Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.2 Bogotá Apr./June 2014
Update on Approaches to Patients with Dyspepsia and Functional Dyspepsia
William Otero R., MD. (1), Martín Gómez Zuleta, MD. (2), Lina Otero P., MD. (3)
(1) Professor of Medicine in the Gastroenterology Unit at the Universidad Nacional de Colombia and Gastroenterologist at the Clínica Fundadores in Bogotá, Colombia.
(2) Professor of Medicine in the Gastroenterology Unit at the Universidad Nacional de Colombia and Gastroenterologist at the Hospital El Tunal in Bogotá, Colombia.
(3) Gastroenterology Fellow at the Universidad Nacional de Colombia in Bogotá, Colombia.
Received: 24-06-13 Accepted: 08-05-14
Abstract
Dyspepsia (DP) is pain or discomfort in the upper abdomen. It may be uninvestigated dyspepsia or functional dyspepsia (FD) which is diagnosed after diagnostic tests such as upper endoscopy and occasionally upper abdominal ultrasound find no organic changes that explain the condition. Despite the wide dissemination of the classifications of dyspepsia, functional dyspepsia and postprandial dyspepsia, recent findings controvert these classifications. This article discusses the approach to patients with dyspepsia and the pathophysiology and drug treatment of functional dyspepsia.
Keywords
Dyspepsia, functional dyspepsia, Helicobacter, gastroprokinetic agents, antisecretory drugs.
INTRODUCTION
The term dyspepsia comes from the Greek words dys (poor) and pepsis (digestion). Dyspepsia (DP) refers to chronic or recurrent pain or discomfort located in the central part of the upper abdomen (epigastrium). It is often replaced incorrectly by the terms "chronic gastritis" or "acid-peptic disease."
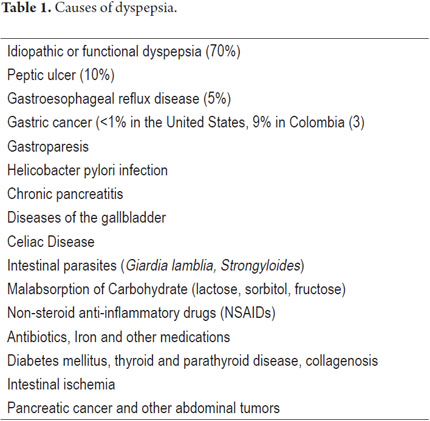
When a patient presents DP and its cause has not yet been investigated, it is referred to as uninvestigated dyspepsia (UDP). When it has been subjected to tests, specifically upper endoscopy and occasionally upper abdominal ultrasound, it can be classified as functional or idiopathic dyspepsia (FD) when there are no disorders to explain the symptoms or as secondary or organic dyspepsia when the cause is due to an organic gastrointestinal disease such as a peptic ulcer or tumors (either systemic or metabolic) (See Table 1) (1). After causes have been studied, FD explains more than 70% of previously uninvestigated cases if dyspepsia (2). In the past this type of dyspepsia was called non-ulcer dyspepsia, but this is an incorrect name because there are other entities besides peptic ulcers that can produce this clinical picture.
Since the pattern of symptoms by itself does not allow the physician to distinguish between an organic and functional disease, further investigation is needed to exclude an organic disease.
EPIDEMIOLOGY
Approximately 15% to 40% of the population have chronic or recurrent symptoms of dyspepsia which have gone uninvestigated. About a third of them will have secondary or organic dyspepsia (4). In some countries it has been found that the annual incidence is about 1%, and it is estimated that one in two people will consult with a physician at some point in their life about dyspeptic symptoms. Dyspepsia accounts for 5% of general practice consultation and about 20% to 30% of gastroenterology consultations (2, 5, 6). In addition to its high prevalence, it is important because it significantly decreases the quality of life of individuals who have the disease (2, 5, 6).
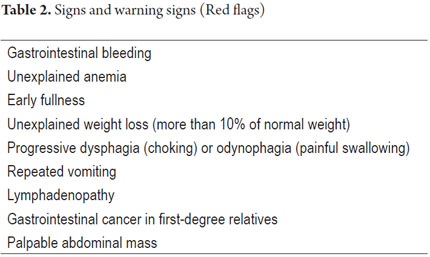
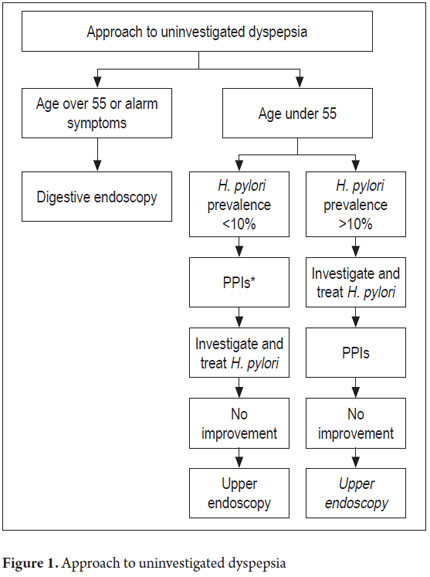
The diagnostic approach to DP depends the relation between cost effectiveness and whether there is concern or suspicion that there is a serious entity (4). Indications for immediate endoscopy are the presence of alarming symptoms and signs (Table 2) and patient age over 50-55 which is the onset age for gastric cancer (GC) in the developed countries (5-7). In developed countries the approach is different from that in developing countries. The differences are primarily based on the prevalence and incidence of GC which is about 4 to 5 times more common in countries such as Colombia, Venezuela, and Costa Rica than in countries like the United States (3). The American Gastroenterological Association and the American College of Gastroenterology recommend the algorithm in Figure 1.
In Colombia, a prospective study of UDP patients who underwent upper endoscopies found that 9% of the patients had GC and that this disease was the second leading cause of organic dyspepsia (3). Characteristically, gastric cancer becomes more common after people reach 30 years of age, so our recommendation is that upper endoscopies should be performed on all patients with uninvestigated dyspepsia over the age of 30 years and on those patients under that age who have alarming symptoms. Although one possible explanation for the high prevalence of GC in this study is the fact that many of the patients were evaluated at a referral hospital, we believe that this recommendation is justified by the high prevalence of GC throughout Colombia and also by the low cost of upper endoscopy (USD 40.00 to USD 100.00). According to recent statistics, the prevalence of GC here of 17.4 to 48.2 cases per 100,000 people is among the highest in the world with (8).
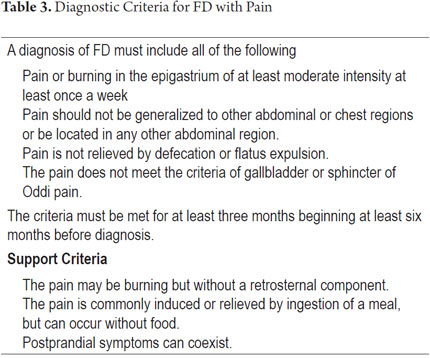
When gastrointestinal endoscopy and hepatobiliary ultrasound (when indicated) reveal no organic pathologies, the diagnosis is FD. Diagnostic criteria for FD are shown in Tables 3 and 4. Since the Rome III consensus, FD has been divided into two categories: FD with pain, and FD with postprandial distress.
A recent study by Vakil et al. has called into question the widespread acceptance of these criteria, particularly the subdivision of FD into two categories (pain and postprandial distress). Although these criteria have been confirmed in studies in various parts of the world (9), Vakil et al. found that 66% of FD patients straddle the two subtypes and that only 10% can be placed solely into the FD with postprandial distress category while only 24% of patients can be placed solely into the FD with epigastric pain category. These authors believe that the subdivision into these two categories is questionable and suggest that a new classification of FD is needed (10). In addition, they note that GERD patients often present symptoms that could classify them as FD patients (10).
PATHOPHYSIOLOGY
The mechanisms which cause FD are not completely understood. Why do symptoms occur when there is no organic disease? Recent clinical studies have found the following abnormalities (11-17):
1. Altered gastric accommodation
2. Delayed gastric emptying
3. Gastric hypersensitivity
4. Duodenal hypersensitivity to acids and lipids
5. Low-grade inflammation
6. Stress, psychosocial factors, trauma, genetic predispositions, prior viral infections, etc.
7. Helicobacter pylori infections
8. Precipitation due to infectious gastroenteritis
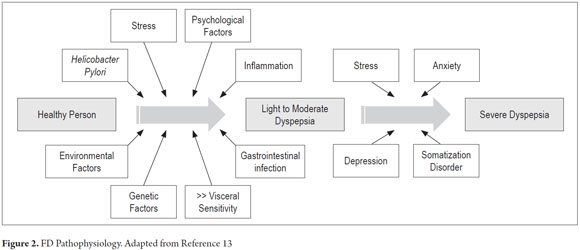
A recent meta-analysis found that the risk of FD significantly increases after infectious gastroenteritis (OR: 1.97 CI 95% 1.51-2.56) (17). These findings have led to the supposition that inflammation and immune disorders may be important. Genetic epidemiological studies have also identified polymorphisms of the GNB3 which influences the activation of immune cells (12). A model of the involvement of different factors from healthy individuals to patients with severe FD is shown in Figure 2 (13).
EVALUATION OF PATIENTS WITH DYSPEPSIA AND TREATMENT OF FD
An evaluation of an FD patient should include a careful medical history emphasizing "alarm" symptoms. These symptoms may suggest the presence of tumors or complicated organic diseases. Contrary to the consensus of Rome III which says that heartburn and/or regurgitation exclusively indicate gastroesophageal reflux disease (GERD), there is now new evidence that these symptoms often occur in FD even after GERD has been confirmed by currently available tests (10). If, in the absence of alarm symptoms, dyspeptic symptoms include upper abdominal pain relieved by defecation or if it is associated with a change in stool frequency or form, these symptoms are more likely to be derived from irritable bowel syndrome (1). If the predominant symptom is upper abdominal pain that worsens with food intake, it may indicate chronic pancreatitis or gallbladder disease. If there is steatorrhea, the probability of chronic pancreatitis with malabsorption is reinforced. If upper gastric pain is relieved by food, antacids, etc., the symptom is suggestive of a peptic ulcer. The warning symptoms in Table 1 suggest the presence of organic or secondary dyspepsia.
These four steps should be followed for DP patients:
1. Immediate endoscopy when there are signs/symptoms of alarm, when the patient is over 50 years old, and when the epidemiological characteristics of gastric cancer are present, no matter what the patient's age.
2. Empirical antisecretory therapy
3. H. pylori tests and treatment if positive
4. H. pylori tests and upper endoscopy if positive (4).
In scenarios 3 and 4, if there is no improvement following treatment, another upper endoscopy is indicated.
TREATMENT OF FUNCTIONAL DYSPEPSIA
Based on the pathophysiological mechanisms involved (6, 7, 18, 19), current recommendations for management of this entity are:
1. Tricyclic antidepressants to block visceral hypersensitivity have an NNT of 2 (95% CI 1.5-5) or 5 (95% CI 3-250) (20).
2. Acid secretion inhibitors such as anti H2 and proton pump inhibitors (PPIs) are designed to control hypersensitivity. The NNT for anti H2 is 8 (95% CI 5-24) and for PPI is 9 (95% CI 6-19). In tests of PPIs, 34% of patients treated with PPIs respond while. 25% of patients treated placebos respond (21). PPIs may be considered the drugs of choice for patients who, in addition to DP, have GERD symptoms or those who present burning-type epigastrium pain. The recommendation is to administer the drugs for at least four weeks (19).
3. H. pylori eradication does not produce improvement in most patients. The NNT is 14 (95% CI 10-29), but this small benefit is statistically significant, and long-term studies have shown that it is cost-effective (18, 19). The therapeutic gain over placebos ranges from 6% to 14% (22). The eradication of H. pylori also has other benefits as long as no advanced histological alterations such as atrophy or intestinal metaplasia occur when eradication takes place. These other benefits include elimination of gastritis, reduction or elimination of GC risk, and reduction of the risk of peptic ulcers (23, 24).
4. Prokinetics aim to correct alterations of gastric motility. Molecules of this group that stimulate smooth muscle activity are the choice for gastroparesis treatment. Overall, the use of prokinetic agents has an NNT of 5 (95% CI 3-11). Within this category, cisapride has been recalled because of adverse cardiovascular effects, and metoclopramide is not a good alternative because it causes serious neurological side effects. They are being replaced by domperidone, a D2 receptor antagonist which does not cross the blood brain barrier (19). Mosapride, a 5HT4 receptor agonist and 5HT3 antagonist, was no better than a placebo in a large European study (25). Itopride is a new drug that combines the anti D2 effect with cholinesterase inhibition. It increases levels of acetylcholine which produces improvements in gastric emptying (19). A recent meta-analysis found that it produces benefits in terms of overall feelings of well-being, postprandial fullness, and early fullness (26). Levosulpiride, the L isomer of sulpiride, has a dual mechanism to correct motility. As an antagonist of D2 dopamine receptors in upper gastrointestinal smooth muscles it decreases the activity of dopamine, an inhibitory substance. This allows acetylcholine's action to predominate resulting in increased gastric contractions (27).
From the results above for the efficacies of drugs currently recommended for FD, it would appear that the least effective treatment is H. pylori eradication (NNT 14, 95% CI 10 to 20) (16). Nevertheless, the quality of studies assessing different prokinetic treatments is poor, very poor for those that assess antidepressants, moderate for those that assess anti H2, and very good for those that assess H. pylori (18). The appearance that H. pylori eradication is less effective is related directly to the excellent quality of the studies of its efficacy. The rigor and quality of controlled clinical trials has an important impact on the quality of the results. Poor quality studies may overestimate the magnitude of an effect. When they score two points or less on the Jadad scale, they produce favorable effects 35% more often than do clinical trials with three or more points (30). Although H. pylori eradication improves symptoms in only 5% to 10% of cases (1 in 14) (16), other studies have shown that eliminating the infection is cost-effective in the long term and that long-term eradication has additional benefits including reducing or preventing the risk of gastric cancer (16, 23, 31), and preventing peptic ulcers (and thus bleeding peptic ulcers) (32, 33). Therefore, the saying of many experts that, "the only good Helicobacter is a dead Helicobacter," is justified. Patients who recover from dyspepsia as the result of H. pylori eradication did not have functional dyspepsia, but rather "H. pylori dyspepsia" which is an organic dyspepsia. Theoretical mechanisms involved in the generation of DP are shown in Figure 3 (16). What are often ignored are the factors that are responsible for FD among all infected people plus how many infected people present this clinical condition even though 100% of them present chronic gastritis (24). Eradication of H. pylori infections should be verified by non-invasive methods such as the urea breath test or stool antigen test four weeks after completion of treatment with antibiotics (23).
Since no treatment is effective for all patients, various strategies for treatment may be used sequentially or in combination. For example, the reflux symptoms of patients with symptoms of GERD and dyspepsia improve with administration of PPIs, but symptoms of dyspepsia persist. In these cases, a prokinetic or tricyclic antidepressant should be administered (34).
OTHER TREATMENTS
There is no evidence about the utility of recommending special diets to prevent FD (35). Psychological therapies and hypnotherapy need skilled specialists dedicated to this type of approach, and we apparently lack them (35). Acotiamide is a new drug that increases the release of acetylcholine, inhibits cholinesterase and may further increase gastric accommodation (36). It seems that its effectiveness is limited to postprandial-distress FD and not to pain related FD (37).
REFERENCES
1. Tack J, Talley NJ, Camilleri M, Holtmann G, Hu P, Malagelada J-R, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130(5):1466-79. [ Links ]
2. Halder SLS, Talley NJ. Functional Dyspepsia: A New Rome III Paradigm. Curr Treat Options Gastroenterol. 2007;10(4):259-72. [ Links ]
3. Pineda LF, Otero W, Gómez M, Arbeláez V. Enfermedad estructural y valor predictivo de la Historia Clínica en pacientes con dispepsia no investigada. Rev Col Gastroenterol 2004;19:13-25. [ Links ]
4. Graham DY, Rugge M. Clinical practice: diagnosis and evaluation of dyspepsia. J Clin Gastroenterol. 2010;44(3):167-72. [ Links ]
5. Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129(5):1756-80. [ Links ]
6. Talley NJ, Vakil N, Practice Parameters Committee of the American College of Gastroenterology. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100(10):2324-37. [ Links ]
7. Gisbert JP, Calvet X, Ferrándiz J, Mascort J, Alonso-Coello P, Marzo M, et al. [Clinical practice guideline on the management of patients with dyspepsia. Update 2012]. Atencion Primaria Soc Esp Med Fam Comunitaria. 2012;44(12):727.e1-727.e38. [ Links ]
8. Globocan 2012 - Home [Internet]. [citado 29 de mayo de 2014]. Recuperado a partir de: http://globocan.iarc.fr/Default.aspx [ Links ]
9. Tack J, Talley NJ. Functional dyspepsia--symptoms, definitions and validity of the Rome III criteria. Nat Rev Gastroenterol Hepatol. 2013;10(3):134-41. [ Links ]
10. Vakil N, Halling K, Ohlsson L, Wernersson B. Symptom overlap between postprandial distress and epigastric pain syndromes of the Rome III dyspepsia classification. Am J Gastroenterol. 2013;108(5):767-74. [ Links ]
11. Lee K-J, Kindt S, Tack J. Pathophysiology of functional dyspepsia. Best Pract Res Clin Gastroenterol. 2004;18(4):707-16. [ Links ]
12. Holtmann G, Siffert W, Haag S, Mueller N, Langkafel M, Senf W, et al. G-protein beta 3 subunit 825 CC genotype is associated with unexplained (functional) dyspepsia. Gastroenterology. 2004;126(4):971-9. [ Links ]
13. Lacy BE. Functional dyspepsia and gastroparesis: one disease or two? Am J Gastroenterol. 2012;107(11):1615-20. [ Links ]
14. Miwa H. Why dyspepsia can occur without organic disease: pathogenesis and management of functional dyspepsia. J Gastroenterol. 2012;47(8):862-71. [ Links ]
15. Vanheel H, Farré R. Changes in gastrointestinal tract function and structure in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10(3):142-9. [ Links ]
16. Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10(3):168-74. [ Links ]
17. Pike BL, Porter CK, Sorrell TJ, Riddle MS. Acute gastroenteritis and the risk of functional dyspepsia: a systematic review and meta-analysis. Am J Gastroenterol. 2013;108(10):1558-1563; quiz 1564. [ Links ]
18. Moayyedi P. Dyspepsia. Curr Opin Gastroenterol. 2012;28(6):602-7. [ Links ]
19. Oustamanolakis P, Tack J. Dyspepsia: organic versus functional. J Clin Gastroenterol. 2012;46(3):175-90. [ Links ]
20. Wu JC, Cheong PK, Chan Y, Lai LH, Ching J, Chan A, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Low Dose Imipramine for Treatment of Refractory Functional Dyspepsia (FD). Gastroenterology. 2011;140(5):S-50. [ Links ]
21. Moayyedi P, Shelly S, Deeks JJ, Delaney B, Innes M, Forman D. WITHDRAWN: Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2011;(2):CD001960. [ Links ]
22. Lacy BE, Talley NJ, Locke GR 3rd, Bouras EP, DiBaise JK, El-Serag HB, et al. Review article: current treatment options and management of functional dyspepsia. Aliment Pharmacol Ther. 2012;36(1):3-15. [ Links ]
23. Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon ATR, Bazzoli F, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61(5):646-64. [ Links ]
24. Otero W. , Trespalacios AA, Otero E. Helicobacter pylori: Tratamiento actual. Un importante reto en gastroenterología. Rev Col Gastroenterol 2009:24:279-292. [ Links ]
25. Hallerbäck BI, Bommelaer G, Bredberg E, Campbell M, Hellblom M, Lauritsen K, et al. Dose finding study of mosapride in functional dyspepsia: a placebo-controlled, randomized study. Aliment Pharmacol Ther. 2002;16(5):959-67. [ Links ]
26. Huang X, Lv B, Zhang S, Fan Y-H, Meng L-N. Itopride therapy for functional dyspepsia: a meta-analysis. World J Gastroenterol WJG. 2012;18(48):7371-7. [ Links ]
27. Serra J. [Levosulpiride in the management of functional dyspepsia and delayed gastric emptying]. Gastroenterol Hepatol. 2010;33(8):586-90. [ Links ]
28. Corazza GR, Tonini M. Levosulpiride for dispepsia and emesis: A review of its pharmacology, efficacy and tolerability. Clin Drug Invest 2000;12:151-62. [ Links ]
29. Mearin F, Rodrigo L, Pérez-Mota A, Balboa A, Jiménez I, Sebastián JJ, et al. Levosulpiride and cisapride in the treatment of dysmotility-like functional dyspepsia: a randomized, double-masked trial. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2004;2(4):301-8. [ Links ]
30. Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta-analyses? Lancet. 1998;352(9128):609-13. [ Links ]
31. Sonnenberg A. Cost-benefit analysis of testing for Helicobacter pylori in dyspeptic subjects. Am J Gastroenterol. 1996;91(9):1773-7. [ Links ]
32. Ford AC, Delaney BC, Forman D, Moayyedi P. Eradication therapy in Helicobacter pylori positive peptic ulcer disease: systematic review and economic analysis. Am J Gastroenterol. 2004;99(9):1833-55. [ Links ]
33. Sánchez-Delgado J, Gené E, Suárez D, García-Iglesias P, Brullet E, Gallach M, et al. Has H. pylori prevalence in bleeding peptic ulcer been underestimated? A meta-regression. Am J Gastroenterol. 2011;106(3):398-405. [ Links ]
34. Quigley EMM, Lacy BE. Overlap of functional dyspepsia and GERD--diagnostic and treatment implications. Nat Rev Gastroenterol Hepatol. 2013;10(3):175-86. [ Links ]
35. Camilleri M, Stanghellini V. Current management strategies and emerging treatments for functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10(3):187-94. [ Links ]
36. Tack J, Masclee A, Heading R, Berstad A, Piessevaux H, Popiela T, et al. A dose-ranging, placebo-controlled, pilot trial of Acotiamide in patients with functional dyspepsia. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2009;21(3):272-80. [ Links ]
37. Tack J, Janssen P. Acotiamide (Z-338, YM443), a new drug for the treatment of functional dyspepsia. Expert Opin Investig Drugs. 2011;20(5):701-12. [ Links ]











 text in
text in