Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.3 Bogotá Sept. 2014
Antibiotic Resistance of Helicobacter pylori in Latin America and the Caribbean
Julián David Martínez M, MD. (1), Sandra Consuelo Henao R. MD. (2), Jorge Iván Lizarazo R. MD. (3)
(1) Gastroenterologist, Associate Professor in the Department of Medicine at the Universidad Nacional de Colombia and member of the Gastroenterology Unit at the Hospital Universitario de La Samaritana in Bogotá, Colombia.
(2) Associate Professor in the Department of Microbiology at the Universidad Nacional de Colombia in Bogotá, Colombia.
(3) Gastroenterologist and member of the Gastroenterology Unit of the Hospital Universitario de La Samaritana in Bogotá, Colombia.
Received: 30-07-13 Accepted: 21-07-14
Abstract
Treatment of Helicobacter pylori infections remains an unsolved problem in clinical practice because of the high percentage of failures to eradicate the bacteria. The main cause is that antibiotic resistance varies with the geographical area under study. Several techniques have been developed to study the bacterias sensitivity in vitro, but they are difficult to use in clinical practice, and they are costly. It is also important to recognize that sensitivity in vitro is not always directly related to sensitivity in vivo. Nevertheless, these techniques can help improve the results of eradication. This article reviews the mechanisms of resistance and reviews articles published in Latin America on in vitro resistance to the antibiotics most frequently used in eradication schemes. The review found 35 studies in Latin America with a total of 3,358 isolates. Forty-eight percent of the studies used the E-test for sensitivity, 37% used agar dilution, and 8% used agar diffusion. The studies show considerable heterogeneity with important differences among countries in the region and even among studies in the same country. In vitro resistance to metronidazole was 65.7%, in vitro resistance to amoxicillin was 6.5 in vitro resistance to clarithromycin was 14%, in vitro resistance to tetracycline was 8.3% to, in vitro resistance to levofloxacin was 39% and in vitro resistance to furazolidone was 6.9%.
Keywords
Helicobacter pylori, resistance, antibiotics, Latin America.
INTRODUCTION
Helicobacter pylori (H. pylori) infections are considered to be the main cause of chronic gastritis, peptic ulcers, MALT (mucosa-associated lymphoid tissue) lymphomas and gastric adenocarcinoma (1, 2).
Currently, the most commonly used first line schemes for treating these infections are triple therapies consisting of proton pump inhibitors (PPIs), amoxicillin, metronidazole, and clarithromycin in schemes that last from seven to fourteen days. Other schemes that have been proposed include sequential therapy, concomitant therapy and quadruple therapy. In many parts of the world eradication rates for these schemes are considered unacceptable, as are eradication rates for second and third line schemes that include other antibiotics such as levofloxacin. Bacterial resistance is the main cause of the failure of antimicrobial therapy (6, 7). Poor adherence is another cause of eradication failure (3, 4):
The resistance of H. pylori bacteria might be primary (natural) which means that the antibiotic has an intrinsic incapacity to eradicate the infection from the start, or it could be secondary resistance acquired after treatment with an antibiotic to which the bacteria is sensitive.
The resistance developed by H. pylori is essentially due to chromosomal mutations. Resistance is transmitted vertically within a bacterial population from resistant cells to their offspring which results in progressively increasing resistance. Unlike resistance due to chromosomal mutation, resistance resulting from plasmids is rapidly transmitted through horizontal diffusion throughout any bacterial population that acquires it (11, 49). The genes implicated in chromosomal mutations of H pylori have been identified and have a number of specific mutations that can be detected by molecular methods (49).
Lab techniques that detect resistance in vitro are divided into phenotypic methods and genotypic methods. Most publications report the use of phenotypic methods.
There are two groups of phenotypic methods: diffusion and dilution.
Diffusion techniques, including the Kirby-Bauer method, are qualitative tests that do not determine the minimum inhibitory concentration (MIC) to which the microorganism is sensitive. The MIC is defined as the lowest amount of antibiotic that inhibits bacterial growth and is expressed in micrograms of antibiotic per milliliter of culture medium (ug/ml or mg/l). The Etest (previously known as the Epsilometer test), stands out among diffusion techniques because it has a good correlation with the reference method even though it may overestimate resistance of H. pylori to metronidazole (8).
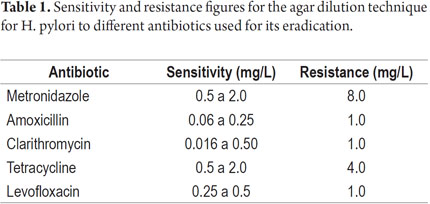
Unlike diffusion techniques, dilution techniques determine the MIC. Table 1 shows the MIC data for each antibiotic employed in treatment of H. pylori infections (9, 10).
Dilution in agar is the most widely used technique and is considered to be the gold standard by the Clinical and Laboratory Standards Institute (CLSI). The interpretation of the MIC of each antibiotic must be compared to cut-off points determined by international committees.
Two genotypic methods are used for resistance detection: polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) molecular tests. Both can be applied in samples of the gastric mucosa or samples of human feces. These methods establish resistance more quickly because of their capacity to determine specific changes in the bacterial genome that provide antibiotic resistance.
This review presents studies published in Latin America and the Caribbean about the resistance of H. pylori found through the use of different in vitro technique. We consider it important to understand the regional situation since it is a fact that resistance varies in different regions of the world.
MATERIALS AND METHODS
Searches of scientific publications in Latin American countries and the Caribbean were done in MEDLINE, BIREME, LILACS and SCIELO databases. Searches targeted articles in Spanish, Portuguese and English about in vitro resistance of H. pylori to the antibiotics most widely used in treatment schemes.
The publications were organized by publication date, country, number of samples, lab technique and antibiotic. The search period was from 1995 and 2002.
RESULTS
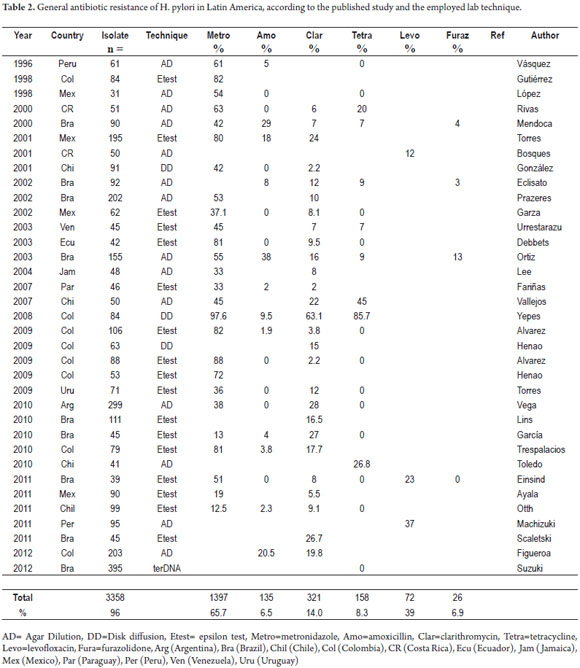
There were 35 published articles found in these data bases that reported tests on a total of 3,358 samples. Of these 3,262 came from adults, and 96 came from children (12-46). The number of samples per study ranged from 395 to 18 with an average of 96 samples.
There were nine articles from Brazil, eight from Colombia, five from Mexico, four from Chile, and one each from Peru, Costa Rica, Ecuador, Jamaica, Paraguay, Uruguay and Venezuela.
The Etest was used in 17 studies (48%), agar dilution in 14 studies (37%) and disc diffusion in three (18%).
The resistance mechanisms and the results from these Latin American countries are presented below.
Metronidazole
Metronidazole is a heterocyclic compound with a five carbon nuclei and one nitro radical (NO2). Nitroimidazoles are activated when they enter into a cell with the help of the NADPH nitroreductase enzyme. Within the cell they are transformed into imidazole intermediaries which cause structural damage to the DNA, inhibit acid synthesis, and lead to cell death (47).
Mutations involving stop codons or substitutions inactivate the rdxA gene which normally encodes oxygen-insensitive NADPH nitroreductase. In some cases, the frxA gene promotes resistance and in other cases it also presents mutations along with rdxA (48).
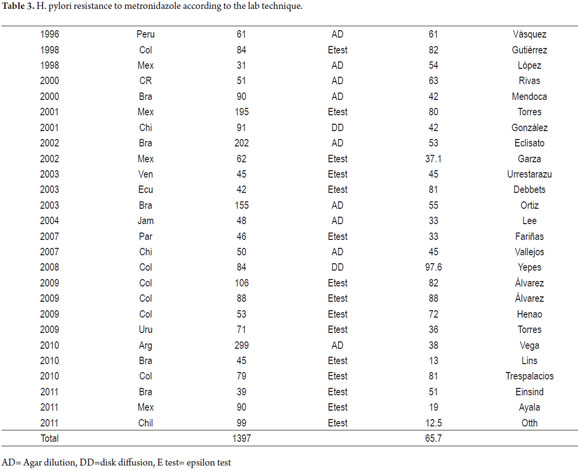
Twenty-six of the studies found in Latin America were for metronidazole. They reported on 2,263 isolate: 2,212 from adults and 51 from children (12-30, 32, 35, 37, 38, 40-42). The prevalences reported range from 97.6% in Colombia (29) to 12.5% in Chile (42). There are also notable differences within countries and within sub-regions.
The prevalence of resistance in three out of four studies, from Mexico and Central America was greater than 50%. In the study from Jamaica it was 33%. The Brazilian studies all reported more than 50%. In Colombia, the prevalence of resistance ranged from 82% to 97.6% (13, 29, 30, 32, 33, 38).
In the south of Latin America the prevalence of resistance reported were 38% in Argentina, 12% in Chile, 33% in Paraguay and 38% in Uruguay (32, 41, 27, 34).
Table 2 presents a summary of these studies.
The prevalence of the resistance published for metronidazole in developed countries shows prevalence of resistance in Europe of 20%, 40% in the USA, and 12% in Japan.
Resistance to metronidazole has been related to its frequent use to treat parasitic diseases and dental infections and to treat gynecological infections in women (table 3)(49, 50).
Amoxicillin
Amoxicillin is a semi-synthetic penicillin that inhibits the synthesis of the bacterial wall which creates an osmotic imbalance and bacterial lysis. The bactericidal action of amoxicillin requires the bacteria to be in its replication phase. Penicillin binding proteins located in the surface of the cellular membrane allow the beta lactam ring contained in the Amoxicillin to block synthesis of the peptidoglycan of the cellular wall. Since amoxicillin is labile to acid, PPIss improve its stability and bio-availability (47).
PBP mutations in the bacteria lead to resistance. The PBP1A mutation presents a greater affinity for the medication and affects its antibiotic effectiveness when manifested. The S414R mutation can create resistant phenotypes by itself. Other mutations that have been reported are S402G, E406A, S417T, T555S, N561Y, S542R, T540I and I562V (51, 52).
Twenty Latin Americas articles about Amoxicillin were found. In total, they reported on 2076 isolates of which 51 came from children. The prevalence of resistance varies from 0% to 38% (25). The highest prevalences of resistance were reported in Brazil (29% and 38%), (16, 25) followed by Colombia (20.5% and 9.5%) and Mexico (18%) (29, 45, 12). In contrast to these results, no resistant strains were found in nine studies which makes it hard to draw conclusions about whether or not resistance to amoxicillin has increased in the region (14, 15, 19, 22, 24, 32, 34, 35, 40).
The high prevalences of resistance found also contrast with prevalences reported in publications from other regions of the world. Megraud has reported prevalences ranging between 0.0% and =.9% from 18 studies in Europe, Asia, the USA and the Middle East.
In Taiwan, an amoxicillin resistant strain with the blaTEM1 gene has been detected. This gene codes for TEM-1 which is a beta lactamase, the class of enzymes responsible for most resistance to penicillin and penicillin derivatives. The MIC of this strain is 256 mg/l (53).
Clarithromycin
Clarithromycin is a synthetic macrolide that inhibits the synthesis of bacterial proteins by binding to 50S ribosomal sub-units where it inhibits the translocation of transfer RNA used in the synthesis of polypeptides.
Resistance to clarithromycin is due to a single point mutation in 23S rRNA. The mutations responsible for 90% of the cases of resistance are produced because of the substitution of adenine by cytosine or guanine in position 2142 (A2142C or A2142G) or by an adenine for a guanine in position 2143 (A2143G). Other mutations such as A2143G are less frequent.
The in vitro frequency of these mutation fluctuates between 3.2 X 10-7 to 6.0 X 10-8, but in vitro results do not always predict in vivo results because in vivo results may be greater due to oxidative stress.
Another resistance mechanism that has been described is the cellular efflux systems. It has been proposed that multiple drug resistance is intrinsic to efflux systems. PPIs may inhibit the activity of bacterial efflux pumps, and their use has decreased MIC values for clarithromycin, metronidazole, amoxicillin and furazolidone in multi-resistant isolate. 27 studies were found in Latin America with a total of 2292 H. pylori isolate.
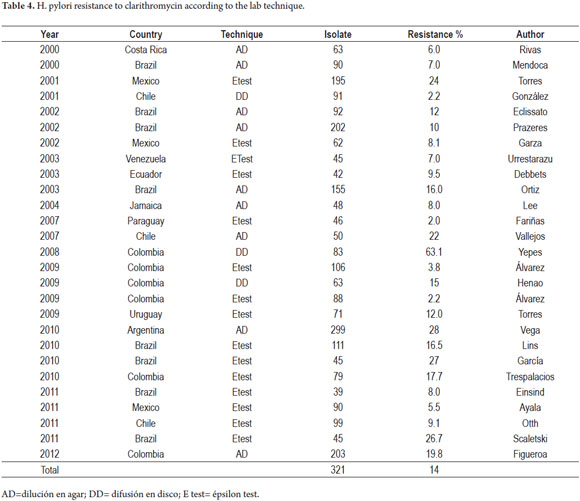
We found a total of 27 Latin American studies that discussed Clarithromycin resistance and a total number of isolate studied of 2,292. The highest prevalences were observed in Mexico, Colombia, Argentina and Brazil (17, 29, 35, 44).
Several studies using phenotypic and genotypic lab techniques have been published in Colombia. The first publication in 2008 used a disc diffusion technique. It reported 63.1% prevalence of resistance. This percentage is considered to be too high, and only one other study, published in Peru in 199, had 50% prevalence of resistance. These high percentages have not been found in more recent studies. Another Colombian study done in the same geographical region in 2009, and which also used a disc diffusion technique, reported a 15% prevalence resistance (12, 29, 31).
Table 4 summarizes the 27 studies of in vitro resistance to Clarithromycin.
Tetracycline
Tetracyclines inhibit bacterial protein synthesis by bonding to ribosome subunit 30S where they inhibit amino acid binding in the process of polypeptide chain formation.
A single point mutation at the rRNA 16S gene is responsible for tetracycline resistance. This gene codes for ribosomal ribonucleic acid 16S. Mutations prevent the binding of aminoacylated transfer RNAs to ribosomal sites. Mutations are found in the base triplet adenine, guanine, adenine in positions 926 to 928 (AGA 926-928) of the rRNA 16S gene in the so-called C box. Mutations in positions 965 to 967 that code for flow protein TetA (p) decrease the affinity of the antibiotic (6, 10, 54).
Twenty Latin American studies discussing tetracycline resistance were found. They reported results for a total of 1,903 isolates. In the studies performed with the agar dilution technique in Brazil between the years 2000 and 2003, the resistance varied from 0% to 9%; in Argentina, Peru and Mexico the resistance was 0%. In Chile, the resistance was 26.8% found an unusually high resistance.
The studies performed with the Etest technique in Colombia, Chile, Ecuador and Uruguay found 0% resistance, but one study using the Etest technique in Venezuela found 7%.
In Colombia, a study performed with the disk diffusion technique with 115 isolates showed resistance in 85.7% of the samples, but this was the only study performed (12, 14, 16, 20, 23-25, 28-30, 32, 34, 35, 42).
Levofloxacin
Fluoroquinolones act by inhibiting bacterial topoisomerases. Topoisomerase IV and DNA gyrase are Type II topoisomerases which consist of two polypeptide subunits, gyrA and gyrB encoded by the gyrA and gyrB genes. DNA gyrase helps remove the DNAs supercoiling at the beginning of replication and maintains the DNAs helical structure. The quinolones stop replication of DNA by inhibiting subunit A of DNA gyrase in the region that determines the resistance to quinolones QRDR (Quinolone Resistance Determining Region). Mutations in the gyRA gene at positions 91 (Asp91Gly, Asn, Ala o Tyr), 87 (Asn87Lys) and 88 (Ala88Val) are in this region. They create resistance to levofloxacin. Mutations in positions 91 and 87 have been found to be present in 100% of the resistant isolates (56). Three publications, one each from from Costa Rica, Brazil and Peru, were found. The highest prevalence of resistance was 37% in Peru (18, 40, 43).
In Europe, two studies in France found prevalence of resistance to be 3.8% and 3.3%. One study in Holland found prevalence of resistance at 4.7%, but one in Portugal found prevalence of resistance to be 20.9% which is the highest level of resistance rate in Europe. The resistance is crossed with other quinolones (42, 55).
In Taiwan, resistance has quadrupled from 3.2% in 2004 to 16.3%. In Europe, the average resistance reported in 2009 was 14.1% (4% to 28%) (5).
Furazolidone
Furazolidone is a synthetic medication derived from furan. Furazolidones action mechanisms intervenes with the enzymatic systems of bacteria by inhibiting monoamine oxidase and decreasing bacterial processes of oxidation. Its secondary metabolites damage RNA. Furazolidone has been used as an alternative antibacterial when high rates of metronidazole resistance have been observed (20).
Mechanisms of resistance to furazolidone are not clear. The bacteria use reductases other than those related to the metronidazole (RdxA, FrxB, FdxB) resistance. Two potential reductases are pyruvate-flavodoxin oxidoreductase and the 2-oxoglutarate reductase which may be essential enzymes for bacteria. This might explain the low mutation rate and therefore the low resistance rate to furazolidone reported in most studies.
There are few studies of furazolidone resistance in Latin America, and most of these have been done in Brazil. Prevalence of resistance was reported at 4% in 2000 and 7.5% in 2002. A study by Queiroz and Colsno in the state of Sao Paulo in 2002 reported no resistant isolates. Godoy Ortiz and collaborators found a prevalence of resistance of 13% in 2003. This is double the prevalence found in other studies performed in the same geographic region (16, 58).
DISCUSSION
The antibiotic resistance of H. pylori is a growing problem worldwide: it is the main cause of eradication failure of all current schemes. Resistances vary geographically, and in vitro studies of resistance are complex and expensive which limits the possibility of performing these studies in clinical practice. The genes compromised by chromosomal mutations that create most of the resistance to antibiotics are transmitted vertically to their offspring in bacterial populations. Most are single point mutations which can be detected by molecular methods based on real time PCR techniques. In the future, these techniques may become more available in clinical practice. This could allow more focused treatment of an infection whose eradication continues to be an unsolved problem.
Since most studies come from other regions in the world, we considered studies in Latin America in the Caribbean that show our continents situation to be especially important. This review spanned a 16 year period and included publications in three different languages. The fact that thirty-five published studies were found shows that the region is not foreign to study of antibiotic resistance.
Resistance to clarithromycin is currently considered to have the greatest impact on eradication failure. The twenty-seven studies found in Latin America reported prevalences of resistance ranging from 2.0% to 63.1% although it should be noted that the results of most studies do not support these extreme percentages.
The most important risk factor for the development of resistance to clarithromycin is previous consumption of macrolides. Increased resistance in children has been related to the frequent use of these antibiotics for respiratory infections (49). The use of clarithromycin must be considered solely for patients who have not been treated previous with macrolides.
The most promising method for detecting clarithromycin resistance is real time PCR detection based on the amplification of mutant gene 23S rRNA.
The 26 studies in Latin America of in vitro resistance to metronidazole also showed varying results that ranged from 97.6% in Colombia (29) to 12.5% in Chile (42). Other notable differences were found between a regions within countries. Unlike studies of resistance to clarithromycin, in vitro studies of resistance to metronidazole have been questioned because phenotypic methods are limited by a high level of reproducibility of failure with discrepancy percentages of 10% to 20% (49).
In the twenty Latin American studies found for tetracycline, resistance rates varied from 0% to 9% except for one study in Chile with 26.8% and one in Colombia of 85.7% which used the disk diffusion technique. This high percentage has not been reproduced in any other study which could have the effect of causing continued use of tetracycline in the belief that resistance to it is low.
The twenty Latin American studies of amoxicillin found reported resistance rates ranging from 0% to 38%. Low resistance rates have been documented worldwide, and a lot of studies in Latin America found 0% resistance. Nevertheless, despite the wide range of reports, if the high resistance percentages of some studies are confirmed, it would be an alarming situation. This could compromise the use of amoxicillin in most schemes even though it is currently considered fundamental due to its effectiveness and low cost.
The three studies of levofloxacin found in Latin America reported high resistance percentages of 37% and 39%. The small number of studies must be taken into account together with the fact that its use is generally considered only for second line schemes since it is an expensive medication. The exceptions are for patients who have previously used macrolides and in geographical regions where resistance to clarithromycin is higher than 20% and resistance to quinolones is less than 10% (57).
The studies of furazolidone found ranges between 0% and 13%. The scant number of studies were mostly done in Brazil. Furazolidone has emerged as a useful antibacterial for treating H. pylori because it is inexpensive and available almost everywhere on the continent even though only selected cases can benefit from its use. The use of furazolidone has been prohibited in European countries due to findings of possible DNA alteration and to its relation to carcinogenesis.
This review assembles a wide number of publications of in vitro resistance to H. pylori in Latin American and the Caribbean. Many of these publications have not been widely disseminated because they are not in English. These studies show that, similar to other continents, in Latin America there are high levels of resistance to many antibiotics (59). This is alarming because of the high prevalence of H. pylori infections combined with the high prevalences of severe diseases that related to H. pylori infection such as pre-neoplastic lesions and gastric adenocarcinoma. Within Latin America there are profound differences in economic development and in the availability and quality of health services. In addition, marked inequality exists between urban and rural areas. Useful and reasonably priced medications are required. The studies conducted in this region pose a continuous challenge to Latin American doctors and create the need to continue research into ways to improve eradication results in our countries.
Conflict of interest
The authors declare no conflicts of interest.
Financing
The costs of this paper were assumed by the authors.
REFERENCES
1. Malfertheiner P, Megraud F, OMorain CA, Atherton J, Axon ATR, Bazzoli F, et al. The European Helicobacter Study Group (EHSG) Management of Helicobacter pylori infection-the Maastricht IV/ Florence Consensus Report Gut 2012; 61: 646-664. [ Links ]
2. Roesler BM, Botelho Costa SC, Robilotta, Zeitune JM. Eradication and Treatment of Helicobacter pylori Infection: Its Importance and Possible Relationship in Preventing the Development of Gastric Cancer. ISRN Gastroenterology 2012. [ Links ]
3. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010; 59: 1143-1153. [ Links ]
4. Chuah S-K, Tsay F-W, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol 2011; 17: 3971-3975. [ Links ]
5. Kuo CH, Kuo FC, Hu HM, Liu CJ, Wang S, Chen YH, et al. The optimal First –Line Therapy of Helicobacter pylori Infection in Year 2012. Gastroenterology Research and Practice 2012. [ Links ]
6. Megraud F. Antibiotic resistance in Helicobacter pylori infection British Medical Bulletin 1998; 54: 207-216. [ Links ]
7. Vakil N, Megraud F. Eradication therapy for Helicobacter pylori. Gastroenterology 2007; 133: 985-1001. [ Links ]
8. Clinical and Laboratory Standards Institute, Performance standards for antimicrobial susceptibility testing. Seventeenth information al supplement. Wayne, Pennsylvania: Clinical and Laboratory Standards Institute; 2007. [ Links ]
9. Megraud F. Whats the relevance of Helicobacter pylori to antimicrobials agents? En Hunt RH, Tygat GNJ, (Eds.). Helicobacter pylori: Basic mechanisms to clinical cure 1996. Dordrecht; Kluwer 1996; 348-356. [ Links ]
10. Zullo V, Hassan C, Giorgio F, Rosania R, Ierardi E. Mechanisms of Helicobacter pylori antibiotic resistance: An updated appraisal. World J Gastrointest Pathophysiol 2011; 2: 35-41. [ Links ]
11. Pajares García JM, Pajares-Villaroya R, Gilsbert JP. Helicobacter pylori: resistencia a los antibióticos. Revista española de Enfermedades Digestivas 2007. [ Links ]
12. Vásquez A, Valdez Y, Gillman RH, McDonald JJ, Westblon TU, Bergg D, et al. Metronidazole and Clarithromycin Resistance in Helicobacter pylori Determinate by measuring MICs of antimicrobial agents in color indicator egg yolk Agar in a miniwell format. J Clin Microbiology 1996; 34: 1232-1234. [ Links ]
13. Gutiérrez O, Otero W. Resistencia de Helicobacter pylori al metronidazol en Colombia. Rev Col Gastroenterología 1998; 12: 31-5. [ Links ]
14. López V. Y, Rangel F, Sigfrido M, Calva JJ. Resistencia a antimicrobiano de Helicobacter pylori en un centro de referencia infectológico. Rv Invest Clin 1998; 50: 19-24. [ Links ]
15. Rivas F, Rivera P, Hernández F, Hevia F, Guillén F, Ricaamayo G. Antibiótico sensibilidad del Helicobacter pylori mediante microdilución. Rev Med Panamá 2000; 25: 19-23. [ Links ]
16. Mendoca S, Ecclissato C, Sartoti MS, Godoy APO; Guerzoni RA, Dregger M, et al .Prevalence of Helicobacter pylori Resistance to Metronidazole, Clarithromicyn, Amoxicillin; Tetraciclyne, and Furazolidone in Brazil. Helicobacter 2000; 5: 79-83. [ Links ]
17. Torres J, Camorlinga M, Pérez G, Madrazo A, Dehesa M, González G, et al. Increasing Multidrug Resistance in Helicobacter pylori Stains Isolates from Children and Adults in Mexico. J Clin Microbiol 2001; 39: 2677-2680. [ Links ]
18. Bosques FJ, Alanís O, Garza E, Maldonado HJ, Tijerina R. Susceptibilidad de Helicobacter pylori a ofloxacina y levofloxacina. MedUniver 2001; 3: 124-127. [ Links ]
19. González C, García A, Daroch F, Kawaguchi F, Solar H, Rivera N,y cols. Suceptibilidad in vitro de cepas de Helicobacter pylori: aislamiento de cepas resistentes a claritromicina. Rev Med Chile, 2001; 129(6). [ Links ]
20. Ecclissato C, Morchioretto MAM, Mendoca S, Godoy APO, Guersoni M, Deguer H, et al. Increased Primary Resistence to Recommended Antibiotics Negatively Afects Helicobacter pylori Eradication. Helicobacter 2002; 7: 53-59. [ Links ]
21. Prazeres P, Magalhaes DM, Campos DV, Aguiar G, Nogueria E, Santos A, et al. Helicobacter pylori Primary Resistanceto Metronidazole and Clarithromicyn in Brazil. Antimicrob Agents Chemother 2002; 46: 2021-2023. [ Links ]
22. Garza-González E, Pérez-Pérez GI, Alani-Aguilar O, Tijerina-Menchaca R, Maldonado-Garza HJ, Bosque-Padilla FJ. Antibiotic susceptibility of Helicobacter pylori strains isolated from northeastern Mexico. J Chemother 2002; 14: 342-345. [ Links ]
23. Urrestarazu MI, Serrano N, Piñero R, Cavazza ME. Susceptibilidad del Helicobacter pylori a los antimicrobianos. Rev Soc Ven Microbiol 2003; 14-15. [ Links ]
24. Debets-Ossemkopp Y, Reyes G, Mulder J. van de Stegge B, Peters J, Savelkoul P, et al. Characteristic of clinical Helicobacter pylori strains from Ecuador. J Antimicrobial Chemother 2003; 51: 141-145. [ Links ]
25. Ortiz AP, Ribeiro ML, Borges YH, Vitiello L, Bueno M, Mendoca S, et al. Analysis of antimicrobial susceptibility and virulence factor in Helicobacter pylori clinical isolates. BMC Gastroenterology 2003; 3: 20. [ Links ]
26. Lee MG, Arthurs M, Smikle M, Dowe G, Levy W, Barton EN. Antibiotic Sensibility of Helicobacter pylori in Jamaica. West Med Indian J 2004; 53: 374-377. [ Links ]
27. Fariña N, Kasamatsu E, Samudio M, Moràn M, Sanabria R, Laspina F. Susceptibilidad antibiótica de cepas de Helicobacter pylori aisladas en pacientes con enfermedad gastroduodenal. Rev Med Chile 2007; 135: 1009-1014. [ Links ]
28. Vallejos C, Garrido L, Cáceres D, Madrid AM, Defilippi C, Defilippi C y cols. Prevalencia de la resistencia a metronidazol, claritromicina y tetraciclina en Helicobacter pylori aislado de pacientes de la Región Metropolitana. Rev Med Chile 2007; 135: 287-293. [ Links ]
29. Yepes CA, Rodríguez A, Ruiz A, Ariza B, Resistencia antibiótica del Helicobacter pylori en el hospital universitario San Ignacio de Bogotá. Acta Médica Colombiana 2008; 33: 11-14. [ Links ]
30. álvarez A, Moncayo JI, Santacruz JJ, Santacoloma M, Corredor LF, Reinosa E. Antimicrobial Susceptibility and Mutations Involved in Clarithromicyn Resistance Helicobacter pylori from Patients in the Western Central Region of Colombia. Antimicrob Agents Chemother 2009; 53: 4022-4024. [ Links ]
31. Henao R. SC, Quiroga A, Martínez M. JD, Otero W. Resistencia primaria a la claritomicina en aislamientos de Helicobacter pylori. Rev Col Gastroenterol 2009; 24: 110-114. [ Links ]
32. álvarez A, Moncayo JI, Santacruz JJ, Corredor LF, Reinosa E, Martínez JW, y cols. Resistencia a Metronidazol y Claritromicina en aislamientos de Helicobacter pylori de pacientes dispépticos de Colombia, Rev Med Chile 2009; 137: 1309-1314. [ Links ]
33. Henao R, Otero W, ángel LA, Martínez M. JD. Resistencia primaria al metronidazol en aislamientos de Helicobacter pylori en pacientes adultos de Bogotá, Colombia. Rev Col Gastroenterol 2009; 24: 10-15. [ Links ]
34. Torres ME, Pérez G, Olivares A, Fernández L, Raisler K, González N, et al. Antimicrobial susceptibility of Helicobacter pylori and mechanisms of clarithromycin resistance in strains isolated from patients in Uruguay, Rev Esp Enferm Dig 2009; 101: 757-762. [ Links ]
35. Vega AE, Cortiñas TI, Puig ON, Silva HJ. Molecular characterization and susceptibility testing of Helicobacter pylori stains isolated in western Argentina. Intern J of Infectious Dis 2010; 14S: 85-92. [ Links ]
36. Lins AK, Lima RA, Magalhaes M. Clarithromycin-Resistant Helicobacter pylori in Recife, Brazil, Directly Identified from Gastric Biopsies by Polymerase Chain Reaction. Arq Gastroenterol 2010; 47: 379-382. [ Links ]
37. García GT, Aranda KRS, Goncalves M, Cardoso SR, Kiyosi I, Neusa S. High prevalence of Clarithromicyn Resistance and cagA, vacA, iceA2and babA2 Genotypes of Helicobacter pylori in Brazil Children J Clin Microbiol 2010; 48: 4266. [ Links ]
38. Trespalacios A, Otero W, Mercado M. Resistencia del Helicobacter pylori a metronidazol, claritromicina y amoxicilina en pacientes colombianos. Rev Col Gastroenterol 2010; 25: 31-38. [ Links ]
39. Toledo H, López-Solis R. Tetracycline resistance in Chilean isolates of Helicobacter pylori. J Antimicrob Chemother 2010; 65: 470-473. [ Links ]
40. Eisig JN, Silva FM, Barbuti RC, Navarro T, Moraes J, Pedrazzoli J. Helicobacter pylori antibiotic resistance in Brazil: clarithromycin is still a good option. Arq Gastroenterol 2011; 48: 261-264 [ Links ]
41. Ayala G, Galván M, Chihu L, Fierros G, Sánchez A, Carrillo B, et al. Resistance to antibiotics and characterization of Helicobacter pylori strains from antrum and body from adults in Mexico. Microb Drugs Resist 2011; 17: 149-155. [ Links ]
42. Otth L, Wilson M, Fernández H, Otth C, Toledo C, Cárcamo V, et al. Isolation of Helicobacter pylori in Gastric Mucosa and Susceptibility to Five Antimicrobial Drugs in Southern Chile. Braz J Microbiology 2011; 42: 442-447. [ Links ]
43. Mochizuki H, Nogueira AP. Determinación de la susceptibilidad de cepas de Helicobacter pylori a levofloxacino en formato pequeño y método de difusión en disco usando agar yema de huevo. Rev Gastroenterol Perú 2011; 31: 224-229. [ Links ]
44. Scalestky I, Aranda K, García G, Goncalves M, Cardoso S, Iriya K, et al. Application of Real Time PCR Stool Assay for Helicobacter pylori Detection And Clarthromycin Susceptibility Testing in Brazilian Children. Helicobacter 2011; 16: 311-315. [ Links ]
45. Figueroa M, Cortés A, Pazos A, Bravo LE. Sensibilidad in vitro a amoxicilina y claritromicina de Helicobacter pylori obtenido de biopsias gástricas de pacientes de zona de bajo riesgo para cáncer gástrico. Biomédica 2012; 32: 32-42. [ Links ]
46. Suzuki RB, Almeida CM, Speranca MA. Absence of Helicobacter pylori high tetracycline resistant 16S rDNA AGA929-928TTC genotype in gastric biopsy specimens from dyspeptic patients in the interior of city Sao Paulo, Brazil. BMC Gastroenterology 2012; 12: 49-54. [ Links ]
47. Van der Wouden EJ, Thijs JC, Kusters JG, Van Zwett AA, Klieibeur JC. Mechanism and clinical significance of metronidazole resistance in Helicobacter pylori. Scand J Gastroenterol 2001; 36: 10-14. [ Links ]
48. Solca NM, Bernasconi MV, Piffaretti JC. Mechanism of metronidazol resistance in Helicobacter pylori: comparison of the rdxA gene sequences in 30 straints. Antimicrob Agents Chemother 2000; 44: 2207-2210. [ Links ]
49. Megraud FH. Pylori antibiotic resistance: Prevalence, Importance, and advances in testing. GUT 2004; 53: 1374-1384. [ Links ]
50. Glupczynski Y, Megraud F, López-Brea M, et al. European multicenter survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur J Clin Microbiol Infect Dis 2000; 11: 820-823. [ Links ]
51. Ortiz AP, Reis FC, Ferraz LFC, Gerrits MM, Mendoca S, Kuster JG, et al. Differentially expressed genes in responses to amoxicillin in Helicobacter pylori analyzed by RNA arbitrarily primed PCR. FEMS Inmunol Med Microbiol 2006; 60: 226-230. [ Links ]
52. Gerrits MM, Godoy AP, Kuipers EJ, Ribeiro ML, Stoof J, Mendoca S, et al. Multiple mutations in or adjacent to the conserved penicillin-binding protein motifs of thepenicilin-bindingprotein1A confer amoxicillin resistance to Helicobacter pylori, Helicobacter 2006; 11: 181-184. [ Links ]
53. Tseng YS, Wu DC, Chang CY, Kuo CH, Yang YC, Jan CM, et al. Amoxicillin resistance with beta lactamase production in Helicobacter pylori. Eur J ClinInvest 2009; 39: 807-812. [ Links ]
54. Hernández M, Reyes O, Rodríguez J, González BL. La resistencia a antibióticos en Helicobacter pylori. Rev Cubana Med 2008; 47(4): 10-14. [ Links ]
55. Megraud F, Lehours. Helicobacter pylori detection and susceptibility testing. Clinical microbiology reviews 2007; 20 (2): 280-322. [ Links ]
56. De Francesco V, Ierardi E, Zullo A, Hassan C, Zullo A. Helicobacter pylori therapy: Present and future. World J Gastrontest 2012; 3: 68-73. [ Links ]
57. Berning M, Krasz S, Miehker S. Should quinolones come first in Helicobacter pylori therapy". Therapeutic Advances in Gastroenterology 2011; 4: 103-114. [ Links ]
58. Alarcón P, de la Obra D, García-Campos JA, Díaz-Regañón J y López-Brea M. Actividad in vitro de la furazolidona y la nitrofurantoína en aislamientos clínicos de Helicobacter pylori y estudio de la tasa de mutación. Rev Esp Quimioterap 2005; 18: 313-318. [ Links ]
59. Ramírez RA, Sánchez SR. Helicobacter pylori 25 años después (1983-2008). Rev gastroenterol Perú 2009; 29: 158-170. [ Links ]











 text in
text in