Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.1 Bogotá Jan./Mar. 2014
An incidental finding of a gastrointestinal stromal tumor (GIST) in the stomach following laparotomy: case report and literature review
Mauricio Melo Peñaloza MD. (1), Dolly Williamson (2), Lady Vargas (2)
1. Internist-Gastroenterologist of the Universidad Nacional, Adult Medicine Coordinator of the School of Medicine of the Universidad Cooperativa de Villavicencio in Villavicencio, Colombia.
2. Student of Adult Medicine in the School of Medicine of the Universidad Cooperativa de Villavicencio in Villavicencio, Colombia.
Received: 30-04-13 Accepted: 19-12-13
Abstract
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal neoplasms of the gastrointestinal tract. The annual frequency of this pathology in developed countries is estimated to be between 6.5 and 14.5 cases per million people. Most GISTs (50-70%) originate in the stomach. Symptoms vary from nonspecific abdominal complaints to abdominal pain with or without a palpable abdominal mass. Some of these patients are diagnosed incidentally when they undergo screening or upper endoscopy to diagnose bleeding. We present a case of GIST which was initially discovered because of upper gastrointestinal bleeding during a laparoscopic cholecystectomy.
Key words
GIST, Gastrointestinal stromal tumor, gastrointestinal bleeding, incidental finding.
INTRODUCTION
GISTs are neoplasms that arise from the gastrointestinal mesenchymal stroma. GISTs probably originate in totipotent stem cells which also form interstitial cells of Cajal (ICC) which are the gastrointestinal pacemakers of Auerbach's plexus that activate smooth muscle function through autonomic neural modulation. These stem cells differentiate into cell groups that do not express smooth muscle markers like desmin and actin. GISTs represent 1% to 2% of all gastric neoplasms and nearly 30% of them are potentially malignant (2, 6, 33).
The first recorded use of the term GIST was by Mazur and Clark in 1983. They used it to designate non-epithelial tumors of the gastrointestinal tract which lacked ultrastructural patterns of smooth muscle but which also had unexpected neural markers closely related to ICCs which were detected by immunohistochemistry techniques. Mazur and Clark initially proposed the term stromal tumors, but the term was later modified to gastrointestinal stromal tumors (3, 4, 5, 18, 19, 20).
In 1998, Hirota et al. showed that the presence of the c-KIT proto-oncogene, a tyrosine kinase III receptor, was required for the normal development of ICCs. The human genome has about 90 tyrosine kinase (TK) receptors and 43 genes associated with them. Their products regulate cell proliferation, survival, differentiation, function and motility. The C-Kit gene is localized in chromosome 4 and codes for a transmembrane TK type III receptor which is also called KIT (CD 117 antigen). They are expressed by ICCs. More than 90% of GISTs and ICCs express the KIT receptor (c-KIT).
Morphological studies and immunohistochemistry staining are used for histological identification of these tumors. Macroscopically, GISTs are structures that range from few millimeters across to many centimeters in diameter. Some of these tumors are more than 30 cm wide. They can be diffuse, encapsulated or multinodular. When they are cut, they seem fibrous with areas of hemorrhaging, cystic degeneration and central necrosis. GISTs are often intramural tumors and usually compromise the submucosal and muscularis propria layers. Microscopically seventy percent of cases are fusiform, 20% are epithelioid cells, and the rest are mixed cell types. Less than 5% of cases have marked cellular polymorphisms (12).
Immunochemistry determination uses many antibodies including monoclonal and polyclonal antibodies able to detect CD117 epitope localized in the external domain of the oncoprotein. The most frequently used is the pAbA4502 (DakoCytomation) (11, 12).
The most common localizations of GIST are in the stomach (60% to 70%), small intestine (20% to 25%) and colon (5%). Approximately 20% of GISTs are small and asymptomatic and are discovered as incidental findings. Their incidence peaks between the ages of 50 and 80 years, with no differences between men and women (1).
CLINICAL CASE
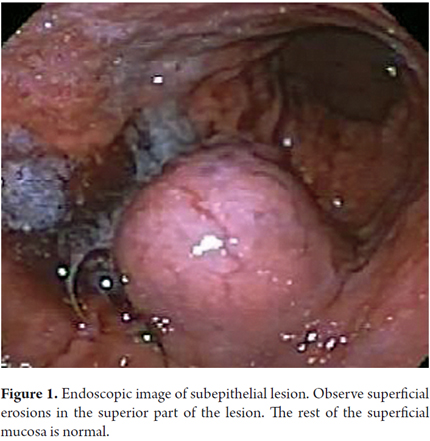
On October 16, 2012 a 57 year-old woman who had had a cholecystectomy performed on October 6, 2012 came to the emergency room of the departmental hospital of Villavicencio, Meta. She had nausea, abdominal distention and fever. Her surgical wound was hot, swollen and red and was oozing a purulent discharge. She was examined by a general surgeon who initially started antibiotics. On the second day of treatment he decided to perform a laparotomy to drain the abscessed hemoperitoneum. The day after surgery the patient presented coffee grounds emesis, diarrhea, melena and pain in the upper abdominal hemisphere. An endoscopy showed an epithelial mass of 6 cm diameter which was compatible with GIST. It had eroded areas and stigmas of recent bleeding located in the posterior proximal third of the mucosa of the fundus 6 cm distal from esophagogastric junction (Figure 1).
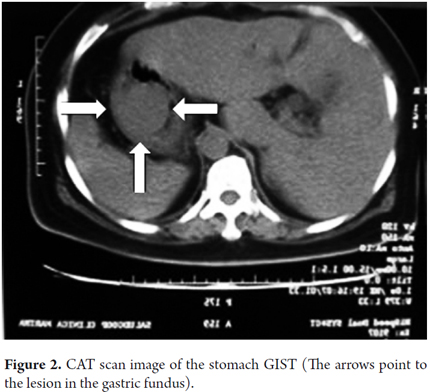
An abdominal CAT scan showed the lesion in the location described above (Figure 2). During surgery a tumor mass was found in the posterior fundus of the stomach. The lesion was resected with wide margins and the material was sent to pathology.
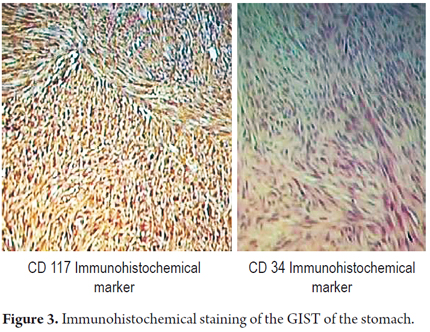
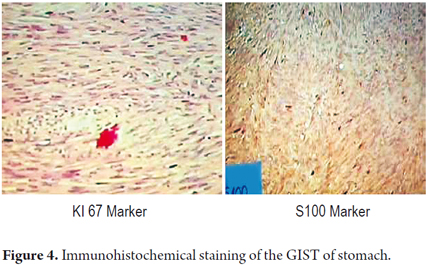
Macroscopic pathology findings showed a mass with a smooth brilliant brownish-grey surface that weighed 42 grams and measured 8cm x 4cm x 2cm.Vascular markings could be seen on its exterior surface. When cut its interior was whitish with areas of hemorrhaging. One border had brownish-grey friable content. Cuts 1 to 3 produced microscopic findings with four areas of friable content, a mitotic count of 1 per 50 high power fields (HPF). A fragment of the greater omentum that measured 12cm x 5cm x 1cm had areas free of congestive tumor. Immunohistochemistry studies reported positively for CD117, S100, and focal CD34 with smooth muscle actin. It also showed a high rate of cell proliferation with Ki167 at 1% (Figure 3 and 4). There was no clear description of the lesion's edges.
The patient was discharged and treatment with 400mg of orally administered Imatinib once in day was initiated. Three months later a follow up examination using endoscopic ultrasound showed a hypoechoic homogeneous lesion in the cardia. It measured 12.6mm x 6.9 mm and was in the 4th hypoechoic layer which is the muscularis propria. This is compatible with GIST. The patient is currently being monitored and treated with 400mg Imatinib once a day. She has responded favorably to treatment and was examined with endoscopic ultrasound again on April 5, 2013 at which time a biopsy of the lesion in the fundus was performed.
DISCUSSION
Since the biological behavior of GISTs cannot usually be predicted easily, it is difficult to classify them. The most important prognostic morphological factors are the mitotic count and the size of the tumor which are related to progression of the disease. Hence, use of the terms prognostic and risk group is better than benign and malignant lesion.
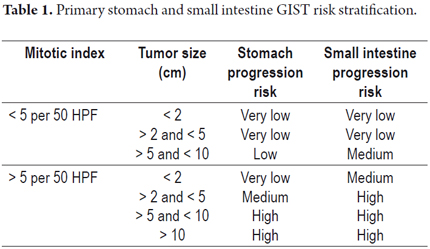
In our case, we employed the Fletcher risk table (2002) as modified in 2006 on the basis of the Miettienen and Lasota surveys (16, 23, 29, 36, 40). The classification has two categories according to the mitotic rate and seven categories according to tumor size: the greater the mitotic rate and tumor size, the greater the risk of tumor progression (Table 1). The tumor described in this article had a low risk of progression because its mitotic count was 1 in 50 high power fields and its dimensions were 8cm x 4cm x 2 cm.
It has also been proposed that a positive result for Ki67 is an indicator of GIST and that when more than 10% of nuclei tint positively for Ki167 it is an indicator of metastasis. In this instance, the tumor had a Ki67 index of 1% (23, 24, 29).
Subepithelial lesions, often seen in upper endoscopies, have an estimated incidence for 0. 3%. Lipomas, vascular structures, cysts, pancreatic remains and extramural structures are included in this category. One of every five subepithelial lesions is a neoplasm such as a GIST. Diagnosis is difficult (17%), even when jumbo forceps are used for biopsies. The diagnostic procedure of choice for submucosal tumors is endoscopic ultrasound because it differentiates between lesions in the gastrointestinal wall lesions and extrinsic compressions and because it also determines the layer of origin and the size and presence of adenopathies with a sensitivity of 64% to 80% (38).
Ulceration of the mucosa occurs frequently in both benign and malignant GISTs. Ulceration has no predictive value but may be related to tumor size. In our case, the symptom that suggested an endoscopic evaluation was gastrointestinal bleeding. We found an 8 cm diameter tumor with multiple superficial erosions indicating a relationship between size and bleeding.
Surgery is still the preferred method of managing this neoplasm. Ideally, the entire tumor can be resected without rupturing it or its pseudocapsule with microscopic tumor free margins. The importance of establishing whether or not the surgical margin contains any tumor lies in the fact that taken together with tumor size and the presence or absence of bleeding it can help determine the prognosis of the GIST and its response to Imatinib (22, 39). In this case, the tumor was 8 cm and there were signs of bleeding, but the pathology of the resected borders was not clear.
In general, the 5-year survival rate for patients with partially resected tumors is between 28% and 35%.
Given the risk of invasion to nearby structures with locally advanced GISTs, neoadjuvant therapy with tyrosine kinase inhibitors is indicated. Once the maximum response from the tumor has been obtained, it is necessary to determine the possibility of surgical resection (22, 41).
Imatinib is a competitive inhibitor of ATP which binds to the tyrosine kinase domain. It inhibits activation and phosphorylation of this domain thereby delaying tumor growth.
The first description of the use of Imatinib (2001) was in the treatment of a woman with a progressively metastasizing tumor that recurred after she had undergone surgery and chemotherapy. The size of the tumor was reduced by 50% in the first two months of treatment, and by 78% after eighteen months. A CAT scan taken four weeks after treatment began showed a significant decrease in the tumor's glucose uptake. The spread of the use of Imatinib for GISTs after this first description has been one of the most rapid in the history of oncological treatments.
The ideal duration of treatment is still unknown. Some authors recommend lifelong treatment (17, 21). The main adverse effects of Imatinib are periorbital swelling (74%), nausea (52%), diarrhea (45%), myalgia and rheumatic pain (40%) and headaches (26%). Nevertheless, treatment is discontinued in only 7% of all cases because of adverse effect (31).
One study of 106 cases established that Imatinib use is successful when GIST is totally eradicated, but its utility is uncertain for high risk GISTs (26, 32).
The clinical practice guidelines of the National Comprehensive Cancer Network (NCCN) suggest that Imatinib be used after surgery for at least 36 months in patients with high risk GISTs (tumor size > 5 cm and high mitotic index >5 mitosis/50 HPF) (41).
CONCLUSIONS
1. The case reviewed was a GIST of the stomach which is its most frequent location.
2. The patient became symptomatic after a complicated cholecystectomy.
3. The main symptom of this GIST was upper gastrointestinal bleeding which has been associated with greater tumor size and poorer therapeutic response.
4. Non-radical surgery was performed but the margins were not reported in the pathology report. Consequently, the patient will need close follow up to prevent dissemination of the neoplasm which has been described in the literature for partial tumor resections with positive margins.
5. The patient received Imatinib mesylate, a drug that has demonstrated its utility for metastasizing lesions. This could eventually improve the long term prognosis for this case because this tumor presented a medium risk of progression according to pathology, Fletcher classification and presence of bleeding.
6. A follow-up endoscopic ultrasound examination found a second lesion of smaller size which could represent short term metastasis.
REFERENCES
1. Eisenberg BL, Pipas JM. Gastrointestinal stromal tumor--background, pathology, treatment. Hematol Oncol Clin North Am. 2012;26(6):1239-59. [ Links ]
2. Gómez M. Paciente con lesión gástrica subepitelial. Rev Col Gastroenterol. 2010;25 (4)371-378. [ Links ]
3. Mazur MT, Clark HB. Gastric stromal tumors. Reappraisal of histogenesis. Am J Surg Pathol. 1983;7(6):507-19. [ Links ]
4. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279(5350):577-80. [ Links ]
5. Romero A, Mesa O, Melo M, Chinchilla S. Tumor del estroma gastrointestinal gástrico con metástasis inusual al cráneo. Rev Col Gastroenterol. 2011; 26: 311-315. [ Links ]
6. Barreda BF, Liu BH, Sánchez L, Landeo A, Sánchez Rodríguez Z. Survival factors in 152 patients with gastrointestinal stromal tumors. Rev Gastroenterol Peru. 2010;30(4):305-23. [ Links ]
7. Toro J, Vasquez l, Madrid J. Tumores del estroma gastrointestinal (GIST): papel del cirujano en la era de la medicina molecular.Abr,26-2010,Facultad de Medicina Universidad de Antioquia iatreia@medicina.udea.edu.co [ Links ]
8. Krause DS, Van Etten RA. Tyrosine kinases as targets for cancer therapy. N Engl J Med. 2005;353(2):172-87. [ Links ]
9. Hewavisenthi SDS, De Silva M. Gastrointestinal stromal tumours (GIST) revisited. Journal of Diagnostic Pathology [Internet]. 21 de abril de 2011 [citado 26 de febrero de 2014];3(1). Recuperado a partir de: http://www.sljol.info/index.php/JDP/article/view/2988. [ Links ]
10. Fresno M, Determinación inmunohistoquímica del CD 117 /c-kit en el GIST, Oncología, 2004; 27 (4):242-245. [ Links ]
11. Bohórquez P, Neveu R. Tumores del estroma gastrointestinal (GIST), un particular tipo de neoplasia. Rev Méd Chile. 2008;136:921-929. [ Links ]
12. Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 15 de septiembre de 2004;22(18):3813-25. [ Links ]
13. Vargas C, Cardona A, Carranza H, Otero J, Reveiz L, Ospina E, et al. Tumores del estroma Gastrointestinal, experiencia en dos instituciones hospitalarias de Bogotá D.C. Colombia. Rev Col Gastroenterol. 2008;23(3)213-223. [ Links ]
14. Oliveros R, Quintero A, Sánchez R, Mesa J. Tumores estromales gastrointestinales (GIST) en el Instituto Nacional de Cancerología, Bogotá D.C., Colombia 2000-2008. Rev Col Cancerol 2011;15(4):202-211. [ Links ]
15. Gómez G, Lombo M, Peñaloza F. Tumores estromales gástricos (GIST) malignos. Presentación de dos casos. Rev Col Gastroenterol. 2004;19(2):137-142. [ Links ]
16. Fletcher CDM, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol. 2002;33(5):459-65. [ Links ]
17. Buchdunger E, O'Reilly T, Wood J. Pharmacology of imatinib (STI571). Eur J Cancer. 2002;38 Suppl 5:S28-36. [ Links ]
18. Saund MS, Demetri GD, Ashley SW. Gastrointestinal stromal tumors (GISTs). Curr Opin Gastroenterol. 2004;20(2):89-94. [ Links ]
19. Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol. 2003;54(1):3-24. [ Links ]
20. Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM. Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol. 1998;152(5):1259-69. [ Links ]
21. Joensuu H, Roberts PJ, Sarlomo-Rikala M, Andersson LC, Tervahartiala P, Tuveson D, et al. Effect of the tyrosine kinase inhibitor STI571 in a patient with a metastatic gastrointestinal stromal tumor. N Engl J Med. 2001;344(14):1052-6. [ Links ]
22. McCarter MD, Antonescu CR, Ballman KV, Maki RG, Pisters PWT, Demetri GD, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: analysis of risk factors and tumor recurrence. J Am Coll Surg. 2012;215(1):53-59. [ Links ]
23. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000;231(1):51-8. [ Links ]
24. Miettinen M, El-Rifai W, H L Sobin L, Lasota J. Evaluation of malignancy and prognosis of gastrointestinal stromal tumors: a review. Hum Pathol. 2002;33(5):478-83. [ Links ]
25. Blair SL, Al-Refaie WB, Wang-Rodriguez J, Behling C, Ali M-W, Moossa AR. Gastrointestinal stromal tumors express ras oncogene: a potential role for diagnosis and treatment. Arch Surg. 2005;140(6):543-548. [ Links ]
26. Gutierrez JC, De Oliveira LOP, Perez EA, Rocha-Lima C, Livingstone AS, Koniaris LG. Optimizing diagnosis, staging, and management of gastrointestinal stromal tumors. J Am Coll Surg. 2007;205(3):479-491. [ Links ]
27. Jeong IH, Kim JH, Lee SR, Kim JH, Hwang JC, Shin SJ, et al. Minimally invasive treatment of gastric gastrointestinal stromal tumors: laparoscopic and endoscopic approach. Surg Laparosc Endosc Percutan Tech. 2012;22(3):244-50. [ Links ]
28. Kim MN, Kang SJ, Kim SG, Im JP, Kim JS, Jung HC, et al. Prediction of risk of malignancy of gastrointestinal stromal tumors by endoscopic ultrasonography. Gut Liver. 2013;7(6):642-7. [ Links ]
29. Eizaguirre Zarza B, Burgos Bretones JJ. Tumores GIST. Revisión de la literatura. Revista Española de Patología. 2006;39(4):209-18. [ Links ]
30. Joensuu H, Fletcher C, Dimitrijevic S, Silberman S, Roberts P, Demetri G. Management of malignant gastrointestinal stromal tumours. Lancet Oncol. 2002;3(11):655-64. [ Links ]
31. Poveda A, Artigas V, Casado A, Cervera J, García Del Muro X, Antonio López-Guerrero J, et al. Clinical practice guidelines in gastrointestinal stromal tumours (GEIS): update 2008. Cir Esp. 2008;84 Suppl 1:1-21. [ Links ]
32. Pennacchioli E, Colombo C, Berselli M, Gronchi A. Update on management of GIST and postsurgical use of imatinib. Open Access Surgery. 2010;2010:63-71. [ Links ]
33. Moraila FA. Tumores del estroma gastrointestinal, definición, generalidades y epidemiología. Cirujano General. 2008;30(Suplemento 1):5-10. [ Links ]
34. Koh JS, Trent J, Chen L, El-Naggar A, Hunt K, Pollock R, et al. Gastrointestinal stromal tumors: overview of pathologic features, molecular biology, and therapy with imatinib mesylate. Histol Histopathol. 2004;19(2):565-74. [ Links ]
35. Muñoz C, Sabah S, Navarro A, Planzer M, Silva C, Santander R. Tumores del estroma gastrointestinal (GIST): Revisión de la literatura. Gastr Latinoam. 2006; 17(1): 43-51. [ Links ]
36. Soto G. S, del Pozo L. M, Kuschel H. C, Schultz H. E, Banse E. C. Tumores estromales del tubo digestivo (GIST): A propósito de un caso clínico. Cuadernos de Cirugía. 2003;17(1):37-42. [ Links ]
37. Acín-Gándara D, Pereira-Pérez F, Castaño-Pascual Á, Durán-Poveda M, Antequera-Pérez A, Miliani-Molina C. Tumores estromales gastrointestinales: diagnóstico y tratamiento. Cir Cir. 2012;80:44-51. [ Links ]
38. Cañadas R, Galiano M, Gil Enfoque epidemiológico, clínico y endoscópico. En Oliveros R, ed. Temas Escogidos en gastroenterología. GIST 2013. Asociación Colombiana de gastroenterología, 2013, cap 1 pág 19-34. [ Links ]
39. Andrade R, Panqueva R, Palau M. Diagnóstico patológico y metodologías accesorias. En Oliveros R, ed. Temas Escogidos en gastroenterología. GIST 2013. Asociación Colombiana de gastroenterología. 2013,cap 2, pg 37-60. [ Links ]
40. Miettinen M, Lasota J. Gastrointestinal stromal tumors: review on morphology, molecular pathology, prognosis, and differential diagnosis. Arch Pathol Lab Med. 2006;130(10):1466-78. [ Links ]
41. Von Mehren M, Benjamin RS, Bui MM, Casper ES, Conrad EU 3rd, DeLaney TF, et al. Soft tissue sarcoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012;10(8):951-60. [ Links ]











 text in
text in