Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.2 Bogotá Apr./June 2014
Diagnostic Usefulness of Upper Gastrointestinal Endoscopy for Patients under 18 Years of Age
Martín Alonso Gómez Zuleta, MD. (1), Óscar Fernando Ruiz Morales, MD. (2), Javier Riveros, MD. (2)
(1) Internist, Gastroenterologist and Assistant Professor of Gastroenterology at the Universidad Nacional de Colombia and Hospital El Tunal in Bogotá, Colombia.
(2) Internist and Gastroenterology Fellow at the Universidad Nacional de Colombia in Bogotá, Colombia.
Received: 26-11-13 Accepted: 08-15-14
Abstract
Objective: The objective of this study was to define the diagnostic value of upper gastrointestinal endoscopy for patients under 18 years of age.
Materials and Methods: This was a retrospective cross-sectional study based on findings from upper gastrointestinal endoscopy and resulting histopathology reports. These reports were found in the database of the endoscopy department at a third level medical center of referral in Bogotá, Colombia. Procedures were performs between January 2007 and January 2013. The study population consisted of 113 patients between the ages of five and seventeen years of age for whom all available information regarding age, gender and endoscopic and histopathological diagnoses was collected.
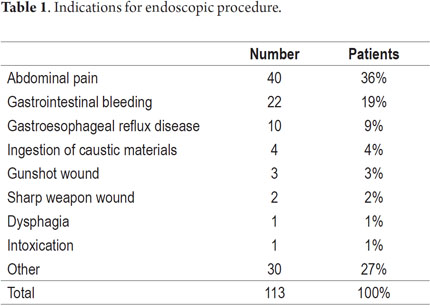
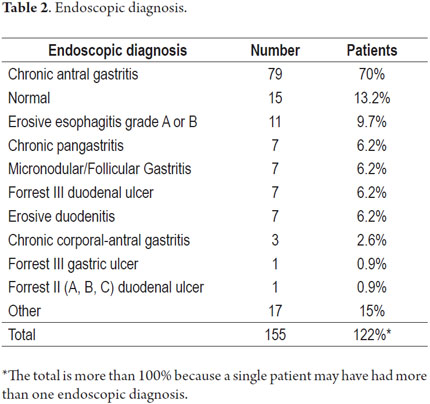
Results: A total of 113 records of patients between 5 and 18 years old were found for the evaluation period between January 2007 and December 2012. Sixty one percent (n = 69) of these patients were female. 16.8% (n = 19) of the patients were under 12 years of age for which reason they required sedation administered by an anesthesiologist. The most common indications for endoscopy were abdominal pain (36 %, n = 40) and gastrointestinal bleeding (19 %, n = 22). The most common endoscopic diagnoses were chronic antral gastritis (70 %, n = 79) and normal endoscopy (11.5 %, n = 13). Of the 22 patients who underwent endoscopies because of bleeding, nine had peptic ulcers. Biopsies were taken from 66 % of the patients (n = 75). Histopathological analysis showed the presence of Helicobacter pylori in 71% (n = 53) of these patients and showed chronic gastritis in 93% (n = 70). The samples examined from this group of patients showed no signs of intestinal metaplasia, gastric atrophy or cancer.
Conclusion: The indications for performing diagnostic upper endoscopy in children should be reconsidered and critically evaluated in order to increase the diagnostic yield and minimize exposure to inherent procedural risks. Current diagnostic yield is very low given that no relevant findings resulted from these procedures for many patients who consulted with a gastroenterologist simply because of abdominal pain, and ulcers were found in only 41% (9/22) of those who presented bleeding.
Keywords
Diagnostic value, upper gastrointestinal endoscopy, underage patients.
INTRODUCTION
Since the 1960s, when the use of fiber optics began in the field of medicine, gastrointestinal endoscopy has become a revolutionary diagnostic and therapeutic tool. It is now commonly used in adults and occasionally in children, but prior to its introduction contrast x-rays were routinely used to study gastrointestinal disorders in pediatric patients (1). In 1972 after a meeting of the European Society of Pediatric Gastroenterology in Hamburg, engineers from Olympus' adapted an industrial fiberscope for use in children. Together with other technical improvements, this made upper gastrointestinal endoscopy in children possible (2). During 1978 and 1979 several studies confirmed the benefits of using diagnostic endoscopy together with biopsies (when required) over imaging studies of the gastrointestinal tract (3, 4). No scope was specifically designed for pediatric endoscopy until 1981 when the first European workshop on pediatric gastrointestinal endoscopy in Bern was held. There, given the growing demand, experts and engineers from Olympus made it a priority to develop a specific device for children. From that moment on over the last 40 years progress has been made in the development of endoscopy equipment and techniques of sedation and anesthesia which have made pediatric endoscopy safer and more useful for treatment and diagnosis (5,6). A 20 year follow-up covering the period from 1985 to 2005 showed that the incidence of conditions requiring endoscopic procedures in patients under 18 increased in such a way that first time upper gastrointestinal endoscopies for patient popuation increased 12 times. During this period of time the proportion of patients with gastrointestinal bleeding also decreased from 34% to 5% while patients with abdominal pain increased from 23% to 43%. Upper gastrointestinal endoscopies with biopsies from the esophagus, stomach and/or duodenum also increased from 18% in 1985 to 95% in 2005 (7). Generally, inclusion of children with less severe clinical symptoms and performance of a greater number of biopsies may have resulted in an increased rate of diagnosis but not necessarily an increased incidence of diseases (8). Upper endoscopic procedures performed in children have high rates of procedures reported as normal (9). In addition, between 2% and 4% of all pediatric visits to the doctor are due to functional gastrointestinal disorders (10). Ten percent of the population has recurrent abdominal pain and it is in this portion of the population that EGDs are less useful for diagnosis because organic diseases are absent in the vast majority of these patients (11). Nevertheless, despite the evidence, these patients are often referred for upper endoscopy, especially in our country.
It is noteworthy that there is almost no knowledge of the pattern of gastrointestinal endoscopy in our population. In addition, there are very few studies on endoscopy in South American children which demonstrate its utility as a diagnostic procedure. In this study we seek to establish the utility of endoscopy in a retrospective cohort of patients aged between 5 and 18 years old who underwent upper gastrointestinal diagnostic endoscopy because of various indications.
MATERIALS AND METHODS
We conducted a descriptive cross-sectional retrospective cohort study based on reports of upper gastrointestinal endoscopy and histopathology reports located in the database of a third-level referral center for endoscopy in a hospital in Bogotá, Colombia. The study covered the period from January 2007 to December 2012 and included patients who were between 5 and 18 years old at the time of that they underwent upper gastrointestinal endoscopy. Endoscopies were performed for diagnostic purposes by gastroenterologists and endoscopists with thorough training in adult gastrointestinal endoscopy. Each of the specialists has performed more than a thousand upper diagnostic endoscopies in adults and children. An Olympus GIF-140 video gastroscope was used for all procedures. It has a 9.8 mm diameter insertion tube, a 2.8 mm biopsy channel and a 103 cm working length (Olympus Inc. Tokyo, Japan).
In accordance with the institutional protocol, endoscopic procedures on patients aged 5-12 years were performed under sedation administered by anesthesiologists. The medication employed was chosen by the specialist in charge of sedation according to individual patient characteristics and procedure site specifications (endoscopy room or operating room). Procedures were performed with patients sedated after insertion of reliable venous access and with monitoring of vital signs and oxygen saturation. All patients received pharyngeal anesthesia during the endoscopic procedure according to the protocol of our hospital. These procedures were performed on both an outpatient and an inpatient basis. At least two nurses trained in endoscopy assisted in all procedures. Procedures were performed only after patients' parents or legal guardians had signed informed consent forms. Data obtained from the patient population were included in virtual data tables from Google Drive, and calculations of frequencies were performed with Microsoft Excel.
RESULTS
A total of 113 upper gastrointestinal endoscopy cases in patients aged 5 to 18 years between 2007 and 2012 were collected and analyzed. The population distribution by gender was 61% (n = 69) female and 39% (n = 44) male, and the median age was 15 years old with an arithmetic mean of 14.61 years. Nineteen patients (16.8%) under 12 years old required sedation which was carried out by an anesthesiologist. The most frequent indications for upper endoscopy were dyspepsia (26%, n = 29) and gastrointestinal bleeding (19%, n = 22) (Table 1). The most common endoscopic diagnoses were chronic antral gastritis (70%, n = 79) and normal endoscopy (11.5%, n = 13) (Table 2). Biopsies were performed on samples taken during endoscopic procedures from 66% of the patients (n = 75). Seventy one percent of the histopathological samples (n = 53) revealed Helicobacter pylori while 93% (n = 70) revealed chronic gastritis and intestinal metaplasia. No cases of gastric atrophy or cancer were found in the samples examined.
DISCUSSION
Currently, diagnostic endoscopy is one of the most important tools of gastroenterology for both adults and children. It has expanded our understanding of the pathophysiology and treatment of common gastrointestinal disorders in children.
Parallel to increasing availability of endoscopy for the pediatric population the number of procedures performed has risen (12). Because the indications and performance of this procedure are still being studied, studies that support its use in this population are scarce. To identify differences and similarities between this study and those conducted in other countries, we have compared this study with studies conducted in Saudi Arabia, Sudan, Jordan, Brazil and the USA. The latter, only recently published, studied a population of more than 1000 patients.
Among the patients in our study who underwent upper endoscopy, most were female. The male-female ratio was 1.78:1.0. This differs from the other studies: the US study had a male-female ration of 1.0:1.0, the Saudi Arabian study's was 0.7:1.0 and the study from Sudan had a male female ratio of 1.2:1.0 (13, 14).
The use of sedation and general anesthesia also varied from study to study. In our study, 16.8% of patients required sedation or anesthesia, but in Saudi Arabia 99% of procedures are carried out under sedation and in Brazil 80% are performed under general anesthesia (15). This results in a reduction of derivative complications and adverse reactions in patients older than 12 years (16, 17).
The most common indication in our study was abdominal pain (36%) which is similar to the percentage found in the US study (28.7%) In that study abdominal pain and epigastric abdominal pain were listed separately with respective frequencies of 28.7% and 8.5% (12). In Sudan, where schistosomiasis is endemic, the main indications were hematemesis 24% and portal hypertension 21% (13). In Brazil, suspicion of malabsorption accounted for 56% of the cases while recurrent abdominal pain accounted for 37% (15). The only significant endoscopic abnormalities found in our study were ulcers (8%) and esophageal erosions (9.7%). These percentages are smaller than those found in the U.S. where endoscopic abnormalities were found in 34.7% of patients. Nevertheless, our most frequent endoscopic diagnosis was chronic antral gastritis (70%) which is much higher than the percentages found in Brazil and the USA where the gastritis was found in 19% and 10.4% of cases respectively (12-15). This difference is probably related to the high rate of H. pylori infection. In our study, histopathological examinations were performed for 66% of patients and 71% of these patients were positive for Helicobacter pylori. This exceeds the recently published rate found in children from Iran of 64.2% (18), and far exceeds that found in the USA of only 2.4%. This information is associated with estimated rates of H. pylori infection in children over 10 years of more than 60% in our population and confirms that infection occurs early in childhood. In developing countries this infection is linked to socioeconomic status (70% -78%) and to the high rate of gastric cancer (20-21).
In our study, no duodenal biopsies were performed patients because it is speculated that celiac disease has no significant impact in our country. This contrasts with performance of duodenal biopsies on 9% of patients in the study from Sudan (14), 46% of the patients in the study from Jordan (22), 29% of the patients in the study from Saudi Arabia (13) and 6.5% of the patients in the study from the USA (12).
In our study 11.5% of the upper gastrointestinal endoscopies were considered to be normal from the endoscopic point of view. This is a smaller percentage than those reported in the study from Jordan (38%) (22), a US study in 2005 (44%) and another65.3% in 2013 (12-18) and Brazil (42%) (15), the frequency of peptic ulcer was 8% (duodenal and gastric ulcer) that is twice the findings of Brazil (4 %) (15). These findings are consistent with a higher rate of H. pylori infection in our population (which can be diagnosed by non-invasive methods with similar performance). Despite the higher frequency of H. pylori and gastritis in these children, no histological findings related to metaplasia or atrophy were found because these lesions require an extended development period. The current recommendation is that pediatric patients who undergo upper endoscopy should have routine biopsies (12). In our study no complications related to the endoscopic procedure or anesthesia were reported. Nevertheless, it should be remembered that this is an invasive procedure, so the procedure itself could result in an emergency. In addition, complications related to this procedure may occur more readily in pediatric patients, so this is not an innocuous procedure (23).
CONCLUSIONS
The findings of this study support the theses that the diagnostic yield of upper gastrointestinal endoscopy in children is modest. Its limited use is not surprising and is similar to that found in similar studies in other geographic areas. We believe that given its limited diagnostic effectiveness, upper gastrointestinal endoscopy should not be performed in children. Indications should be considered and critically evaluated by pediatricians in order to prevent exposure to the inherent, although low, risks in the endoscopic procedure and sedation.
REFERENCES
1. K. Kawai KM. Endoscopical Observations on Gastric Ulcers in Teenagers. Endoscopy. 1970;02(04):206-8. [ Links ]
2. Cremer M, Peeters JP, Emonts P, et al. Fiberendoscopy of the gastrointestinal tract in children. Experience with newly designed fiberscopes. Endoscopy 1974;6:186-9. [ Links ]
3. Graham DY, Klish WJ, Ferry GD, Sabel JS. Value of fiberoptic gastrointestinal endoscopy in infants and children. South Med J. 1978;71(5):558-60. [ Links ]
4. Hartemann E, Rigal D, Sassolas F, Louis JJ, Briand H. [Upper digestive system hemorrhage in the child. Value of emergency fiberscopy]. Pédiatrie. 1979;34(6):649-58. [ Links ]
5. Govaerts MJM, Cadranel S. Premedication for pediatric gastroenterologic procedures. In: Dinari G, Rozen P, Bujanover Y, Lebenthal E. editors. Newer tests and procedures in pediatric gastroenterology. Basel: Karger;1989. [ Links ]
6. Hassall E. Should pediatric gastroenterologists be i.v. drug users? J Pediatr Gastroenterol Nutr. mayo de 1993;16(4):370-2. [ Links ]
7. Franciosi JP, Fiorino K, Ruchelli E, Shults J, Spergel J, Liacouras CA, et al. Changing indications for upper endoscopy in children during a 20-year period. J Pediatr Gastroenterol Nutr. 2010;51(4):443-7. [ Links ]
8. Friedt M, Welsch S. An update on pediatric endoscopy. Eur J Med Res. 2013;18(1):24. [ Links ]
9. O'Loughlin EV, Dutt S, Kamath R, Gaskin K, Dorney S. Prospective peer-review audit of paediatric upper gastrointestinal endoscopy. J Paediatr Child Health. 2007;43(7-8):551-4. [ Links ]
10. Bonilla S, Deli Wang, Saps M. The prognostic value of obtaining a negative endoscopy in children with functional gastrointestinal disorders. Clin Pediatr (Phila). 2011;50(5):396-401. [ Links ]
11. Saps M, Seshadri R, Sztainberg M, Schaffer G, Marshall BM, Di Lorenzo C. A prospective school-based study of abdominal pain and other common somatic complaints in children. J Pediatr. 2009;154(3):322-6. [ Links ]
12. Sheiko MA, Feinstein JA, Capocelli KE, Kramer RE. Diagnostic yield of EGD in children: a retrospective single-center study of 1000 cases. Gastrointest Endosc. 2013;78(1):47-54.e1. [ Links ]
13. El-Mouzan MI, Al-Mofleh IA, Abdullah AM, Al-Rashed RS. Indications and yield of upper gastrointestinal endoscopy in children. Saudi Med J. septiembre de 2004;25(9):1223-5. [ Links ]
14. Mudawi HMY, El Tahir MA, Suleiman SH, Eltaybe NH, Gamer NM, Abdallha FA, et al. Paediatric gastrointestinal endoscopy: experience in a Sudanese university hospital. East Mediterr Health J Rev Santé Méditerranée Orient Al-Majallah Al-iīyah Li-Sharq Al-Mutawassi. 2009;15(4):1027-31. [ Links ]
15. Ferreira CT, Berti MR, Pires AL, Wieczorek C, Alves J. [Upper gastrointestinal endoscopy in children: indications and results]. J Pediatr (Rio J). 1998;74(1):39-44. [ Links ]
16. Asge Standards Of Practice Committee, Lee KK, Anderson MA, Baron TH, Banerjee S, Cash BD, et al. Modifications in endoscopic practice for pediatric patients. Gastrointest Endosc. 2008;67(1):1-9. [ Links ]
17. Flóres LS, Villalobos DC, Rodríguez R, et al. Endoscopia digestiva superior en pediatría, Colomb Med 2005; 36(Supl 1):42-51. [ Links ]
18. Gilger MA, Gold BD. Pediatric endoscopy: new information from the PEDS-CORI project. Curr Gastroenterol Rep. 2005;7(3):234-9. [ Links ]
19. Jafar S, Jalil A, Soheila N, Sirous S. Prevalence of helicobacter pylori infection in children, a population-based cross-sectional study in west iran. Iran J Pediatr. 2013;23(1):13-8. [ Links ]
20. Gutiérrez O, Aponte D, Páramo D, et al. Seroprevalencia y factores de riesgo asociados con la infeccion por Helicobacter pylori en niños, Rev Col Gastroenterol. 2001;16:19-22. [ Links ]
21. Bravo LE, Cortés A, Carrascal E, Jaramillo L, García L, Bravo P, et al. Helicobacter pylori: patología y prevalencia en biopsias gástricas en Colombia. Colomb Med. 2003;34:124-31. [ Links ]
22. Rawashdeh MO, Abu-Farsakh N, al-Jaberi TM. Paediatric upper gastro-intestinal endoscopy in developing countries. Ann Trop Paediatr. 1996;16(4):341-6. [ Links ]
23. Kim YJ. General considerations and updates in pediatric gastrointestinal diagnostic endoscopy. Korean J Pediatr. 2010;53(9):817-23. [ Links ]











 text in
text in