerative colitis. Gut. 2008;57(7):893-902. [ Links ]
27. Hanauer, S.B., G.R. Lichtenstein, M.A. Kamm, W.J. Sandborn, K. Lees, K. Barrett, et al. MMX Mesalamine for Induction and Maintenance Therapy in Mild-to-Moderate Ulcerative Colitis. Gastroenterol Hepatol. 2009;5(7):494-500. [ Links ]
28. Prantera, C., A. Kohn, M. Campieri, R. Caprilli, M. Cottone, F. Pallone, et al. Clinical trial: ulcerative colitis maintenance treatment with 5-ASA: a 1-year, randomized multicentre study comparing MMX with Asacol. Aliment Pharmacol Ther. 2009;30(9): 908-18. [ Links ]
29. D'Haens, G., D. Hommes, L. Engels, F. Baert, L. Van Der Waaij, P. Connor, et al. Once daily MMX mesalazine for the treatment of mild-to-moderate ulcerative colitis: a phase II, dose-ranging study. Alimentary Pharmacology & Therapeutics. 2006;24(7): 1087-1097. [ Links ]
30. Sandborn, W.J., M.A. Kamm, G.R. Lichtenstein, A. Lyne, T. Butler, R.E. Joseph.MMX Multi Matrix System® mesalazine for the induction of remission in patients with mild-to-moderate ulcerative colitis: a combined analysis of two randomized, double-blind, placebo-controlled trials. Alimentary Pharmacology & Therapeutics. 2007;26(2):205-215. [ Links ]











 text in
text in