Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.1 Bogotá ene./mar. 2014
Pathological aspects of fatty liver disease
Rocío del Pilar López Panqueva MD. (1)
(1) Medical Pathologist at the Hospital Universitario Fundación Santa Fe de Bogotá and the Universidad de Los Andes in Bogotá, Colombia.
Received: 11-02-14 Accepted: 21-02-14
Abstract
In a normal human liver 5% of its mass consists of lipids. When deposition of fat increases, the terms most often used are fatty liver or steatosis. This includes non-alcoholic fatty liver disease whose acronym is NAFLD and alcoholic liver disease (ALD). A liver biopsy is still considered to be the gold standard for determining the severity of liver damage in either of these entities.
Keywords
Steatosis, steatohepatitis, non-alcoholic steatosis, non-alcoholic steatohepatitis, NASH, alcoholic steatohepatitis, ALD, liver biopsy.
INTRODUCTION
Although numerous twentieth century studies showed a well-documented association between steatosis, cirrhosis, obesity, diabetes and alcohol, it was not until 1980 that Ludwig et al. at the Mayo Clinic published a study where the nature of nonalcoholic hepatic changes in steatosis and steatohepatitis were shown. In that study they coined the term NASH (Non-alcoholic steatohepatitis).
Since those first publications, fatty liver has become an increasingly common disease especially because of the increased prevalence of metabolic syndrome (obesity, hypertension, dyslipidemia, microalbuminuria and diabetes mellitus type II) in the general population. Currently, NAFLD is one of the most prevalent diseases worldwide.
These entities are accompanied by a spectrum of pathological changes ranging from simple steatosis to steatosis accompanied by inflammation with or without significant fibrosis to steatohepatitis. This can evolve into advanced chronic liver disease that progresses to cirrhosis, liver failure and increased risk of hepatocellular carcinoma (HCC). Approximately 30% of patients who have the sole initial finding of steatosis progress to cirrhosis. Of these 10% will develop HCC, and - over a period of 10 years - 30% to 40% will develop liver failure or die from their underlying disease (3).
Steatosis has been observed in 50% to70% of patients with risk factors for NAFLD, especially obesity and type 2 diabetes. Of these 20% to 30% progress to steatohepatitis, and 2% to 3% progress to cirrhosis. The occurrence of cirrhosis has also been reported in between 5% and 15% of (missing word in original), with an overall mortality rate that ranges from 12% to 36% and a rate of mortality related to liver disease of between 2% and 7% which is significantly higher than in the general population. Morphological changes can be identical in both alcoholic and non-alcoholic etiologies (2, 4, 5).
There are still no clinical, diagnostic, laboratory or imaging methods that can establish with certainty the difference between simple steatosis and steatohepatitis or which can determine the degree of inflammatory activity and fibrosis and evaluate the existence of other coexisting disease processes. This is where the role of liver biopsy becomes importance for this condition.
DEFINITION
Fatty liver disease has traditionally been divided into alcoholic and non-alcoholic. Although their macro and microscopic appearances are very similar, their clinical signs and pathophysiology are different.
NAFLD is defined as the accumulation of liver fat that exceeds 5% to 10% of the liver's weight. It is indispensable to discard substantial alcohol consumption (less than 20 g/day for women and less than 40 g/day for men). This directly correlates with the presence of fatty vacuoles in the cytoplasm of hepatocytes.
The condition known as nonalcoholic fatty liver disease (NAFL) represents a spectrum of changes starting from a simple finding of steatosis alone which may be very mild and even have no inflammation. NAFL's evolution is benign, and its risk of chronicity with progression to cirrhosis is less than 5%. Steatohepatitis (NASH) steatosis and inflammation of variable intensity is always accompanied by hepatocellular lesions (ballooning and/or pericellular fibrosis) with progression to chronic disease and cirrhosis in 21% to 28% of cases. Progression to liver failure and hepatocellular carcinoma with its associated mortality occurs in 12% of cases (6, 7).
More than half of patients with cryptogenic cirrhosis have previously had a diagnosis of NASH or histological changes that suggest NAFLD. Up to 13% of them are associated with hepatocellular carcinoma (3).
RISK FACTORS
Many conditions are associated with the histological phenotype of NAFLD, but the vast majority of patients seen in clinical practice have metabolic syndrome with insulin resistance being the most important characteristic. This is the most common link of all etiologies associated with NAFLD. Metabolic syndrome, present in 22% to 30% of the population, increases the risk of cardiovascular morbidity and mortality and the risk of progression to fibrosis in nonalcoholic steatohepatitis (NASH). 90% of patients with NASH have metabolic syndrome. Between 70% and 80% of people with BMI >30 kg/m2 have hepatic steatosis (8, 9, 10).
There are many other causes of fatty liver disease that are considered to be secondary. The size of the vacuole can help us in the search for an etiologic factor. For instance, a large vacuole is seen in rapid weight loss and malnutrition and is associated with Hepatitis C; genetic metabolic disorders such as Wilson's disease, tyrosinemia and abetalipoproteinemia; other conditions such as congenital lipodystrophy and lipodystrophy acquired through HIV infection; large intestinal resections; gastroplasties and jejunoileal bypasses. Small vacuole steatosis is found in Reye syndrome, acute fatty liver during pregnancy, Hellp syndrome, metabolic errors such as acyl transferase lecithin deficiency, Wolman's disease and deposition of cholesterol esters (10, 11).
Hepatic steatosis and steatohepatitis have also been linked with drugs. For example 1% to 3% of patients who use Amioradone for prolonged periods develop this condition. Perhexiline maleate (Pexid) and diethyl aminoethyl hexestrol hydrochloride (Coralgil) which are widely used in Europe and Japan can lead to the development of these conditions, as can calcium channel blockers such as nifedipine. Tamoxifen, isoniazid, estrogens, glucocorticoids, steroids and diethylstilbestrol have questionable etiologic associations, but their use has been proven to exacerbate NASH especially in patients with other risk factors. Methotrexate is also well known to cause steatosis, fibrosis and eventually cirrhosis. It has been suggested that its use increases chances of steatosis (12, 13, 14). Later we will expand on the findings observed in the parenchyma of the liver and those secondary to drug toxicity.
Predictors of severity and a greater probability of progression include ages over 45, AST/ALT greater than 1.0, obesity, type 2 diabetes mellitus, hypoalbuminemia, hypertension and the presence of liver fibrosis (15).
WHAT IS THE ROLE OF A LIVER BIOPSY?
Fatty liver disease has been identified as the most frequent cause of unexplained elevation of aminotransferases, but since a liver biopsy may also reveal other accompanying conditions in about 30% of cases, it remains the gold standard for confirmation of a diagnosis of NAFLD. A liver biopsy will show the clinical and pathological spectrum, and in many cases it will allow the clinician to establish the appropriate treatment. Repeated biopsies are used for evaluating treatment and for monitoring response to treatment especially in the context of research or use of new therapeutic methods.
When should a liver biopsy be performed? This remains a question that involves a lot of controversy. It is seen as a costly, invasive procedure with potential morbidity and vital risks. Therefore this decision should be made on an entirely individual basis that takes into account diagnostic images and especially the risk factors demonstrating progression of the condition. The main objectives of the biopsy should be to diagnose the type of steatohepatitis (NASH versus NAFL), to determine the stage of inflammatory activity or fibrosis, and to assess comorbid conditions and potential dual etiologies which are present in up to 20% of cases of ALD (16, 17).
HISTOPATHOLOGICAL FINDINGS
We will focus on the morphological changes necessary to establish a diagnosis and remember that in both alcoholic and non-alcoholic etiologies the histological criteria of liver compromise may be indistinguishable. An NAFLD diagnosis is based on two major criteria. The first is determining the presence of fatty liver or steatohepatitis, the second is determining the nonalcoholic nature of the disease.
The morphological spectrum, and the severity and extent of each of the criteria evaluated varies from case to case. In early stages the changes are concentrated in zone 3 or the centrilobular zone. Then it progresses to the acinus, destroys the normal architecture, and becomes cirrhosis.
The main findings are given by steatosis, inflammation, hepatocellular lesions and fibrosis (17, 18).
Steatosis
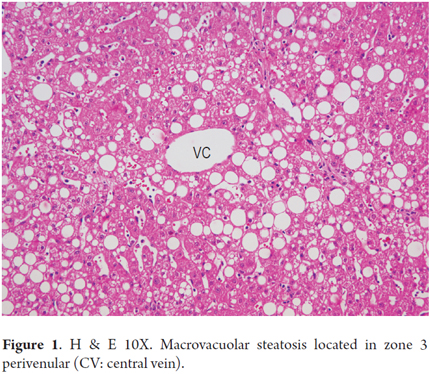
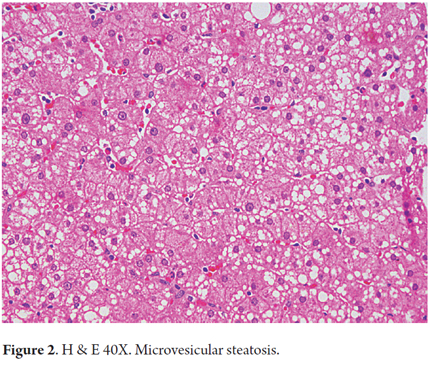
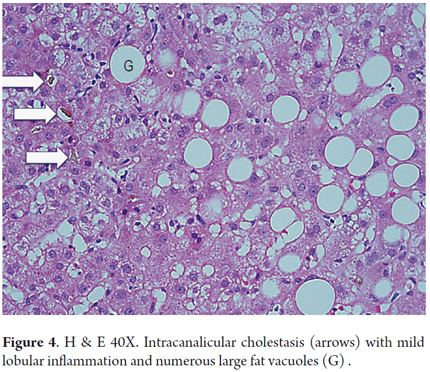
Steatosis is present in 90% to 100% of cases. It is predominantly either macrovesicular or a combination of microvesicular and macrovesicular. It is localized primarily in zone 3 (Figures 1 and 2). A limit of more than 5% is used as a cutoff point to define significant steatosis, lesser amounts are considered to be within normal histological limits. Sometimes fat is minimal or practically disappears. This occurs in cases with significant fibrosis, some stages of cirrhosis, and when an alcoholic patient has stopped drinking (12). Steatosis alone is not a specific pattern and may have some mild inflammation.
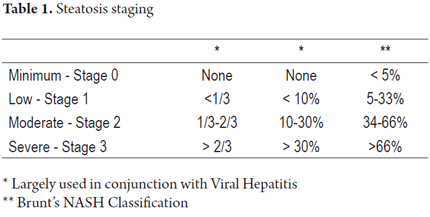
The severity of steatosis is graduated by considering the amount of hepatocytes involved in fatty vacuoles. There are a number of qualitative and quantitative classifications for this some of which are used primarily when steatosis accompanies conditions such as viral hepatitis C. A comparison of criteria is shown in Table 1 (4, 13, 19, 20, 21).
Inflammation
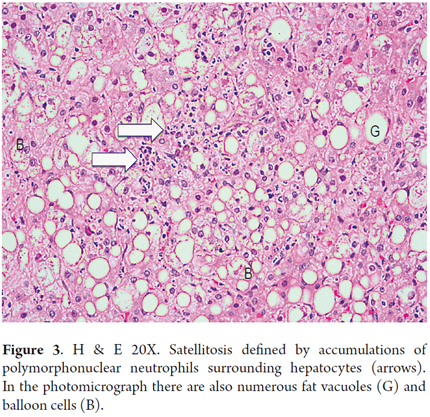
A polymorphonuclear neutrophil (PMN) is a cell that is typically present in small clumps or sinusoids. The finding of PMNs surrounding ballooned hepatocytes is known as satellitosis (Figure 3). Lobular or acinar inflammation, usually mild with mixed infiltrates (lymphocytes and polymorphonuclear neutrophils), is considered to be one of the diagnostic features of steatohepatitis (18, 19). There are usually small amounts of acinar infiltrates, portal inflammation of lymphocytes and histiocytes, but these increase when they are accompanied by necroinflammatory activity superimposed on conditions such as viral hepatitis C, or cholestasis in acute alcoholic hepatitis (Figure 4) (20, 21).
Hepatocellular lesions
Hepatocellular lesions are essential for diagnosis of steatohepatitis. Steatosis alone, with or without inflammation, is not a sufficient criterion for diagnosis NAFLD: it must be accompanied by hepatocellular lesions. Lesion occurs in two forms: hepatocellular ballooning and/or subsinusoidal or pericellular fibrosis.
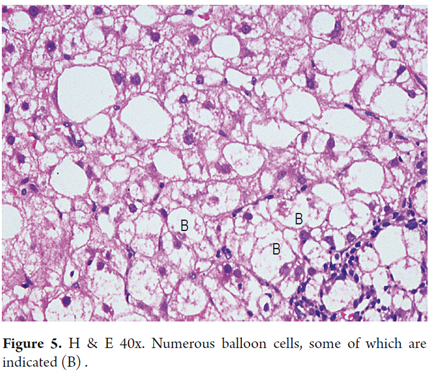
Ballooning is characterized by edema and cytoplasmic rarefaction resulting in enlarged cells with clear appearances with intracytoplasmic residual granular material located in Zone 3. In advanced stages the disease is adjacent to fibrous septa (Figure 5). As in other forms of hepatitis, isolated hepatocellular necrosis, acidophilic bodies and apoptotic bodies may be observed (18, 19).
Fibrosis
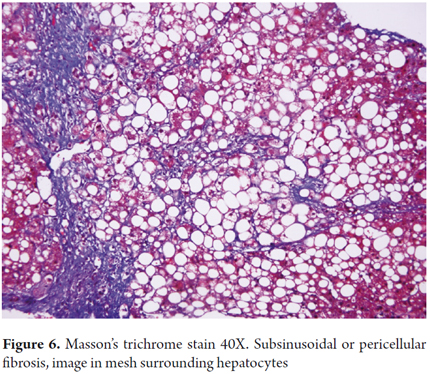
The distinctive fibrosis of steatohepatitis is pericellular and subsinusoidal. It is called chicken wire fibrosis due to the pattern caused by the collagen deposits in the space of Disse. It starts in zone 3 and can spread to the portal space. It occurs in the early stages of liver compromise (Figure 6).
In addition, fibrosis also develops around the terminal hepatic venules. This perivenular fibrosis or phlebosclerosis eventually completely obstructs the vein. In more advanced stages the combination of these progresses to replace scarred areas resulting in zone 3 sclerosing hyaline necrosis.
In endophlebitis there is inflammatory cells permeation areas next to terminal, sublobular or intercalated hepatic venules. Then it obliterates the lumen with subintimal proliferation and formation of veno-occlusive lesions. Portal and periportal fibrosis is unusual in early stages, although there may be evidence of veno-occlusive disease in portal veins.
In advanced stages of the disease bridging fibrosis extends from the perivenular areas to the portal tracts. Characteristically cirrhosis is micronodular, but a mixed pattern of macronodular and micronodular can also be observed especially in those cases in which the initial stimulus is removed e.g. massive alcohol intake (22, 23).
Other findings
Megamitochondria can be observed in both NASH and ALH as small eosinophilic bodies between 3 and 10 microns. When they are negative with PAS staining it is indicative of high oxidative stress of the hepatocytes.
Glycogenated nuclei are observed in diabetic patients and in children with NASH.
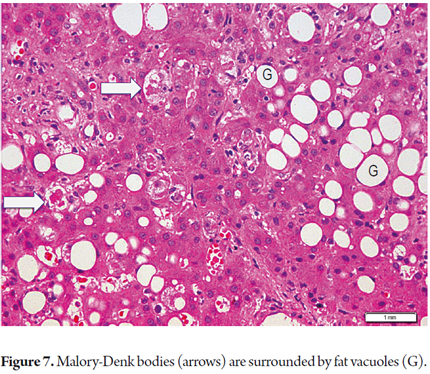
Mallory bodies, also called Mallory-Denk bodies, appear in ballooned cells and are more abundant in zone 3. They are not well developed and are inconspicuous in NASH, but are prominent and well developed in AML (Figure 7).
For the diagnosis of nonalcoholic steatohepatitis take into account the recommendations made by the NASH conference of the American Association for the Study of Liver Diseases in Atlanta, GA, in September 2002. (11)
The alterations that are indispensable for diagnosis are steatosis localized in zone 3, lobular inflammation and ballooning hepatocellular lesions.
Changes usually present but which are not necessary for diagnosis include subsinusoidal or pericellular fibrosis in zone 3, lipogranulomas, glycogenated nuclei in zone 1, acidophilic bodies and presence of hypertrophic Kupffer cells with PAS positive diastase materials.
Changes that may occur but are not necessary for diagnosis include Mallory bodies, iron deposits in hepatocytes or in sinusoidal cells, usually in small numbers and more closely related to AML, and megamitochondria.
Unusual findings that are more frequently found as part of the morphological chart of other conditions include microvesicular steatosis, less than 30% non-zonal macrovesicular steatosis, prominent portal or acinar inflammation, presence of an inflammatory infiltrate rich in plasma cells or eosinophils, epithelioid granulomas, portal or periportal fibrosis in the absence of pericellular fibrosis, chronic cholestasis with ductular proliferation and copper deposits and acute cholestasis with bile plug formation. Findings such as perivenular fibrosis, veno occlusive vascular lesions, phlebosclerosis and hyaline sclerosis. Intrahepatic iron deposits gradient from zone 1 to zone 3 (22, 23, 24, 25).
SPECIAL CONDITIONS
Steatohepatitis in children
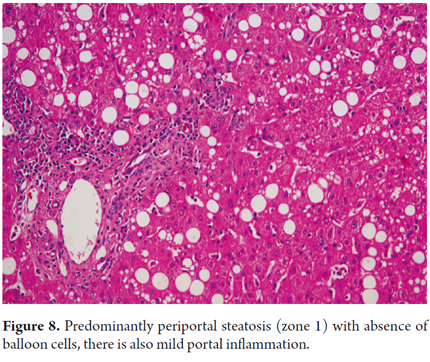
For some authors the morphological differences observed in children suggest that this could be a different disease. Type 1 steatohepatitis occurs in approximately 17% of pediatric cases, and is most common among white girls. Changes are very similar to those observed in the adult population. Type 2 has slightly different features. 51% of cases occur in older children or adolescents, especially Hispanic, Asian and Native American males. It is more closely related to obesity in children. Morphological changes occur in zone 1 with increased inflammation and lymphocyte portal fibrosis with no ballooning (Figure 8). In the remaining 32% of cases we can observe a mixed pattern with overlapping of the two patterns described above (25, 26).
Alcoholic etiology
As mentioned, histopathological histories may be indistinguishable, but there is usually increased inflammatory activity. We will list some of the findings that may help suggest this etiology.
The presence of sclerosing hyaline necrosis is a combination of large quantities of well-formed Mallory-Denk bodies, perivenular fibrosis with type III collagen deposits, hepatocyte necrosis in zone 3 and veno-occlusive disease. This is the so called alcoholic foamy degeneration corresponding to microvacuolar steatosis in zone 3 and cholestasis.
Noninvasive models have been proposed for differentiating between NASH and ALD. They useg an ANI index that assesses the mean corpuscular volume, AST/ALT ratio, body mass index and gender. When this index is greater than 0 it supports an alcoholic etiology. However, these models have not been validated yet, and therefore liver biopsy is still used for diagnosis (16, 27).
GRADING AND STAGING
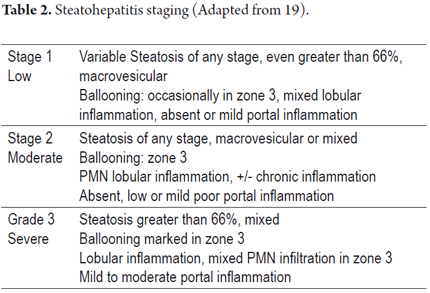
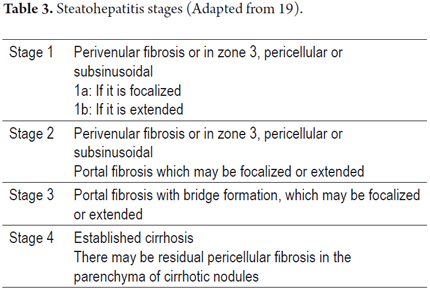
As with most liver diseases determining the stage of inflammatory activity is important for defining the prognosis and determining the appropriate therapy. The grade is defined by the extent of inflammation and hepatocellular lesions, and its stage is defined as the length and extent of fibrosis and architectural changes. Several groups have worked to create grading and staging schemes. The most frequently used is the Brunt's semi-quantitative modified classification. No matter what system is used, it is advisable to always perform the most objective and proper possible classification of the morphological findings observed (18, 22, 23, 25, 28, 29). Tables 2 and 3 summarize the criteria to consider.
REFERENCES
1. Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55(7):434-8. [ Links ]
2. Levene AP, Goldin RD. The epidemiology, pathogenesis and histopathology of fatty liver disease. Histopathology. 2012;61(2):141-52. [ Links ]
3. Shimada M, Hashimoto E, Taniai M, Hasegawa K, Okuda H, Hayashi N, et al. Hepatocellular carcinoma in patients with non-alcoholic steatohepatitis. J Hepatol. 2002;37(1):154-60. [ Links ]
4. Neuschwander-Tetri BA, Caldwell SH. Nonalcoholic steatohepatitis: summary of an AASLD Single Topic Conference. Hepatology. 2003;37(5):1202-19. [ Links ]
5. Kakar Sanjay. Medical Liver Disease: Problem Diagnoses for Practicing Pathologists. Short Course # 14. XXVI International Congress of the International Academy of Pathology. Montreal, Quebec, Canada. September 20, 2006. [ Links ]
6. Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40(6):1387-95. [ Links ]
7. Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. julio de 2005;129(1):113-21. [ Links ]
8. Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36(6):1349-54. [ Links ]
9. Chitturi S, Abeygunasekera S, Farrell GC, Holmes-Walker J, Hui JM, Fung C, et al. NASH and insulin resistance: Insulin hypersecretion and specific association with the insulin resistance syndrome. Hepatology. 2002;35(2):373-9. [ Links ]
10. Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917-23. [ Links ]
11. Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005-23. [ Links ]
12. Stravitz RT, Sanyal AJ. Drug-induced steatohepatitis. Clin Liver Dis. 2003;7(2):435-51. [ Links ]
13. Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology. 2006;49(5):450-65. [ Links ]
14. Patel V, Sanyal AJ. Drug-induced steatohepatitis. Clin Liver Dis. 2013;17(4):533-546, vii. [ Links ]
15. Idrovo CV, Gozalo GL. Enfermedad hepática grasa no alcohólica: NAFLD. Rev Col Gastroenterol. 2004;19(1):44-9. [ Links ]
16. O'Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Am J Gastroenterol. 2010;105(1):14-32; quiz 33. [ Links ]
17. Burt AD, Mutton A, Day CP. Diagnosis and interpretation of steatosis and steatohepatitis. Semin Diagn Pathol. 1998 [ Links ]
18. Brunt EM. Histopathology of non-alcoholic fatty liver disease. Clin Liver Dis. 2009;13(4):533-44. [ Links ]
19. Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16(42):5286-96. [ Links ]
20. Castéra L, Hézode C, Roudot-Thoraval F, Bastie A, Zafrani E-S, Pawlotsky J-M, et al. Worsening of steatosis is an independent factor of fibrosis progression in untreated patients with chronic hepatitis C and paired liver biopsies. Gut. 2003;52(2):288-92. [ Links ]
21. Fartoux L, Chazouillères O, Wendum D, Poupon R, Serfaty L. Impact of steatosis on progression of fibrosis in patients with mild hepatitis C. Hepatology. 2005;41(1):82-7. [ Links ]
22. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94(9):2467-74. [ Links ]
23. Bedossa P, Poitou C, Veyrie N, Bouillot J-L, Basdevant A, Paradis V, et al. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56(5):1751-9. [ Links ]
24. Rafiq N, Younossi ZM. Nonalcoholic fatty liver disease: a practical approach to evaluation and management. Clin Liver Dis. 2009;13(2):249-66. [ Links ]
25. Loomba R, Sirlin CB, Schwimmer JB, Lavine JE. Advances in pediatric nonalcoholic fatty liver disease. Hepatology. 2009;50(4):1282-93. [ Links ]
26. Hsu E, Murray K. Is nonalcoholic fatty liver disease in children the same disease as in adults? Clin Liver Dis. 2012;16(3):587-98. [ Links ]
27. Schwartz JM, Reinus JF. Prevalence and natural history of alcoholic liver disease. Clin Liver Dis. 2012;16(4):659-66. [ Links ]
28. Pagadala MR, McCullough AJ. The relevance of liver histology to predicting clinically meaningful outcomes in nonalcoholic steatohepatitis. Clin Liver Dis. 2012;16(3):487-504. [ Links ]











 texto en
texto en