Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.3 Bogotá set. 2014
Cooperative Endoscopic Laparoscopy: a new alternative for management of early gastric cancer
Martín Gómez MD. (1), Camilo Ortiz MD. (2), Giovanni Muñoz MD. (3)
(1) Assistant Professor in the Gastroenterology Unit of the Department of Internal Medicine at the Universidad Nacional de Colombia and Gastroenterologist at Hospital El Tunal in Bogotá, Colombia.
(2) Laparoscopic surgeon at Hospital El Tunal and Universidad de la Sabana in Bogotá, Colombia.
(3) Resident in general surgery at Hospital El Tunal in Bogotá, Colombia.
Received: 14-01-14 Accepted: 21-07-14
Abstract
Usually early gastric cancer is treated by submucosal resection, but for a case in which the lesion is very large, is located in a very difficult place for resection, or has a very aggressive histological pattern, a gastrectomy is indicated. This procedure is too mutilating and results in poor patient quality of life. For these cases cooperative endoscopic laparoscopy may be a new alternative for management of the condition. This series of cases presents our hospitals experience with this technique and shows the variables faced with the procedure so that it can be applied elsewhere in our country.
Keywords
Early gastric cancer, laparoscopy, endoscopy, submucosal dissection.
INTRODUCTION
Advanced gastric cancer is the malignancy with the highest mortality rate in our environment, hence the importance of detecting it during its early stages during which survival rates are very high (1). Traditionally, treatment with the intent to heal early gastric cancer is surgical, but approximately 15 years ago endoscopic submucosal dissection (ESD) began to be accepted as the current treatment of choice (2). However, the first treatment causes mutilation and results in poor patient quality of life. Also, ESD, is expensive and the learning curve is steep, moreover, since it is not widely available, other alternatives such as combinations of surgery and endoscopy are often resorted to (3). Cooperative surgery combining laparoscopic and endoscopic techniques is a method which can be used to manage sub epithelial lesions. It consists of locating the lesion through endoscopy and then resecting it through laparoscopy. Many studies have been published about the utility of this technique for subepithelial lesions. It has been suggested for management of early gastric cancer, and has had positive results in the small number of cases published (4-6). Several techniques have been described including laparoscopic and endoscopic cooperative surgery (LECS), non-exposed endoscopic wall-inversion surgery (NEWS), combination of laparoscopic and endoscopic approaches to neoplasia with a non-exposure technique (CLEAN-NET), and EFTR (endoscopic full-thickness resection).
SURGICAL PROCESS
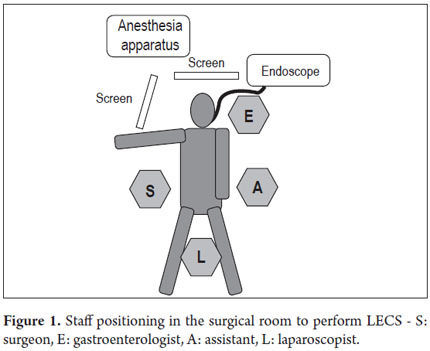
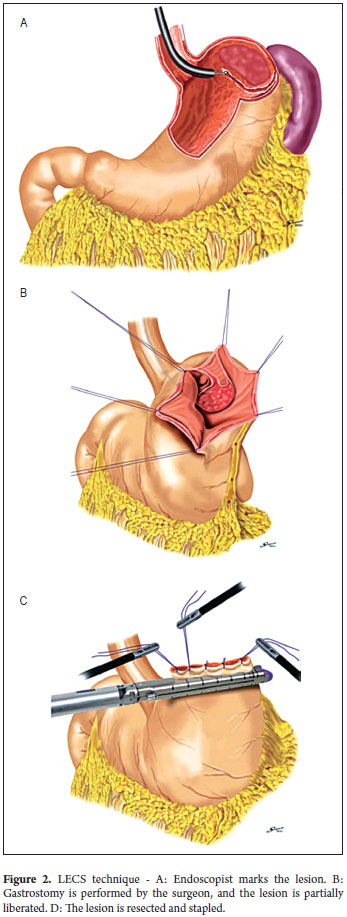
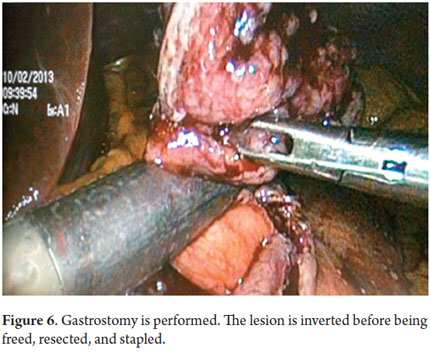
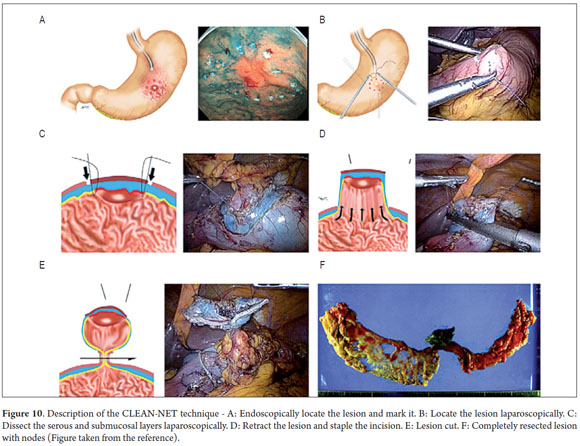
With the patient under general anesthesia (Figure 1) the endoscope is introduced. The lesion is located in the gastric mucosa under direct endoscopic view. A two mm margin around the tumor is then marked with a pointed papillotome. This process can partially dissect and free the lesion. The surgeon then performs the laparoscopy and identifies the site of the lesion in the external face of the stomach with the help of endoscopy. There are two important variations in the technique at this point: a gastrostomy can be performed to trap the lesion with the LECS technique (Figure 2), or the stomach can remain closed and the CLEAN-NET technique can be used. In this case the point of the papillotome is placed in the center of the serous membrane to remove the lesion. In both techniques, the next step is stapling the lesion under direct endoscopic view. This ensures that the entire lesion has been resected and that the margins of the cut are adequate. Finally, the endoscope is used to verify that there are no perforations and the stomach opening is adequate to avoid stenosis.
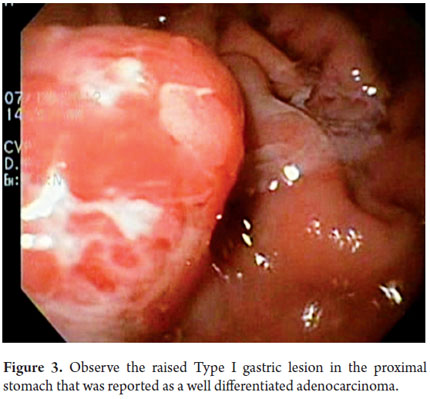
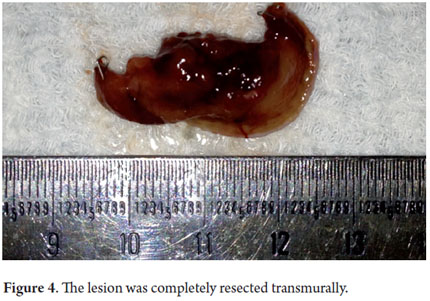
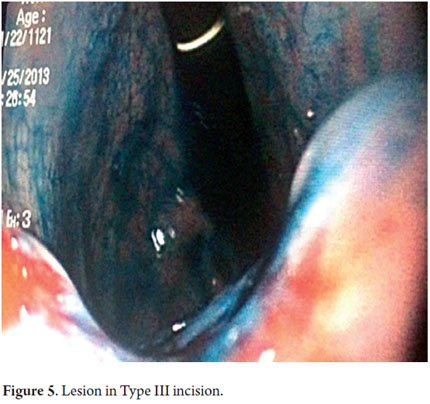
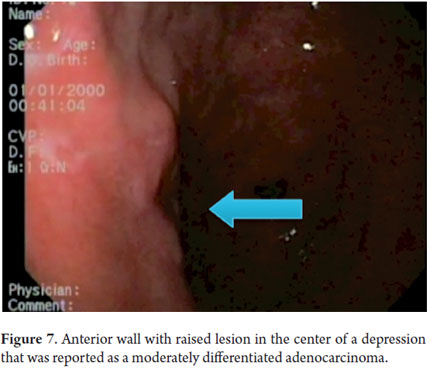
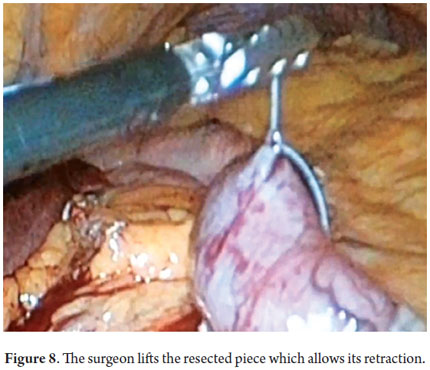
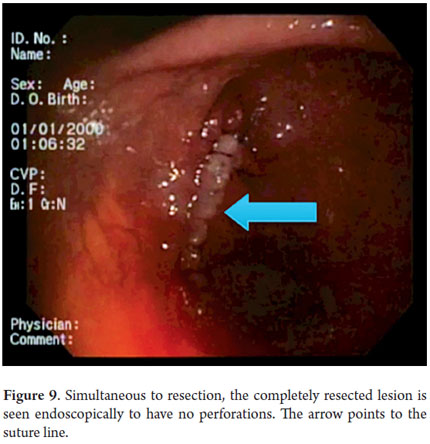
Next, we present the experience at the Hospital El Tunal with four patients: three men and one woman. Their average age was 64.7 years. Three tumors were located in the distal stomach and one in the proximal stomach. There were two Type I lesions (Figures 3 and 4) and two Type III ulcerated lesions (Figures 5 and 6). All lesions were early (within the upper third of the submucosa: SM1). Two lesions were resected using the LECS technique, and the other two were resected using the CLEAN-NET technique (Figures 7, 8 and 9). All patients were discharged 48 hours after procedures were performed.
DISCUSSION
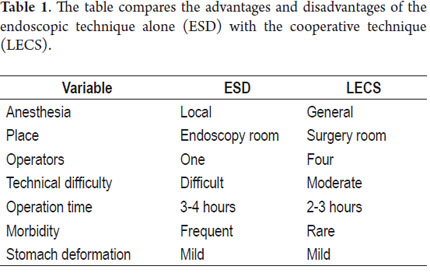
As endoscopies are performed more and more frequently to assess dyspepsia, and as the incidence of gastric cancer increases in our environment, it is probable that more gastric cancer cases will be detected early. Since surgery has been abandoned as the method of choice due to the rate of morbidities and the resulting poor quality of patient life, we have to be prepared to treat these patients in some other way. Although endoscopic treatment through ESD is the ideal treatment for these patients, this technique is very demanding, requires the use of multiple accessories, is expensive, and is not widely available (7). This means that other cheaper and more frequently available alternatives need to be found. LECS is one such technique: it is easier to perform, cheaper and can be performed in most health institutions (8).
This study shows our experience with four patients who were successfully treated with both of the variations of this technique. These patients did not present any complications and were discharged 48 hours following the procedures. Two patients were treated with the LECS technique in which the surgeon opened the gastric cavity to remove the lesion after it had been previously located by endoscopy. The tumors were inverted and removed and the incisions stapled. This technique has a potential risk in that tumor cells can fall into the peritoneal cavity. This makes the closed technique used in the other two cases useful. In one of these cases the lesion was marked by endoscopy, then a suture was placed and retracted by the surgeon who then stapled the incision guided by endoscopy. In the other case, the CLEAN-NET technique was used. When the lesion is too big, the serous and submucosal membranes are dissected by the surgeon in order to resect as little gastric tissue as possible when retracting the lesion (Figure 10) (9). The main advantages of LECS over the ESD techniques is that it can be used for lesions located in any part of the stomach and does not take as long to perform (Table 1).
In conclusion, the different LECS techniques are interesting alternatives for treatment of early gastric cancer. Rather than condemning patients to mutilating surgery such as total or partial gastrectomies when ESD is not possible or available, other gastroenterology groups should take advantage of LECs techniques and the patient benefits they offer and begin to implement these techniques.
REFERENCES
1. Otero W, Gómez M, Castro D. Carcinogénesis gástrica. Rev Col Gastroenterol 2009; 24: 314-329. [ Links ]
2. Sugano K. Gastric cancer: pathogenesis, screening and treatment. Gastrointest Endosc Clin N Am. 2008; 18(3): 513-522. [ Links ]
3. Toyonaga T, Man-i M, Fujita T, et al. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for laterally spreading tumors of the colorectum. Endoscopy 2010; 42: 714-22. [ Links ]
4. Wilhelm D, von Delius S, Burian M, Schneider A, Frimberger E, Meining A, Feussner H. Simultaneous use of laparoscopy and endoscopy for minimally invasive resection of gastric subepithelial masses - analysis of 93 interventions. World J Surg 2008; 32: 1021-1028. [ Links ]
5. Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc 2008; 22: 1729-1735. [ Links ]
6. Walsh RM, Ponsky J, Brody F, Matthews BD, Heniford BT. Combined endoscopic/laparoscopic intragastric resection of gastric stromal tumors. J Gastrointest Surg 2003; 7: 386-392. [ Links ]
7. Nakamoto S, Sakai Y, Kasanuki J, et al. Indications for the use of endoscopic mucosal resection for early gastric cancer in Japan: a comparative study with endoscopic submucosal dissection. Endoscopy 2009; 41(9): 746-50. [ Links ]
8. N. Hiki Æ Y. Yamamoto Æ T. Fukunaga Æ T. Yamaguchi Æ S. Nunobe Æ M. Tokunaga Æ A. Miki Æ S. Ohyama Æ Y. Seto. Laparoscopic and endoscopic cooperative surgery for gastrointestinal stromal tumor dissection,Surg Endosc 2008; 22: 1729-1735. [ Links ]
9. Abe N, Mori T, Takeuchi H, et al. Successful treatment of early gastric cancer by laparoscopy-assisted endoscopic full-thickness resection with lymphadenectomy. Gastrointest Endosc 2008; 68: 1220–4. [ Links ]
10. Inoue H, Minami H, Ogata N, et al. How can we decide treatment of early stage gastric cancer? Choosing EMR/ESD, laparoscopic surgery or another. Endoscopia Digestiva 2009; 21: 749-54. [ Links ]
11. Haruhiro Inoue, Haruo Ikeda, Toshihisa Hosoya, Akira Yoshida, Manabu Onimaru, Michitaka Suzuki. Endoscopic Mucosal Resection, Endoscopic Submucosal Dissection, and Beyond: Full-Layer Resection for Gastric Cancer with Nonexposure Technique (CLEAN-NET). Surg Oncol Clin N Am 2012; 21: 129-140. [ Links ]











 texto em
texto em