Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.2 Bogotá Apr./June 2014
Probiotics for Specific Treatment of Pain in Irritable Bowel Syndrome: A Review
María Ortiz Lucas, Lic. (1), Aurelio Tobías (2), Pablo Saz Peiró MD. (3), Juan José Sebastián, MD. (4)
(1) BSc and Credential in Biochemistry, Professor in the Faculty of Health Sciences at the Universidad San Jorge in Zaragoza, Spain. Mail: mariaortizlucas@gmail.com
(2) Investigator at IDAEA (Instituto de Diagnóstico Ambiental y Estudios del Agua -Institute of Environmental Assessment and Water Research, CSIC. Barcelona, Spain. Mail: aurelio.tobias@gmail.com
(3) Professor in the Faculty of Medicine at the Universidad de Zaragoza in Zaragoza, Spain. Mail: pablosaz@unizar.es
(4) Professor in the Faculty of Medicine and Chief of Gastroenterology at the Hospital Royo Villanova in Zaragoza, Spain. Mail: jjsebastian@salud.aragon.es
Received: 04-10-13 Accepted: 08-15-14
Abstract
Abdominal pain is one of the least well tolerated symptoms in patients with irritable bowel syndrome (IBS). Studies conducted in recent years suggest that dysbiosis in these patients may be responsible, at least in part, for these symptoms. This literature review indicates that probiotics may be an effective therapy for the relief of pain in patients with IBS and recognizes that the effects of probiotics are specific to the strain used. In this article we review the effect that each strain or mixture of probiotics has for relieving abdominal pain according to published clinical trials and we also discuss possible mechanisms of action. New perspectives are proposed for research to elucidate the mechanisms of probiotic action for relief of abdominal pain in these patients.
Keywords
Probiotics, irritable bowel syndrome, abdominal pain, mechanism of action.
IRRITABLE BOWEL SYNDROME AND MICROBIOTA
Irritable Bowel Syndrome (IBS) is the most prevalent functional digestive disorder. A diagnosis of IBS is established according to the consensual clinical criteria (Rome Criteria) which has changed throughout the years. The last consensus (Rome III, 2006) ratifies that patients with IBS must present: "Recurrent pain or abdominal discomfort at least 3 times a month during the last three months associated with 2 or more of the following elements: improvement with defecation, onset of pain associated with a change in bowel movement frequency, onset of pain associated with a change in appearance of the feces" (1). In recent years, increasing amounts of evidence have suggested the presence of intestinal dysbiosis in these patients, even though there is no uniform alteration in the composition of the microbiota, the group of patients with IBS or the various subtypes of IBS. There are even some authors that suggest categorizing patients into subtypes according to microbiotic profiles (2-7).
THE ACTION MECHANISM OF PROBIOTICS
Probiotics are defined as live microorganisms which provide a health benefit to the host when administered in adequate quantities (8). They have different effects in the host. Modulation of intestinal microbiota by probiotics is attributed to their capacity of transiently colonizing the gastrointestinal tract and releasing antimicrobial elements. Probiotics compete against other pathogens, preventing their replication and weakening their virulence. They also affect the functioning of the intestinal barrier by adhering to intestinal cells and maintaining the integrity and resistance of the epithelial barrier which prevents the enteric pathogens from adhering to the intestinal cells thus preventing their effects. The anti-inflammatory effects of the probiotics have been attributed to the recruitment of immune cells and the activation of the immune response through the alteration of cytokine and chemokine release. Finally, probiotics might play a role in decreasing visceral hypersensitivity. Besides these local effects, probiotics also have the systemic effect of increasing immune protection (9-16).
In the particular case of IBS, probiotics might modify the intestinal microbiota by increasing the amount of beneficial bacteria in the gastrointestinal tract and decreasing bacterial overgrowth in the intestine. This improves functioning of the intestinal barrier by increasing intestinal permeability, inverting the imbalance between pro- and anti-inflammatory cytokines, delaying intestinal transit and modifying visceral hypersensitivity (17-21).
Although the effects of probiotic intake on microbiota have been studied in IBS patients, the studies reach different conclusions. Some authors could not demonstrate any change in intestinal microbiota after administration of probiotics (22-24). Others have found that the microbiota stabilized (25-27). The latter authors considered that stabilization of the microbiota is a positive effect since unstable microbiota have been found more frequently in IBS patients than in healthy patients (28, 29). In contrast, a recently published study has found that a change in microbiota occurred in patients treated with probiotics (30).
The scientific evidence indicates that the action mechanisms of probiotics are specific to the individual species and even to the strain of probiotic. Also, there are multiple effects on the host (9, 11, 21, 31). Abdominal pain, one of the least tolerated symptoms for IBS patients, negatively affects their quality of life (32). The literature suggests that probiotics could be an effective therapy for treating pain in some patients with IBS (33-35), however, what specific species or strains of probiotic are responsible for pain relief in IBS patients?
ANTINOCICEPTIVE EFFECT OF PROBIOTICS
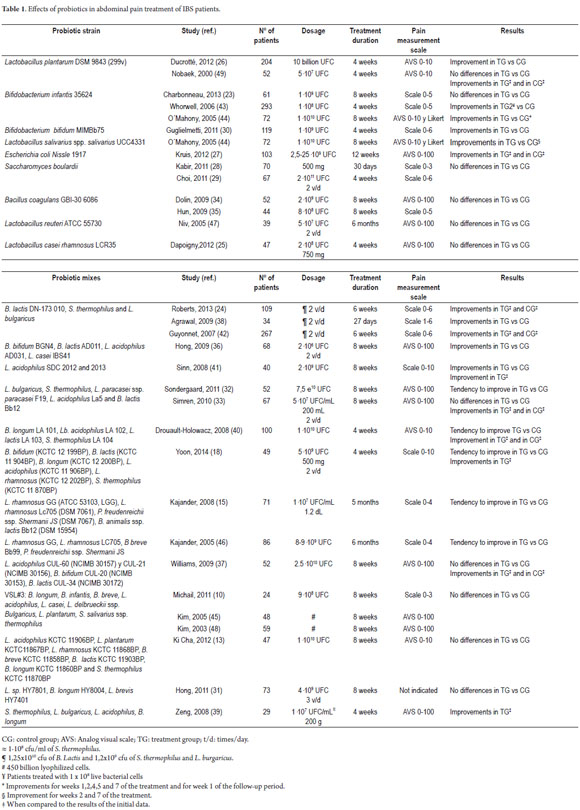
Table 1 summarizes results from random clinical trials on abdominal pain relief resulting from administration of probiotics to adult patients with IBS. Only two of the studies include information on the probiotic strain or the mix of probiotics used in the studies (22, 27, 30, 36-62).
SPECIES OF PROBIOTICS
The effect of the Lactobacillus plantarum straing DSM 9843 (299v) on abdominal pain relief was measured in two studies (39,62). Ducrotté et al. found that the probiotic diminished pain, while Nobaek et al. found improvements at the beginning of treatment in patients given probiotics, but also in patients given placebos. Combinations of probiotics that contained L. plantarum did not produce any greater improvements than did the administration of placebos (22, 25, 58, 61). A study of the administration of this species of probiotic to healthy animals found both decreased pain and an anti-inflammatory effect. (63) This antinociceptive effect was not found when a painful stimulus was applied (64). Upon evaluating this species of probiotic (strain NCIMB8826) and a mutant of the strain which is deficient in D-alanine, it was found that the regulating effect of probiotics in visceral pain perception in the colon could be related to the degree of D-alanylation of membrane-associated lipoteichoic acid. The researchers proposed that anti-inflammatory cytokines could also be involved in decreasing the perception of visceral pain (63, 65). However, the antinociceptive action mechanism seems to be specific to the combination of probiotic strain and target organ (64).
Two studies have found a positive effect of the Bifidobacterium infantis 35624 strain in the treatment of abdominal pain (56, 57). Nevertheless, Charbonneau et al. were unable to confirm this effect (36). Whorwell et al. found that this effect was significant in patients whose predominant symptom was constipation (56). Administration of the VSL#3 mix of probiotics which contains this species provided no additional abdominal pain relief (22, 58, 61). A visceral antinociceptive effect of strain B. infantis 35624 has been found in healthy rats, rats with an anxiety profile and rats with post-inflammatory colon hypersensitivity (66, 67). O'Mahony et al. found positive relief of abdominal pain accompanied by a modulation in immune response through reestablishment of the IL-10/IL-12 ratio in the probiotic group (57). However, the study provides no data on correlations between these two factors. Duncker et al. suggest that there might be a relation between the levels of cytokines and the nociceptive capacity of the nervous system, which could explain the results of O´Mahonny et al (63).
A positive effect of the Bifidobacterium bifidum MIMBb75 strain has been shown in one abdominal pain treatment study (43). Three other studies have employed other strains of B. bifidum as part of probiotic mixes (30, 49, 50). Patients who were treated with a probiotic mix containing the B. bifidum BGN4 also showed improvements in abdominal pain (49). Those treated with a mix containing B. bifidum (KCTC 12 199BP) strain showed a greater tendency to improve (p=0.07) than did the control group (30). In this case, the group treated with probiotics showed a significant improvement at the beginning of the treatment which was not observed in the control group. A group treated with a probiotic mix that included the B. bifidum CUL-20 (NCIMB 30153) strain had results that did not differ from a group treated with placebos (50). In this case, both the probiotic group and the placebo group showed improvements over pretreatment data for each group.
Lactobacillus salivarius ssp. salivarius UCC4331 strain improved abdominal pain in weeks 2 and 7 of the treatment but not at the end of the 8 week study (57). A study of administration of the Escherichia coli Nissle 1917 strain showed improvements in abdominal pain at the beginning of the treatment in both the treatment group and the placebo group (40). There was also an increase in the visceral sensitivity threshold when this strain was administered to rats with post-inflammatory visceral hyperglasia, but not when it was administered to healthy rats (68).
Other clinical studies have looked at the actions of other individual probiotic strains but have found no significant differences in abdominal pain relief. These strains are Saccharomyces boulardii (41,42), Bacillus coagulans GBI-30 6086 (47), Lactobacillus reuteri ATCC 55730 (60) and Lactobacillus casei rhamnosus LCR35 (38). In healthy rats, the Lactobacillus rhamnosus JB-1 strain* decreased visceral hypersensitivity in colorectal and gastric distention. The study showed that this effect has an impact on the dorsal root ganglia at a medullar level by preventing hyper excitability prior to a painful stimulus (65, 69, 70).
PROBIOTIC MIXES
Agrawal et al. have found that the mixture of Bifidobacterium lactis DN-173 010, Streptococcus thermophilus, and Lactobacillus bulgaricus has a positive effect on abdominal pain relief (51). They also found a correlation between abdominal distension and the colonic and oral-fecal transit times when patients were treated with this mix. However, the authors did not provide data regarding the correlation between abdominal pain and transit time. It might be possible that improvements in the distension observed in these patients could be, at least partially, responsible for alleviation of their abdominal pain. Two other studies of the same probiotic mix found improvements in both groups when comparing the results with the data at the beginning of the study (37, 55).
The following probiotic mixes have shown significant effects relieving pain for IBS patients:
1. B. bifidum BGN4, B. lactis AD011, Lactobacillus acidophilus AD031, and Lactobacillus casei IBS41 (49).
2. L. acidophilus SDC 2012 and 2013. In animal studies, Rousseaux et al. have shown decreased visceral pain when inducing opioid and cannabinoid receptors in epithelial rat cells after administering L. acidophilus NCFM (54, 71).
A study that evaluated the mix of L. bulgaricus, S. thermophilus, Lactobacillus paracasei ssp. paracasei F19, L. acidophilus La5, and B. lactis Bb12 found a tendency toward abdominal pain relief (45), but the authors did not provide any data on the value of p. Another study of the same probiotic mix showed no differences between groups (46), but there both the treatment group and the placebo group had decreased intestinal pain and less frequent pain at the beginning of the study.
The following probiotic mixes have shown tendencies to improve abdominal pain in IBS patients:
1. Bifidobacterium longum LA 101, L. acidophilus LA 102, L. lactis LA 103, and S. thermophilus LA 104 (p=0.054) (53). Improvements were found both in the treatment and the placebo group at the beginning of the study for all patients as well as each of the subgroups of IBS patients. In the subgroup of patients with an alternating pattern, significant relief of abdominal pain was found in both groups.
2. B. bifidum KCTC 12 199BP, B. lactis KCTC 11 904BP, B. longum KCTC 12 200BP, L. acidophilus KCTC 11 906BP, L. rhamnosus KCTC 12 202BP, and S. thermophilus KCTC 11 870BP (p=0.07) (30).
3. L. rhamnosus GG (ATCC 53103, LGG), L. rhamnosus Lc705 (DSM 7061), Propionibacterium freudenreichii ssp. shermanii JS (DSM 7067), and B. animalis ssp. lactis Bb12 (DSM 15954) (p=0.052) (27).
4. Lactobacillus rhamnosus GG, L. rhamnosus LC705, Bifidobacterium breve Bb99, and P. freudenreichii ssp. shermanii JS (p=0.110) (59).
The following probiotic mixes have shown no significant pain relief for patients with IBS (See table 1):
1. L. acidophilus CUL-60 (NCIMB 30157) and CUL-21 (NCIMB 30156), B. bifidum CUL-20 (NCIMB 30153), and B. lactis CUL-34 (NCIMB 30172) (50). In this study there was an improvement at the beginning of the study for the group treated with the probiotic, and abdominal pain relief as well as days with pain for the control group.
2. VSL#3 (B. longum, B. infantis, B. breve, L. acidophilus, L. casei, L. delbrueckii ssp. Bulgaricus, L. plantarum, S. salivarius ssp. thermophilus) (22,58,61). However, Dai et al. found decreased visceral hypersensitivity upon administering this probiotic mix in a rat model that simulate IBS with predominant diarrhea (72). These authors suggest that the antinoceptive effect of VSL#3 could be mediated in part by the empowerment of nitric oxide synthesis. That study also found decreased paracellular permeability and increased numbers of proteins involved in tight membrane junctions after administration of the probiotic. The authors found no relation between the inhibition of nitric oxide synthesis and the changes observed in intestinal permeability for which reason they postulate that there is no correlation between these factors. Distrutti et al. have also found that administration of VSL#3 to rats with visceral hypersensitivity and allodynia has an antinociceptive effect. They also found a reversion in the expression of genes that measure pain and inflammation (73). It also seems that this effect is not due to a change in the state of consciousness or to modification of the tone of the colon.
3. L. acidophilus KCTC 11906BP, L. plantarum KCTC11867BP, L. rhamnosus KCTC 11868BP, B. breve KCTC 11858BP, B. lactis KCTC 11903BP, B. longum KCTC 11860BP and S. thermophilus KCTC 11870BP (25).
4. Lactobacillus sp. HY7801, B. longum HY8004, and L. brevis HY7401 (44).
Zeng et al.(52) found that abdominal pain decreased in group of patients to whom a probiotic mix of S., L. bulgaricus, L. acidophilus and B. longum was administered while there was no improvement in the placebo group in a comparison of before and after data for each of the groups individually. However, the study did not show data comparing the two groups. They also suggest that the decreased permeability of the small intestine found after ingestion of the probiotic mix could partially explain alleviation of IBS symptoms including pain. However, no correlation analysis was provided nor was any significant difference between the probiotic and placebo groups shown.
Other animal studies have shown antinociceptive effects for various probiotic strains. Administration of the Lactobacillus paracasei NCC2461 strain has been found to modulate visceral hypersensitivity in mice with hypersensitivity induced by antibiotics in one study, and in two rat models with stress induced hypersensitivity (74, 75). The study of mice also found that the probiotic normalized levels of substance P. The study of rats also found that after administration of the probiotic intestinal permeability normalized in rats subject to maternal separation. These effects were specific to the probiotic strain employed since no effect was found after administration of B. lactis NCC362 or Lactobacillus johnsonii NCC533. A mix of another strain of B. lactis CNCM I-2494 with Lactococcus lactis CNCM I-1631, L. bulgaricus and S. thermophilus decreased visceral sensitivity of mice subject to stress (76). It was also shown that this effect was dosage dependent. The authors of this study found normalization of paracellular permeability which suggests that it could be one of the factors that explain this antinociceptive effect.
Ait-Belgnaoui et al. have found that administration of the Lactobacillus farciminis CIP 103136 probiotic to stressed induced rats has an antinociceptive effect and increases colonic paracellular permeability (77). The release of nitric oxide by the probiotic could have influenced both of these factors. The probiotic may act to modulate intestinal permeability by preventing phosphorylation of the myosin light chain. The contraction of the cytoskeletons of the epithelial cells may lead to an opening of the tight junctions. This could be due to the presence of nitric oxide. However, as mentioned previously, other authors have not found a relation between increased paracellular permeability and the inhibition of nitric oxide synthesis after administration of the VSL#3 probiotic mix (72). A later study in which Lactobacillus farciminis CIP 103136 strain was administered to stressed induced rats observed decreased visceral hypersensitivity through the visceral nociceptive process at the spinal and supraspinal levels and also found decreasing over-expression of Fos proteins at these levels (78).
NEW PERSPECTIVES FOR STUDYING THE EFFECT OF PROBIOTICS IN ABDOMINAL PAIN RELIEF
It is currently accepted that communication between the central nervous system and the gastrointestinal tract is bidirectional, and it has furthermore been postulated that IBS is a multifactorial entity which includes deregulation of the gut-brain axis. To understand the action mechanism of probiotics in abdominal pain relief it will be necessary to clarify the effect of probiotics with antinociceptive effects on the regulation of this axis. Nevertheless, as this review has shown, there has been great progress in this field in the last 10 years.
Future research could look at the effect of certain probiotics in the regulation of different substances, cells and processes that have been found to be correlated to abdominal pain. These include the mastocytes (81-83), the nerve fibers of transient receptor potential vanilloid 1 (84, 85), serotonin (86), brain-derived neurotrophic factor (87), intestinal permeability (88), tight junction union proteins (86, 90), and cellular adhesion (91).
A correlation between abdominal pain and decreased numbers of tight junction JAM-A proteins and occlusion has been found in IBS patients (89, 90). Since probiotic treatment of animals produces decreased visceral hypersensitivity accompanied by reestablishment of tight junction protein levels, this could be one of the factors that explains the action mechanism of probiotics' antinociceptive effect (72-76).
Other possibilities for research include studying the effects of taking probiotics on patients with IBS and how these effects are related to abdominal pain. Among these effects are decreased permeability of the small intestine and normalization of the immune response (52, 57). Finally, the chronicity of abdominal pain makes it necessary to study the effects of probiotics on the central neural mechanism and psychological factors which may be related to modulation of abdominal pain (79, 92-94). The first steps are already being taken in this direction (70, 78).
A series of probiotic strains including L. plantarum DSM 9843 (299v), B. infantis 35624 and B. bifidum MIMBb75 and three mixes of probiotics (B. lactis DN-173 010, S. thermophilus and L. bulgaricus; B. bifidum BGN4, B. lactis AD011, L. acidophilus AD031 and L. casei IBS41, and L. acidophilus SDC 2012 and 2013) have shown apparent antinociceptive effects and apparent abdominal pain relief in certain subgroups of patients with IBS. Perhaps, in the not so distant future they will be used to develop master formulas to treat this serious symptom which afflicts some patients with this functional disorder.
REFERENCES
1. Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130(5):1480-91. [ Links ]
2. Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2012;24(6):521-530, e248. [ Links ]
3. Chassard C, Dapoigny M, Scott KP, Crouzet L, Del'homme C, Marquet P, et al. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35(7):828-38. [ Links ]
4. Jeffery IB, O'Toole PW, öhman L, Claesson MJ, Deane J, Quigley EMM, et al. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61(7):997-1006. [ Links ]
5. 2012 Probiotics_NEW FINAL_sp - 2012 Probiotics_NEW FINAL_sp.pdf [Internet]. [citado 12 de junio de 2014]. Recuperado a partir de: http://www.worldgastroenterology.org/assets/export/userfiles/2012%20Probiotics_NEW%20FINAL_sp.pdf [ Links ]
6. Rowland I, Capurso L, Collins K, Cummings J, Delzenne N, Goulet O, et al. Current level of consensus on probiotic science--report of an expert meeting--London, 23 November 2009. Gut Microbes. 2010;1(6):436-9. [ Links ]
7. Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC. Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis. 2009;15(2):300-10. [ Links ]
8. Dai C, Zheng C-Q, Jiang M, Ma X-Y, Jiang L-J. Probiotics and irritable bowel syndrome. World J Gastroenterol WJG. 2013;19(36):5973-80. [ Links ]
9. Barbara G, Zecchi L, Barbaro R, Cremon C, Bellacosa L, Marcellini M, et al. Mucosal permeability and immune activation as potential therapeutic targets of probiotics in irritable bowel syndrome. J Clin Gastroenterol. 2012;46 Suppl:S52-55. [ Links ]
10. Michail S, Kenche H. Gut microbiota is not modified by Randomized, Double-blind, Placebo-controlled Trial of VSL#3 in Diarrhea-predominant Irritable Bowel Syndrome. Probiotics Antimicrob Proteins. 2011;3(1):1-7. [ Links ]
11. Kajander K, Krogius-Kurikka L, Rinttilä T, Karjalainen H, Palva A, Korpela R. Effects of multispecies probiotic supplementation on intestinal microbiota in irritable bowel syndrome. Aliment Pharmacol Ther. 2007;26(3):463-73. [ Links ]
12. Charbonneau D, Gibb RD, Quigley EMM. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes. 2013;4(3):201-11. [ Links ]
13. Ki Cha B, Mun Jung S, Hwan Choi C, Song I-D, Woong Lee H, Joon Kim H, et al. The effect of a multispecies probiotic mixture on the symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Clin Gastroenterol. 2012;46(3):220-7. [ Links ]
14. Lyra A, Krogius-Kurikka L, Nikkilä J, Malinen E, Kajander K, Kurikka K, et al. Effect of a multispecies probiotic supplement on quantity of irritable bowel syndrome-related intestinal microbial phylotypes. BMC Gastroenterol. 2010;10:110. [ Links ]
15. Kajander K, Myllyluoma E, Rajilić-Stojanović M, Kyrönpalo S, Rasmussen M, Järvenpää S, et al. Clinical trial: multispecies probiotic supplementation alleviates the symptoms of irritable bowel syndrome and stabilizes intestinal microbiota. Aliment Pharmacol Ther. 2008;27(1):48-57. [ Links ]
16. Maukonen J, Satokari R, Mättö J, Söderlund H, Mattila-Sandholm T, Saarela M. Prevalence and temporal stability of selected clostridial groups in irritable bowel syndrome in relation to predominant faecal bacteria. J Med Microbiol. 2006;55(Pt 5):625-33. [ Links ]
17. Mättö J, Maunuksela L, Kajander K, Palva A, Korpela R, Kassinen A, et al. Composition and temporal stability of gastrointestinal microbiota in irritable bowel syndrome--a longitudinal study in IBS and control subjects. FEMS Immunol Med Microbiol. 2005;43(2):213-22. [ Links ]
18. Yoon JS, Sohn W, Lee OY, Lee SP, Lee KN, Jun DW, et al. Effect of multispecies probiotics on irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. J Gastroenterol Hepatol. 2014;29(1):52-9. [ Links ]
19. Spiegel B, Strickland A, Naliboff BD, Mayer EA, Chang L. Predictors of patient-assessed illness severity in irritable bowel syndrome. Am J Gastroenterol. 2008;103(10):2536-43. [ Links ]
20. Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, et al. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325-32. [ Links ]
21. Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P. A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol. 2009;9:15. [ Links ]
22. McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol WJG. 2008;14(17):2650-61. [ Links ]
23. Charbonneau D, Gibb RD, Quigley EMM. Fecal excretion of Bifidobacterium infantis 35624 and changes in fecal microbiota after eight weeks of oral supplementation with encapsulated probiotic. Gut Microbes. 2013;4(3):201-11. [ Links ]
24. Roberts LM, McCahon D, Holder R, Wilson S, Hobbs FDR. A randomised controlled trial of a probiotic «functional food» in the management of irritable bowel syndrome. BMC Gastroenterol. 2013;13:45. [ Links ]
25. Dapoigny M, Piche T, Ducrotte P, Lunaud B, Cardot J-M, Bernalier-Donadille A. Efficacy and safety profile of LCR35 complete freeze-dried culture in irritable bowel syndrome: a randomized, double-blind study. World J Gastroenterol WJG. 2012;18(17):2067-75. [ Links ]
26. Ducrotté P, Sawant P, Jayanthi V. Clinical trial: Lactobacillus plantarum 299v (DSM 9843) improves symptoms of irritable bowel syndrome. World J Gastroenterol WJG. 2012;18(30):4012-8. [ Links ]
27. Kruis W, Chrubasik S, Boehm S, Stange C, Schulze J. A double-blind placebo-controlled trial to study therapeutic effects of probiotic Escherichia coli Nissle 1917 in subgroups of patients with irritable bowel syndrome. Int J Colorectal Dis. 2012;27(4):467-74. [ Links ]
28. Kabir MA, Ishaque SM, Ali MS, Mahmuduzzaman M, Hasan M. Role of Saccharomyces boulardii in diarrhea predominant irritable bowel syndrome. Mymensingh Med J MMJ. 2011;20(3):397-401. [ Links ]
29. Choi CH, Jo SY, Park HJ, Chang SK, Byeon J-S, Myung S-J. A randomized, double-blind, placebo-controlled multicenter trial of saccharomyces boulardii in irritable bowel syndrome: effect on quality of life. J Clin Gastroenterol. 2011;45(8):679-83. [ Links ]
30. Guglielmetti S, Mora D, Gschwender M, Popp K. Randomised clinical trial: Bifidobacterium bifidum MIMBb75 significantly alleviates irritable bowel syndrome and improves quality of life--a double-blind, placebo-controlled study. Aliment Pharmacol Ther. 2011;33(10):1123-32. [ Links ]
31. Hong Y-S, Hong KS, Park M-H, Ahn Y-T, Lee J-H, Huh C-S, et al. Metabonomic understanding of probiotic effects in humans with irritable bowel syndrome. J Clin Gastroenterol. 2011;45(5):415-25. [ Links ]
32. Søndergaard B, Olsson J, Ohlson K, Svensson U, Bytzer P, Ekesbo R. Effects of probiotic fermented milk on symptoms and intestinal flora in patients with irritable bowel syndrome: a randomized, placebo-controlled trial. Scand J Gastroenterol. 2011;46(6):663-72. [ Links ]
33. Simrén M, Ohman L, Olsson J, Svensson U, Ohlson K, Posserud I, et al. Clinical trial: the effects of a fermented milk containing three probiotic bacteria in patients with irritable bowel syndrome - a randomized, double-blind, controlled study. Aliment Pharmacol Ther. 2010;31(2):218-27. [ Links ]
34. Dolin BJ. Effects of a proprietary Bacillus coagulans preparation on symptoms of diarrhea-predominant irritable bowel syndrome. Methods Find Exp Clin Pharmacol. 2009;31(10):655-9. [ Links ]
35. Hun L. Bacillus coagulans significantly improved abdominal pain and bloating in patients with IBS. Postgrad Med. 2009;121(2):119-24. [ Links ]
36. Hong KS, Kang HW, Im JP, Ji GE, Kim SG, Jung HC, et al. Effect of probiotics on symptoms in korean adults with irritable bowel syndrome. Gut Liver. 2009;3(2):101-7. [ Links ]
37. Williams EA, Stimpson J, Wang D, Plummer S, Garaiova I, Barker ME, et al. Clinical trial: a multistrain probiotic preparation significantly reduces symptoms of irritable bowel syndrome in a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2009;29(1):97-103. [ Links ]
38. Agrawal A, Houghton LA, Morris J, Reilly B, Guyonnet D, Goupil Feuillerat N, et al. Clinical trial: the effects of a fermented milk product containing Bifidobacterium lactis DN-173 010 on abdominal distension and gastrointestinal transit in irritable bowel syndrome with constipation. Aliment Pharmacol Ther. 2009;29(1):104-14. [ Links ]
39. Zeng J, Li Y-Q, Zuo X-L, Zhen Y-B, Yang J, Liu C-H. Clinical trial: effect of active lactic acid bacteria on mucosal barrier function in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2008;28(8):994-1002. [ Links ]
40. Drouault-Holowacz S, Bieuvelet S, Burckel A, Cazaubiel M, Dray X, Marteau P. A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroentérologie Clin Biol. 2008;32(2):147-52. [ Links ]
41. Sinn DH, Song JH, Kim HJ, Lee JH, Son HJ, Chang DK, et al. Therapeutic effect of Lactobacillus acidophilus-SDC 2012, 2013 in patients with irritable bowel syndrome. Dig Dis Sci. 2008;53(10):2714-8. [ Links ]
42. Guyonnet D, Chassany O, Ducrotte P, Picard C, Mouret M, Mercier C-H, et al. Effect of a fermented milk containing Bifidobacterium animalis DN-173 010 on the health-related quality of life and symptoms in irritable bowel syndrome in adults in primary care: a multicentre, randomized, double-blind, controlled trial. Aliment Pharmacol Ther. 2007;26(3):475-86. [ Links ]
43. Whorwell PJ, Altringer L, Morel J, Bond Y, Charbonneau D, O'Mahony L, et al. Efficacy of an encapsulated probiotic Bifidobacterium infantis 35624 in women with irritable bowel syndrome. Am J Gastroenterol. 2006;101(7):1581-90. [ Links ]
44. O'Mahony L, McCarthy J, Kelly P, Hurley G, Luo F, Chen K, et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: symptom responses and relationship to cytokine profiles. Gastroenterology. 2005;128(3):541-51. [ Links ]
45. Kim HJ, Vazquez Roque MI, Camilleri M, Stephens D, Burton DD, Baxter K, et al. A randomized controlled trial of a probiotic combination VSL# 3 and placebo in irritable bowel syndrome with bloating. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2005;17(5):687-96. [ Links ]
46. Kajander K, Hatakka K, Poussa T, Färkkilä M, Korpela R. A probiotic mixture alleviates symptoms in irritable bowel syndrome patients: a controlled 6-month intervention. Aliment Pharmacol Ther. 2005;22(5):387-94. [ Links ]
47. Niv E, Naftali T, Hallak R, Vaisman N. The efficacy of Lactobacillus reuteri ATCC 55730 in the treatment of patients with irritable bowel syndrome--a double blind, placebo-controlled, randomized study. Clin Nutr Edinb Scotl. 2005;24(6):925-31. [ Links ]
48. Kim HJ, Camilleri M, McKinzie S, Lempke MB, Burton DD, Thomforde GM, et al. A randomized controlled trial of a probiotic, VSL#3, on gut transit and symptoms in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2003;17(7):895-904. [ Links ]
49. Nobaek S, Johansson ML, Molin G, Ahrné S, Jeppsson B. Alteration of intestinal microflora is associated with reduction in abdominal bloating and pain in patients with irritable bowel syndrome. Am J Gastroenterol. 2000;95(5):1231-8. [ Links ]
50. Duncker SC, Wang L, Hols P, Bienenstock J. The D-alanine content of lipoteichoic acid is crucial for Lactobacillus plantarum-mediated protection from visceral pain perception in a rat colorectal distension model. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2008;20(7):843-50. [ Links ]
51. Duncker SC, Kamiya T, Wang L, Yang P, Bienenstock J. Probiotic Lactobacillus reuteri alleviates the response to gastric distension in rats. J Nutr. 2011;141(10):1813-8. [ Links ]
52. Grangette C, Nutten S, Palumbo E, Morath S, Hermann C, Dewulf J, et al. Enhanced antiinflammatory capacity of a Lactobacillus plantarum mutant synthesizing modified teichoic acids. Proc Natl Acad Sci U S A. 2005;102(29):10321-6. [ Links ]
53. Johnson AC, Greenwood-Van Meerveld B, McRorie J. Effects of Bifidobacterium infantis 35624 on post-inflammatory visceral hypersensitivity in the rat. Dig Dis Sci. 2011;56(11):3179-86. [ Links ]
54. McKernan DP, Fitzgerald P, Dinan TG, Cryan JF. The probiotic Bifidobacterium infantis 35624 displays visceral antinociceptive effects in the rat. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2010;22(9):1029-1035, e268. [ Links ]
55. Liebregts T, Adam B, Bertel A, Jones S, Schulze J, Enders C, et al. Effect of E. coli Nissle 1917 on post-inflammatory visceral sensory function in a rat model. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2005;17(3):410-4. [ Links ]
56. Kamiya T, Wang L, Forsythe P, Goettsche G, Mao Y, Wang Y, et al. Inhibitory effects of Lactobacillus reuteri on visceral pain induced by colorectal distension in Sprague-Dawley rats. Gut. 2006;55(2):191-6. [ Links ]
57. Ma X, Mao Y-K, Wang B, Huizinga JD, Bienenstock J, Kunze W. Lactobacillus reuteri ingestion prevents hyperexcitability of colonic DRG neurons induced by noxious stimuli. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G868-875. [ Links ]
58. Rousseaux C, Thuru X, Gelot A, Barnich N, Neut C, Dubuquoy L, et al. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13(1):35-7. [ Links ]
59. Dai C, Guandalini S, Zhao D-H, Jiang M. Antinociceptive effect of VSL#3 on visceral hypersensitivity in a rat model of irritable bowel syndrome: a possible action through nitric oxide pathway and enhance barrier function. Mol Cell Biochem. 2012;362(1-2):43-53. [ Links ]
60. Distrutti E, Cipriani S, Mencarelli A, Renga B, Fiorucci S. Probiotics VSL#3 protect against development of visceral pain in murine model of irritable bowel syndrome. PloS One. 2013;8(5):e63893. [ Links ]
61. Verdú EF, Bercik P, Verma-Gandhu M, Huang X-X, Blennerhassett P, Jackson W, et al. Specific probiotic therapy attenuates antibiotic induced visceral hypersensitivity in mice. Gut. 2006;55(2):182-90. [ Links ]
62. Eutamene H, Lamine F, Chabo C, Theodorou V, Rochat F, Bergonzelli GE, et al. Synergy between Lactobacillus paracasei and its bacterial products to counteract stress-induced gut permeability and sensitivity increase in rats. J Nutr. 2007;137(8):1901-7. [ Links ]
63. Agostini S, Goubern M, Tondereau V, Salvador-Cartier C, Bezirard V, Lévèque M, et al. A marketed fermented dairy product containing Bifidobacterium lactis CNCM I-2494 suppresses gut hypersensitivity and colonic barrier disruption induced by acute stress in rats. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2012;24(4):376-e172. [ Links ]
64. Ait-Belgnaoui A, Han W, Lamine F, Eutamene H, Fioramonti J, Bueno L, et al. Lactobacillus farciminis treatment suppresses stress induced visceral hypersensitivity: a possible action through interaction with epithelial cell cytoskeleton contraction. Gut. 2006;55(8):1090-4. [ Links ]
65. Ait-Belgnaoui A, Eutamene H, Houdeau E, Bueno L, Fioramonti J, Theodorou V. Lactobacillus farciminis treatment attenuates stress-induced overexpression of Fos protein in spinal and supraspinal sites after colorectal distension in rats. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2009;21(5):567-573, e18-19. [ Links ]
66. Elsenbruch S. Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms. Brain Behav Immun. 2011;25(3):386-94. [ Links ]
67. Barbara G, Stanghellini V, De Giorgio R, Cremon C, Cottrell GS, Santini D, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693-702. [ Links ]
68. Keszthelyi D, Troost FJ, Jonkers DM, Helyes Z, Hamer HM, Ludidi S, et al. Alterations in mucosal neuropeptides in patients with irritable bowel syndrome and ulcerative colitis in remission: a role in pain symptom generation? Eur J Pain Lond Engl. 2013;17(9):1299-306. [ Links ]
69. Cremon C, Carini G, Wang B, Vasina V, Cogliandro RF, De Giorgio R, et al. Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol. 2011;106(7):1290-8. [ Links ]
70. Yu Y-B, Zuo X-L, Zhao Q-J, Chen F-X, Yang J, Dong Y-Y, et al. Brain-derived neurotrophic factor contributes to abdominal pain in irritable bowel syndrome. Gut. 2012;61(5):685-94. [ Links ]
71. Piche T, Barbara G, Aubert P, Bruley des Varannes S, Dainese R, Nano JL, et al. Impaired intestinal barrier integrity in the colon of patients with irritable bowel syndrome: involvement of soluble mediators. Gut. 2009;58(2):196-201. [ Links ]
72. Wilcz-Villega EM, McClean S, O'Sullivan MA. Mast cell tryptase reduces junctional adhesion molecule-A (JAM-A) expression in intestinal epithelial cells: implications for the mechanisms of barrier dysfunction in irritable bowel syndrome. Am J Gastroenterol. 2013;108(7):1140-51. [ Links ]
73. Bertiaux-Vandaële N, Youmba SB, Belmonte L, Lecleire S, Antonietti M, Gourcerol G, et al. The expression and the cellular distribution of the tight junction proteins are altered in irritable bowel syndrome patients with differences according to the disease subtype. Am J Gastroenterol. 2011;106(12):2165-73. [ Links ]
74. Wilcz-Villega E, McClean S, O'Sullivan M. Reduced E-cadherin expression is associated with abdominal pain and symptom duration in a study of alternating and diarrhea predominant IBS. Neurogastroenterol Motil Off J Eur Gastrointest Motil Soc. 2014;26(3):316-25. [ Links ]











 text in
text in