Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.1 Bogotá ene./mar. 2014
GERD in children: an update
Carlos Alberto Velasco Benítez MD. (1)
(1) Pediatrician, Gastroenterologist, Nutritionist, University teaching specialist, Msc. in Epidemiology, Professor and Director of the GASTROHNUP Research Steering Group at the Universidad del Valle in Cali, Colombia.
Received: 10-08-11 Accepted: 19-12-13
Abstract
Although the definition of Gastro-Esophageal Reflux Disease (GERD) has not changed much over the last ten years, GERD continues to cause high rates of morbidity and mortality. Probably, and in a practical way, it could be said that physiological GERD that is not pathological is usually accompanied by regurgitation, and that its main symptom is vomiting. As in acid peptic disease, in GERD we can talk about certain aggressive and protective factors that can cause damage depending on their prevalence. Signs and symptoms of GERD in children depend on the age of the group studied. Just as every wheezing child is not asthmatic, in gastroenterology not every child that vomits or regurgitates has GERD. Today, certain slowly evolving diseases and conditions are defined as refractory GERD because the natural history of these diseases and their association with increased morbidity and mortality result in prognoses that imply different therapeutic approaches. Sensitivity, specificity and reproducibility vary in accordance to the laboratory tests requested to study a child with GERD. Treatment of children with GERD includes anti-reflux measures, administration of medicine and surgery.
Key words
Update, Gastro-Esophageal Reflux Disease, children.
INTRODUCTION
Gastro-Esophageal Reflux Disease (GERD) is nothing but the return of gastric contents into the esophagus. The contents are usually acidic, but they can also be alkaline. GERD has general gastrointestinal and respiratory repercussions, but unlike non-pathological physiological Gastro-Esophageal Reflux (GER) which is present in between 90% and 95% of infants, its definition has not varied much over the last ten years. Nevertheless, it continues to cause high rates of morbidity and mortality. This is reflected in the descriptive study that we carried out of 31 children hospitalized due to GERD in the Infant Service of the Hospital Universitario Ramón González in Bucaramanga, Colombia. The mortality rate was 26% primarily because of respiratory complications such as irrecoverable massive bronchoaspiration. The purpose of this study to review articles concerning GERD in children published in the last five years and add our experience and the experience of other Colombian authors.
DEFINITIONS
Probably, it can be said that practically physiological non-pathological GER usually occurs with regurgitation and consists of the sudden return of small amounts of gastric contents into the pharynx and mouth when nausea, retching, autonomic symptoms and symptoms of thoracic or abdominal muscle contraction are absent. In contrast, the main presentation symptom present in GERD is vomiting when nausea, retching, autonomic symptoms and/or symptoms of chest or abdominal muscle contraction are present. This does not mean that children with non-pathological physiological GER cannot present vomiting or that children with GERD cannot experience episodes of regurgitation. In general, and in principle, it is always preferred to think in terms of GER rather than GERD in infants. This is reflected in a study of 216 infants with symptoms of regurgitation. They were followed from birth to the first year of life. At two months of age almost 87% of them had the symptom, at four months about 61% of them still regurgitated, at sixth months 46% had the symptom, at 8 months only 23% continued to present this symptom, and after one year only 8% continued to regurgitate (3).
Physiopathology
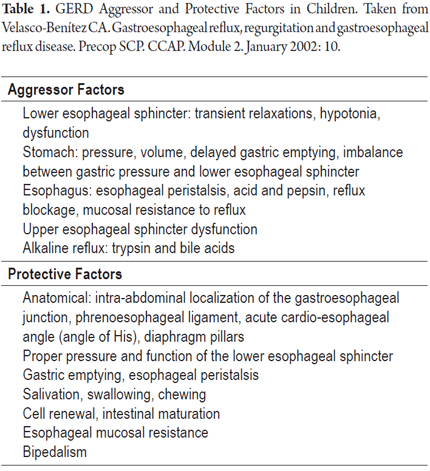
In a way that is similar acid peptic diseases, we can talk about certain aggressor and protective factors in GERD which can cause damage depending on their prevalences (Table 1). Aggressor factors include those that have to do with the upper and lower esophageal sphincters, the esophagus and the stomach. They include acid as well as alkaline flux and include gastric and the esophageal pressures. Protective factors include those related to the anatomy itself such as sphincter pressures, the physiology of esophageal peristalsis and gastric emptying as well as the presence of a buffer system consisting of salivation, cell renewal, esophageal resistance, and bipedalism (4). Bipedalism is deeply connected with changes in intra-abdominal and intra-thoracic pressures (5).
Clinical findings
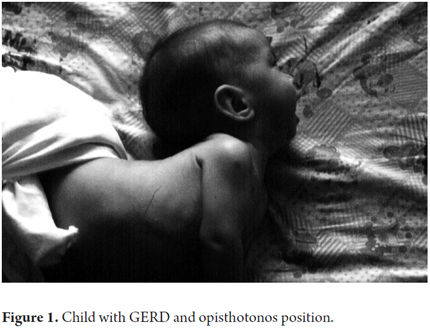
Signs and symptoms of GERD in children are determined by age group. They can be general, digestive and respiratory. Frequently present in infants, they include mucocutaneous pallor; Sandifer syndrome which comprises opisthotonos, anemia and GER esophagitis (Figure 1); loss of appetite; anorexia, irritability, sleep disturbances and failure to thrive. A recent study by Pashankar et al. of 251 obese American children between 7 and 16 years old reported that obese children with body mass indices of more than the 95th percentile for their age and sex are 7.3 times more likely to present GERD than children who are not obese.
Children with GERD may present gastrointestinal signs and symptoms such as vomiting, regurgitation, hematemesis and/or melena, heartburn, colic, epigastric pain, flatulence, burping, rumination, hiccups, retro-sternal pain, protein-losing enteropathy, bloating, dysphagia, odynophagia and oral lesions such as dental caries, dental erosions, mucosal lesions and bacterial plaque (8). Some dyspeptic symptoms present in children with Helicobacter pylori gastritis definitely not solely associated with GERD (9, 10). Finally, respiratory and otolaryngologic signs and symptoms, whose presence classifies a case of GERD as one with an atypical presentation, include apnea, coughing, stridor, pharyngitis, dysphonia, otitis, sinusitis, laryngitis, subglottic stenosis, vocal granulomas, bronchiolitis, bronchoaspiration and difficult-to-treat asthma (11, 12, 13, 14).
When searching for an association between digestive symptoms and/or respiratory symptoms in children with GERD and between nutritional status and 24-hour ambulatory esophageal pH monitoring, we found no association between symptoms and pH test parameters in children with GERD. Nevertheless, the nutritional status and pH test parameters of the group of children with respiratory symptoms showed the greatest compromises (15). We also wanted to find any associations among atypical GERD otolaryngology symptoms such as otitis, sinusitis, croup, dysphonia and vocal cord nodules in a group of 18 children under 14 years old (median age 3 years and 10 months), and then to compare these findings with those from a group of 19 children with GERD digestive symptoms. Although the greatest compromise was found in the number of acid episodes found in the 24-hour ambulatory esophageal pH test, none of the pH test parameters were statistically significant (16).
Differential diagnosis
In pulmonology, not every child who wheezes is an asthmatic, and similarly, in gastroenterology, not every child who vomits or regurgitates has GER. The first possibility to be considered is non-pathological physiological GER present in infants called happy vomiters. They have good growth in terms of weight and height, but have a tendency to regurgitate. These patients will improve promptly with instruction and guidance about anti-reflux measures. An entity that has become increasingly more common is food allergy. Perhaps this is related to abandonment of healthy eating habits and increasing presence of environmental hazards although the genetic predisposition of each individual cannot be forgotten. Although each patient must be identified, and her or his clinical history remains essential for complete an accurate diagnosis, there are certain conditions that can occur with vomiting which require more attention. These include increased intracranial pressure from mass effects, certain metabolic diseases which present persistent vomiting and acid-base disorders such as galactosemia and glycogen storage disease, kidney diseases, urinary tract infections, and vesicoureteral reflux. Finally, there are congenital anomalies which requite prompt and early surgical correction. These include esophageal atresia, achalasia, hiatal hernia, vascular rings, pylorus hypertrophy, duodenal or gastric membranes, intestinal malrotation, intestinal duplication, choledochal cyst, the annular pancreas and congenital adhesions (17).
Refractory GERD
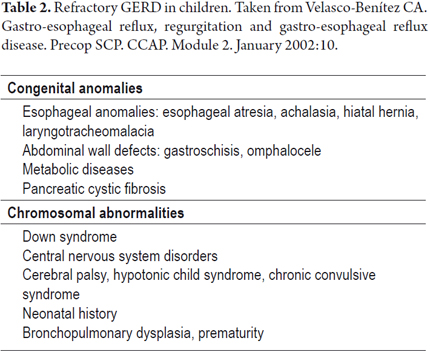
Today, certain diseases and conditions which are known to evolve slowly are classified as refractory GERD because of the natural history of these diseases and their association with increased morbidity and mortality. Their prognoses imply different therapeutic approaches (Table 2). Some congenital anomalies, metabolic diseases, chromosomal abnormalities, central nervous system alterations with certain preponderant neonatal historyies are considered to be refractory GERD (18).
Diagnosis
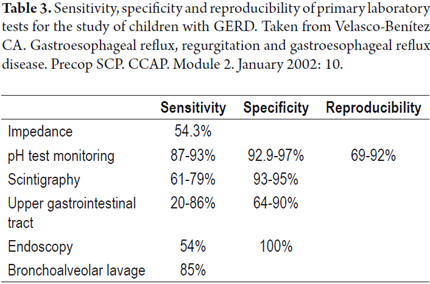
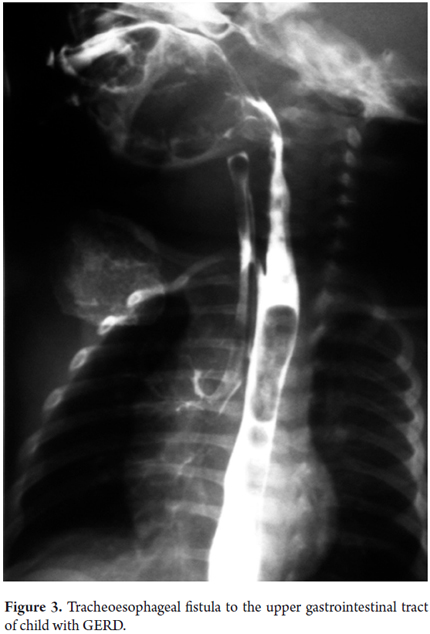
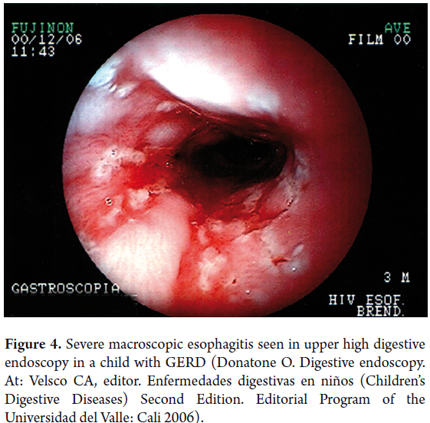
Sensitivity, specificity and reproducibility of some of the lab tests used to study children with GERD are displayed in Table 3 (19). To date, the gold standard for GERD diagnosis is the 24-hour ambulatory esophageal pH monitoring (21). This has now been complemented with impedance monitoring which allows more accurate diagnosis. It shows not only whether the reflux is acid or alkaline, but also shows the progression of reflux through the esophagus and whether there is any association between digestive and/or respiratory/otolaryngologic symptoms and esophageal pH changes. It reports four parameters including reflux rate (number of acid episodes/duration of procedure), number of acid episodes, number of acid episodes that last longer than five minutes, and duration of the longest episode. Results are considered abnormal if they are over percentile 50% of the reference tables for the patient's age (Figure 2). Today, placement of the Bravo endoscopic trans-catheter allows the study of children with a wireless 24-hour ambulatory esophageal pH monitor. Upper gastrointestinal tract scintigraphy can determine the reflux index (Normal is less than 4%), liquid gastric emptying (Normal is greater than 50%) and the presence of pulmonary microaspiration. Using a suction/swallowing mechanism when taking upper gastrointestinal images is an excellent method for detection of anatomical abnormalities such as strictures, fistulas, and hiatal hernias, but does not provide radiological diagnosis of GERD (Figure 3). Upper gastrointestinal endoscopy, besides visualizing esophageal motility and distension, can show possible anatomic abnormalities and the presence of macroscopic esophagitis. It also allows biopsies for determination of whether esophagitis caused by GERD or is eosinophilic or allergic (Figure 4). In search of improvements in the sensitivity of tests such as 24-hour esophageal pH tests and endoscopic diagnosis of esophagitis, we performed a study of 49 children aged 4 months to 13 years (mean age 5 years 4 months' ± 3 years 8 months) who had been diagnosed with GERD. Of these patients 26.5% had respiratory symptoms and 8.2% had digestive symptoms. We found no association between histopathological findings and results from the pH monitoring. Using bronchoalveolar lavage, siderophages carrying refluxed material towards the lungs have been identified. In more recent studies they have been shown to carry dipalmitoylphosphatidylcholine which is a major component of the surfactant (27). Even more recent studies have shown that the presence of bile acids and bronchoalveolar pepsin are associated with aspiration caused by GER in children with chronic lung disease (28). As described by Salazar et al., cineradiography and videofluoroscopy are dynamic examinations which make it possible to analyze the different phases of the sucking/swallowing mechanism in relation to different consistencies. In their study of 68 children between 1 month and 5 years old who had been diagnosed with swallowing disorders at the Hospital Universitario San Vicente de Paul in Medellin, Colombia showed that 84% presented gastrointestinal disturbances (mainly GER). Differential diagnoses of esophageal motility abnormalities were performed using manometry, and direct laryngoscopy identified otolaryngologic problems associated with GERD. Ultrasound is a noninvasive method which has increasingly become more important for diagnosis of GERD. Nevertheless, it requires an expert for its use and interpretation.
MANAGEMENT
Treatment of children with GERD includes measures against reflux, medication, and surgery. This is true even for children with GERD associated with asthma. Treatment must include support from health care professionals for children with GERD and for their parents or guardians as we reported following our descriptive study of families of 11 infants under 24 months of age (mean age: 9 months ± 6 months) with GERD who had been diagnosed by clinical tests, endoscopy and 24-hour ambulatory esophageal pH monitoring. Psychological characteristics inherent to the disease process are described in that study whose main conclusion was that the pediatrician's knowledge of the psychological capacities of the child and her/his family (ego defenses against disease) and recognition of the emotional threat GERD implies for the parents results in extra support in safeguarding the child's health and for making the process more tolerable.
Anti-reflux measures
Anti-reflux measures include nutritional management which involves increasing caloric density by thickening; food fractionation, monitoring risks of becoming overweight risk and avoiding certain foods such as fats, citruses, tomatoes, carbonated beverages, acidic beverages, coffee and chocolate. Another measure is teaching the decubitus supine position at 30° to avoid risk of sudden death. This should preferably be on the left side to improve gastric emptying. Other measures include avoiding certain medications such as anticholinergics, adrenergics, xanthines, calcium channel blockers, prostaglandins and nicotine; and avoiding tight clothing and heavy meals before bedtime. In a crossover controlled clinical trial of anti-regurgitation formulas (which have been misnamed anti-reflux in Colombia), we studied 21 infants with a mean age of 3.5 ± 2.4 months who had GERD which had been diagnosed clinically and with scintigraphy. Thickened anti-regurgitation prescription with amylopectin was administered to these infants who were then monitored with 24-hours ambulatory esophageal pH tests. We found that three was no statistically significant improvement in all four parameters measured in the 24-hour ambulatory esophageal pH test.
Medication
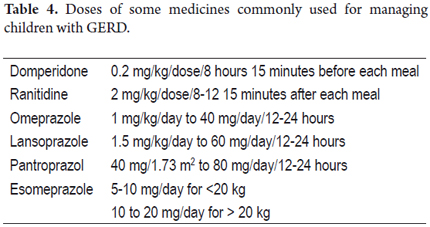
It is worth remembering that antacids were a good pharmacological alternative, as we demonstrated in a comparative study of 60 infants with a mean age of 5.4 ± 3.5 months whose clinical diagnoses of GERD had been confirmed by 24-hours ambulatory esophageal pH testing. Group 1 was treated with an orally administered H2 blocker such as ranitidine plus a prokinetic such as cisapride. Group 2 was treated only with hydrotalcite, an antacid. Group 3 was treated with cisapride plus oral hydrotalcite with a dosage determined by 1 gr/1.73m2 of body surface which was administered in 4 doses over 8 weeks. Clinical symptoms of 94% of the children who received hydrotalcite with or without prokinetic improved. Infants treated in this manner probably require more than 8 weeks of treatment for histological improvement (39). To date, H2 blockers and proton pump inhibitors are preferred over antacids because therapeutic doses of antacids are very close to the toxic dose, and because they must be administered every 4 to 6 hours daily to be effective (This task difficult most of the time). Other medicines used for managing children with GERD are prokinetics such as domperidone (40), although metoclopramide should not be used because its mechanism of action crosses the blood-brain barrier and often causes extrapyramidal effects. In addition, its commercial presentation in drops facilitates mistakes that can lead to poisoning. To avoid esophagitis caused by the return of acid or alkaline contents to the esophagus, H2 blockers such as ranitidine and famotidine, and proton pump inhibitors such as omeprazole, lansoprazole and pantoprazole are advised (41-45). These medicines have been approved for children by the FDA in the United States. Recently, the FDA also approved oral doses of esomeprazole, the S-isomer of omeprazole, for children aged 1 to 17 years old. Dosages are between 5 and 10 mg/day for children weighing less than 20 kilograms, and oral doses are between 10 and 20 mg/day for children weighing more than 20 kilograms. (46)
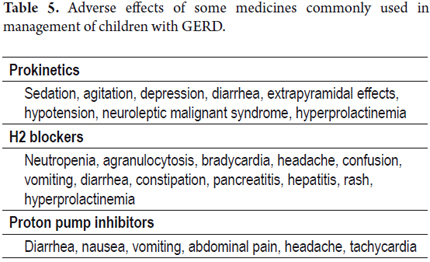
Dosage and adverse effects of the medicines most frequently used in children with GERD can be found in the Tables 4 and 5 (47). It should not be forgotten that domperidone must be administered between 15 and 20 minutes before meals to promote absorption and to engage its action mechanism. Proton pump inhibitors in micropellets makes dilution in acid solutions and domperidone suspension possible. They can be administered through a feeding tube. It should not be forgotten that when administered in a single dose, it must be administered on an empty stomach following fasting and that they must be changed every 8 to 12 weeks during use.
Surgery
About twenty years ago, surgical management was the most commonly used therapeutic approach, but it has fallen into disuse due to increasing understanding of the pathophysiology of the entity due to better diagnostic methods, the emergence of better medicines, and persistently high levels of postoperative morbidity and mortality. The primary indication for surgery on a child with GERD is fundoplication (48, 49). Surgery can be performed laparoscopically or through gastrostomy or pyloroplasty. Prior to surgery pH/impedance monitoring, manometry, and digestive tract scintigraphy are required.
REFERENCES
1. Velasco CA, Acevedo CP, Cortés EP, Castiblanco LA. Enfermedad por reflujo gastro-esofágico en niños. Rev IATREIA 1997;10:120-126. [ Links ]
2. Velasco CA, Acevedo CP, Cortés EP, Contreras C, Gutiérrez LA. Enfermedad por reflujo gastro-esofágico en lactantes del Hospital Universitario Ramón González Valencia de Bucaramanga 1995-1996. Rev Gastrohnup 2001;3:12-17. [ Links ]
3. Osatakul S, Sriplung H, Puetpaiboon A, Junjana C, Chamnongpakdi S. Prevalence and natural course of gastroesophageal reflux symptoms: a 1-year cohort study in Thai infants. J Pediatr Gastroenterol Nutr. 2002;34(1):63-7. [ Links ]
4. Argon M, Duygun U, Daglioz G, Omür O, Demir E, Aydogdu S. Relationship between gastric emptying and gastroesophageal reflux in infants and children. Clin Nucl Med. 2006;31(5):262-5. [ Links ]
5. Gold BD. Epidemiology and management of gastro-oesophageal reflux in children. Aliment Pharmacol Ther 2004; 19 (Suppl. 1): 22–27. [ Links ]
6. Størdal K, Johannesdottir GB, Bentsen BS, Sandvik L. Gastroesophageal reflux disease in children: association between symptoms and pH monitoring. Scand J Gastroenterol. 2005;40(6):636-40. [ Links ]
7. Pashankar DS, Corbin Z, Shah SK, Caprio S. Increased prevalence of gastroesophageal reflux symptoms in obese children evaluated in an academic medical center. J Clin Gastroenterol. 2009;43(5):410-3. [ Links ]
8. Alfaro EV, Aps JKM, Martens LC. Oral implications in children with gastroesophageal reflux disease. Curr Opin Pediatr. 2008;20(5):576-83. [ Links ]
9. Nielsen RG, Bindslev-Jensen C, Kruse-Andersen S, Husby S. Severe gastroesophageal reflux disease and cow milk hypersensitivity in infants and children: disease association and evaluation of a new challenge procedure. J Pediatr Gastroenterol Nutr. 2004;39(4):383-91. [ Links ]
10. Pollet S, Gottrand F, Vincent P, Kalach N, Michaud L, Guimber D, et al. Gastroesophageal reflux disease and Helicobacter pylori infection in neurologically impaired children: inter-relations and therapeutic implications. J Pediatr Gastroenterol Nutr. 2004;38(1):70-4. [ Links ]
11. Velepic MM, Velepic MS, Starcevic R, Manestar D, Rozmanic V. Gastroesophageal reflux and sequelae of chronic tubotympanal disorders in children. Acta Otolaryngol. 2004;124(8):914-7. [ Links ]
12. Misra S, Macwan K, Albert V. Transpyloric feeding in gastroesophageal-reflux-associated apnea in premature infants. Acta Paediatr. 2007;96(10):1426-9. [ Links ]
13. Mousa H, Woodley FW, Metheney M, Hayes J. Testing the association between gastroesophageal reflux and apnea in infants. J Pediatr Gastroenterol Nutr. 2005;41(2):169-77. [ Links ]
14. Gold BD. Asthma and gastroesophageal reflux disease in children: exploring the relationship. J Pediatr. 2005;146(3 Suppl):S13-20. [ Links ]
15. Velasco CA, Valencia AG, Sánchez MP. Asociación entre síntomas digestivos y/o respiratorios y parámetros de la pHmetría intraesofágica ambulatoria de 24 horas en niños. Rev Colomb Med 2007;38(Supl 1):14-18. [ Links ]
16. Velasco CA, Pérez ML, Sánchez MP. pHmeter in children with gastroesophageal relfux and otolaringology symptoms. J Pediatr Gastroenterol Nutr 2006;43:E17. [ Links ]
17. Hassall E. Decisions in diagnosing and managing chronic gastroesophageal reflux disease in children. J Pediatr. 2005;146(3 Suppl):S3-12. [ Links ]
18. Dhillon AS, Ewer AK. Diagnosis and management of gastro-oesophageal reflux in preterm infants in neonatal intensive care units. Acta Paediatr. 2004;93(1):88-93. [ Links ]
19. Salvatore S, Hauser B, Vandemaele K, Novario R, Vandenplas Y. Gastroesophageal reflux disease in infants: how much is predictable with questionnaires, pH-metry, endoscopy and histology? J Pediatr Gastroenterol Nutr. 2005;40(2):210-5. [ Links ]
20. Velasco CA. Acerca de la pHmetría intraesofágica ambulatoria de 24 horas. Actualizaciones Pediátricas FSFB 2000;10:2-5. [ Links ]
21. Velasco CA. pH-metría en el diagnóstico de enfermedad por reflujo gastroesofágico pediátrico. Rev Col Gastroenterol 1996;11:59-62. [ Links ]
22. Wenzl TG, Moroder C, Trachterna M, Thomson M, Silny J, Heimann G, et al. Esophageal pH monitoring and impedance measurement: a comparison of two diagnostic tests for gastroesophageal reflux. J Pediatr Gastroenterol Nutr. 2002;34(5):519-23. [ Links ]
23. Harris P, Muñoz C, Mobarec S, Brockmann P, Mesa T, Sánchez I. Relevance of the pH probe in sleep study analysis in infants. Child Care Health Dev. 2004;30(4):337-44. [ Links ]
24. Gunnarsdóttir A, Stenström P, Arnbjörnsson E. Wireless esophageal pH monitoring in children. J Laparoendosc Adv Surg Tech A. 2008;18(3):443-7. [ Links ]
25. Cleghorn G. Eosinophilic esophagitis: an emerging condition. In: Velasco CA, editor. Topics in Pediatric Gastroenterology, Hepatology and Nutrition. Distribuna Editorial: Bogotá 2007:33-42. [ Links ]
26. Velasco CA, Sánchez MP. Esophagitis and 24-hour pHmeter on children with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr 2005;41:501. [ Links ]
27. Chang AB, Hills YC, Cox NC, Cleghorn GJ, Valery PC, Lewindon PJ, et al. «Free» surfactant in gastric aspirates and bronchoalveolar lavage in children with and without reflux oesophagitis. Intern Med J. 2006;36(4):226-30. [ Links ]
28. Starosta V, Kitz R, Hartl D, Marcos V, Reinhardt D, Griese M. Bronchoalveolar pepsin, bile acids, oxidation, and inflammation in children with gastroesophageal reflux disease. Chest. 2007;132(5):1557-64. [ Links ]
29. Salazar OF, Serna D, Múnera A, Mejía MM, Álvarez P, Cornejo W, et al. Características clínicas y videofluoroscópicas de la disfagia orofaríngea en niños entre un mes y cinco años de vida. Hospital Universitario San Vicente de Paúl, Medellín, Colombia, 2004. Rev IATREIA 2008;21:13-20. [ Links ]
30. Carr MM, Nagy ML, Pizzuto MP, Poje CP, Brodsky LS. Correlation of findings at direct laryngoscopy and bronchoscopy with gastroesophageal reflux disease in children: a prospective study. Arch Otolaryngol Head Neck Surg. 2001;127(4):369-74. [ Links ]
31. Carroll AE, Garrison MM, Christakis DA. A systematic review of nonpharmacological and nonsurgical therapies for gastroesophageal reflux in infants. Arch Pediatr Adolesc Med. 2002;156(2):109-13. [ Links ]
32. McPherson V, Wright ST, Bell AD. Clinical inquiries. What is the best treatment for gastroesophageal reflux and vomiting in infants? J Fam Pract. 2005;54(4):372-5. [ Links ]
33. Scarupa MD, Mori N, Canning BJ. Gastroesophageal reflux disease in children with asthma: treatment implications. Paediatr Drugs. 2005;7(3):177-86. [ Links ]
34. Velasco CA, Jiménez AM. Psychological characteristics in families with gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2005;41:501-2. [ Links ]
35. Aggett PJ, Agostoni C, Goulet O, Hernell O, Koletzko B, Lafeber HL, et al. Antireflux or antiregurgitation milk products for infants and young children: a commentary by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2002;34(5):496-8. [ Links ]
36. Khoshoo V, Ross G, Brown S, Edell D. Smaller volume, thickened formulas in the management of gastroesophageal reflux in thriving infants. J Pediatr Gastroenterol Nutr. 2000;31(5):554-6. [ Links ]
37. Velasco CA. Manejo nutricional de niños con enfermedad por reflujo gastroesofágico. Rev Salud UIS. 1997;25:122-3. [ Links ]
38. Velasco CA, Cortés EP, Cortés JG. Parámetros de la pHmetría intraesofágica ambulatoria de 24 horas ante una fórmula antiregurgitación en lactantes con enfermedad por reflujo gastroesofágico. J Pediatr Gastroenterol Nutr. 2001;33:420. [ Links ]
39. Velasco CA, Herrán OF. Enfermedad por reflujo gastro-esofágico en niños tratados con hidrotalcita y cisaprida. Hosp Pract. 1998;2:60-3. [ Links ]
40. Pritchard DS, Baber N, Stephenson T. Should domperidone be used for the treatment of gastro-oesophageal reflux in children? Systematic review of randomized controlled trials in children aged 1 month to 11 years old. Br J Clin Pharmacol. 2005;59(6):725-9. [ Links ]
41. Salvatore S, Hauser B, Salvatoni A, Vandenplas Y. Oral ranitidine and duration of gastric pH >4.0 in infants with persisting reflux symptoms. Acta Paediatr. 2006;95(2):176-81. [ Links ]
42. Marier J-F, Dubuc M-C, Drouin E, Alvarez F, Ducharme MP, Brazier J-L. Pharmacokinetics of omeprazole in healthy adults and in children with gastroesophageal reflux disease. Ther Drug Monit. 2004;26(1):3-8. [ Links ]
43. Springer M, Atkinson S, North J, Raanan M. Safety and pharmacodynamics of lansoprazole in patients with gastroesophageal reflux disease aged <1 year. Paediatr Drugs. 2008;10(4):255-63. [ Links ]
44. Croom KF, Scott LJ. Lansoprazole: in the treatment of gastro-oesophageal reflux disease in children and adolescents. Drugs. 2005;65(15):2129-37. [ Links ]
45. Fiedorek S, Tolia V, Gold BD, Huang B, Stolle J, Lee C, et al. Efficacy and safety of lansoprazole in adolescents with symptomatic erosive and non-erosive gastroesophageal reflux disease. J Pediatr Gastroenterol Nutr. 2005;40(3):319-27. [ Links ]
46. Croxtall JD, Perry CM, Keating GM. Esomeprazole: in gastroesophageal reflux disease in children and adolescents. Paediatr Drugs. 2008;10(3):199-205. [ Links ]
47. Vandenplas Y. Gastroesophageal reflux: medical treatment. J Pediatr Gastroenterol Nutr. 2005;41 Suppl 1:S41-42. [ Links ]
48. Allal H, Captier G, Lopez M, Forgues D, Galifer RB. Evaluation of 142 consecutive laparoscopic fundoplications in children: effects of the learning curve and technical choice. J Pediatr Surg. 2001;36(6):921-6. [ Links ]
49. Esposito C, Langer JC, Schaarschmidt K, Mattioli G, Sauer C, Centonze A, et al. Laparoscopic antireflux procedures in the management of gastroesophageal reflux following esophageal atresia repair. J Pediatr Gastroenterol Nutr. 2005;40(3):349-51. [ Links ]
50. Gatti C, di Abriola GF, Villa M, De Angelis P, Laviani R, La Sala E, et al. Esophagogastric dissociation versus fundoplication: Which is best for severely neurologically impaired children? J Pediatr Surg. 2001;36(5):677-80. [ Links ]
51. Di Lorenzo C, Benninga MA, Forbes D, Morais MB, Morera C, Rudolph C, et al. Functional gastrointestinal disorders, gastroesophageal reflux and neurogastroenterology: Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2004;39 (Suppl 2):S616-625. [ Links ]











 texto en
texto en