Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.3 Bogotá Sept. 2014
A Review of Helicobacter Pylori and Colon Cancer
José Urrego Díaz MD. (1), William Otero Regino MD. (2), Martín Gómez Zuleta MD.(3)
(1) Student of Medicine at the National University of Colombia in Bogotá, Colombia.
(2) Professor of Medicine, Gastroenterology Unit, National University of Colombia. Gastroenterologist Clinic Founders. Bogotá, Colombia.
(3) Professor of Medicine, Gastroenterology Unit, National University of Colombia. Gastroenterologist, El Tunal Hospital. Bogotá, Colombia.
Received: 19-07-13 Accepted: 21-07-14
Abstract
Helicobacter pylori are gram-negative bacteria that colonize the gastric epithelia of approximately 50% of the worlds population. This infection is considered to be the leading known cause of chronic gastritis, peptic ulcers, gastric MALT and gastric cancer. In addition, there is evidence linking the bacteria to several extra-gastric diseases. The association of extra-gastric diseases with colon cancer and other colonic neoplasms has been the subject of much debate since it was first suggested. Although some studies have cast doubt on this association, others have sustained it. These include a recent meta-analysis and cross-sectional study of more than 150,000 patients which is the largest on this subject so far. In addition, there are numerous articles that support the biological plausibility of this association. In this article we review the available evidence regarding this association and the mechanisms of causality that have been proposed.
Keywords
Helicobacter pylori, polyps, cancer, inflammation.
INTRODUCTION
Helicobacter pylori (H. pylori) is a spiral gram-negative bacillus that infects human gastric epithelia (1). It was first cultivated by Warren and Marshall in 1983 (2). It infects approximately 50% of the worlds population (3, 4), although its prevalence varies significantly among geographical areas, social classes, ages and races (5-7). Fifteen years ago this bacteria was classified as a Type 1 human carcinogen, by the World Health Organizations International Agency for Research on Cancer (IARC) (8). H. pylori, hepatitis B, hepatitis C and human papillomavirus are the four infectious agents that cause more than 90% of cancers associated with infections (9). These bacteria have causal relationships with a series of gastric diseases including chronic gastritis, gastric cancer, peptic ulcers and MALT lymphoma (10-12). However, the percentage of infected people who develop clinically significant disease is low (20%) and development of clinically significant disease requires coexistence with the bacteria for decades, individual genetic susceptibility and depends on bacterial virulence factors (13, 14). In addition to the gastric diseases mentioned, it has been suggested that H. pylori can also be associated with extraintestinal entities such as coronary disease, neurodegenerative diseases and hematological diseases. Certainty is greatest for hematological diseases (14-23). Although published data are contradictory polyps and adenocarcinoma of the colon have recently been associated with H. pylori infections (24-27). This review aims to bring together and discuss the currently available evidence concerning the relationship between H. pylori and colon cancer and the mechanisms proposed for this association.
METHODOLOGY
The following search strategy was used in the PubMed database: (((Colonic Neoplasms OR Colonic Neoplasm OR Neoplasm, Colonic OR Neoplasms, Colonic OR Colon Neoplasms OR Colon Neoplasm OR Neoplasm, Colon OR Neoplasms, Colon OR Cancer of Colon OR Colon Cancers OR Cancer of the Colon OR Colonic Cancer OR Cancer, Colonic OR Cancers, Colonic OR Colonic Cancers OR Colon Cancer OR Cancer, Colon OR Cancers, Colon))) AND ((Helicobacter pylori OR campylobacter pylori)). The search was limited to the last 5 years. Titles and abstracts of articles were reviewed, and those that were going to be fully reviewed were selected. Later, articles referred to in the selected articles were also reviewed.
ASSOCIATION BETWEEN H. PYLORI AND COLON CANCER
Some prospective studies have not found any associations between H. pylori and colorectal cancer (CRC) (25, 28-30). For example, in one recent prospective case-control study, blood samples from 93 fasting people who had been diagnosed with CRC were assessed for levels of gastrin antibodies, antibodies against H. pylori and expression of the CagA protein by H. pylori. They were then compared with 20 age-matched controls (31). This study found no significant differences between the two groups for any of the three variables assessed.
In contrast a 2006 meta-analysis of 11 papers that studied this association found that those infected with H. pylori have an odds ratio of 1.4 for CRC (32). The possibility exists that this result could have been influenced by bias. Another meta-analysis of case and control studies up to 2007 again found a statistically significant association between H. pylori infection or the presence of anti-H. Pylori IgG antibodies and CRC risk with odds ratios of 1.49 and 1.56 respectively (33). More recently Sonnenberg and Genta conducted the largest case-control study to date that has analyzed the possibility of this association (34). Their study reviewed the results of gastric and colon biopsies of more than 156 000 patients. Biopsies had been performed on the same day that patients had undergone colonoscopies and upper endoscopies. The presence of gastritis due to H. pylori was defined as evidence of active chronic inflammation in the gastric mucosa and presence of the bacteria. This was evaluated using immunohistochemistry which is the most reliable method for detecting H. pylori in gastric biopsies (35, 36). Patients with H. pylori gastritis were more likely than patients without H.Pylori to have all of the following conditions in the colon: hyperplastic polyps (OR = 1.24), adenomatous polyps (OR = 1.52), advanced adenomas (OR = 1.80), villous adenomas with high grade dysplasia (OR = 1.97) and adenocarcinoma (OR = 2.35). As this evidence shows, the strength of the association increased with increasing severity of the neoplasia. Despite the methodological rigor of the study, some authors believe that the results of this important study should be interpreted with caution (37). They point to the following limitations of its design (7, 38):
1. The group studied consists only of individuals with indications for endoscopy, so it is not necessarily representative of the general population.
2. Since it is a cross-sectional study, it can demonstrate association but not causation.
3. Most of the ORs reported in the study were not adjusted for possible confounding factors such as age and sex. This is of great importance given that the prevalence of H. pylori varies with age, and its prevalence decreases progressively in successive generational cohorts of those born in developed countries.
Finally, some studies have shown a positive association between CRC and CagA positive strains of H. pylori (39, 40). For example, the case-control study of Shmuely and colleagues found an increased risk for this disease in the infection by CagA + strains with an OR = 10.6 by comparing those infected with H. pylori CagA + with those infected with H. pylori cagA- in a group of 67 patients diagnosed with CRC (40).
PROPOSED MECHANISMS FOR COLON CANCER RISKS IN PATIENTS WITH H. PYLORI INFECTIONS
It is considered that the increased risk of colonic neoplasia in individuals infected with H. pylori is associated with the rise of production of gastrin triggered by this bacteria (34, 41). This is the best supported proposal for this mechanism. For many years, it has been known that H. pylori infections lead to a rise in gastric gastrin production primarily in response to food intake. This can be reversed by removing the bacteria (42-44). H. pylori, especially CagA positive strains, induce inflammatory responses in the colonized gastric mucosa that causes D cell deficiencies in the gastric antrum. It also leads to chronic atrophic gastritis with consequent reduction in the production of acid. Both situations cause hypergastrinemia (31, 45, 46). In addition, H. Pylori exerts stimulates gastrin production by G cells due to excessive production of proinflammatory cytokines, ammonia and growth factors such as TGF-α and EGF (33, 47, 48). Gastrin causes trophic activity in certain gastric cells with production of gastric polyps and neuroendocrine tumors (49, 50). This is the mechanism that has been invoked for the development of benign and malignant neoplasias in the colon (41, 51, 52). High levels of serum gastrin could act as a hormone which directly promotes proliferation of cells in the mucosa of the colon leading to an increase in the risk of carcinogenesis (53-56). Another mechanism that may play a role in this proliferation could be early expression and activation of gastrin receptors in colonic polyps and more advanced neoplasias (57, 58). This would condition its accelerated progression in the presence of hypergastrinemia secondary to the H. pylori infection. It has also been shown that gastrin is related to high levels of inflammatory mediators such as COX-2 and IL-8 which might contribute to the development of colon cancer (39, 59). In addition, increased expression of the inducible enzyme COX-2 secondary to H. pylori infection is considered to be involved in the risk of developing CRC, since (48), an association between the expression and tissue activity of COX-2 and development and progression of colon cancer and other neoplasias has been shown to exist (60, 61). For these reasons, the use of enzyme inhibitors such as NSAIDs have an important effect that reduces the risks of CRC and mortality due to CRC (62-64). H. pylori may increase the expression of COX-2 by increasing proinflammatory cytokines and by increasing gastrin levels (48). The ultimate effect of this would be increased angiogenesis in the proliferation and mutagenesis of the mucosal cells of the colon while decreasing their rate of apoptosis both of which are related to carcinogenesis (61, 65).
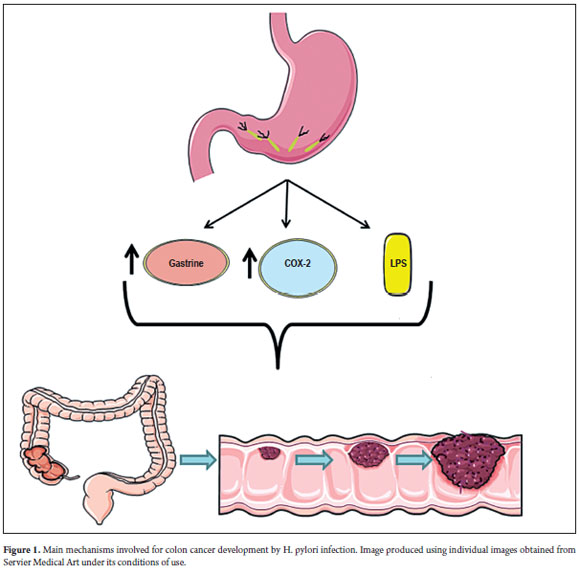
On the other hand, there is the possibility that H. pylori acts directly on the mucosa of the colon to promote the development of neoplastic changes (66, 67). Nevertheless, the statistically significant association that exists does not imply causation. A recent experimental in vitro study demonstrated that the lipopolysaccharides (LPS) of H. pylori, the major constituent of its outer membrane, may interfere with the DNA repair system of the cells of the colon. This may cause genotoxicity and influence the development of colon cancer (68). The same study has shown that cellular production of nitric oxide (NO) increases in response to the LPS of H. pylori has a central role in this genotoxicity process. Nitric oxide causes chronic inflammation thus mediating carcinogenesis (69, 70). The carcinogenic effect of NO in colon cells could occur through inhibition of DNA repair enzymes and through pro-apoptotic effector proteins as the result of nitrosylation of their cysteine and tyrosine residues, as has been shown to occur in other cells of the gastrointestinal system (71, 72). The mechanisms involved in the genesis of CRC in patients infected with H. pylori are shown in Figure 1.
Finally, some authors have suggested that alterations in intestinal microbial flora secondary to decreased secretion of acid in atrophic gastritis caused by H. pylori infections may be another factor leading to progression of CRC (31, 73).
Increased production of gastrin and COX-2 in colon cells and increased production of lipopolysaccharides on the cell walls of H. pylori lead to the development of polyps and progression of CRC in individuals infected with H. pylori.
CONCLUSIONS
There are statistically significant associations between H. pylori and CRC in published metaanalyses and in large case-control studies (32, 33, 34). Nevertheless, the possibility of bias cannot be discarded in metaanalyses, and confounding variables may not have been controlled for in the work of Sonnenberg and Genta (34). Therefore, causality cannot definitively be established even though there is avery b association together with biological plausibility.
The pathophysiological model contains three mechanisms:
1. Inflammation caused by H. pylori leads to increased tissue activity of COX-2 which could promote the development of colonic neoplasias. This mechanism that has sufficient evidence (48, 61, 65).
2. Detection of H. pylori in colonic neoplasias (66, 67) and the recent discovery of the carcinogenic activity of the components of its membrane on colonic cells allow us to talk of a direct effect by the bacteria (68).
3. The main mechanism, supported by multiple investigations, has to do with hypergastrinemia secondary to H. pylori infections which would be the trigger for neoplastic colonic alterations (34, 41-44, 53-56).
In conclusion, although so far no one can say with certainty that the association between H. pylori and colon cancer is causal, current evidence and biological plausibility support this possibility. These bacteria may well be one of the factors in the complex multifactorial process that leads to the development of CRC.
REFERENCES
1. Marshall B. Helicobacter pylori: 20 years on. Clin Med 2002; 2: 147-52. [ Links ]
2. JR W, BJ M. Unidentified curved bacilli on gastric epithelium in active chronic gastritis. Lancet 1983; 1: 1273-5. [ Links ]
3. Dunn BE, Cohen H, Blaser MJ. Helicobacter pylori. Clin Microbiol Rev 1997; 10: 720-41. [ Links ]
4. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002; 347: 1175-86. [ Links ]
5. Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol 2012; 175: 54-9. [ Links ]
6. Epplein M, Signorello LB, Zheng W, Peek RM, Michel A, Williams SM, et al. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer Epidemiol Biomarkers Prev 2011; 20: 826-34. [ Links ]
7. Jafar S, Jalil A, Soheila N, Sirous S. Prevalence of helicobacter pylori infection in children, a population-based cross-sectional study in west iran. Iran J Pediatr 2013; 23: 13-8. [ Links ]
8. Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 1994; 61: 1-241. [ Links ]
9. de Martel C, Ferlay J, Franceschi S, Vignat J, Bray F, Forman D, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13: 607-15. [ Links ]
10. Otero W, Gómez M, Castro D. Carcinogénesis gástrica. Rev Col Gastroenterol 2009; 24: 314-29. [ Links ]
11. Herrera V, Parsonnet J. Helicobacter pylori and gastric adenocarcinoma. Clin Microbiol Infect 2009; 15: 971-6. [ Links ]
12. Otero W, Trespalacios A, Otero E. Helicobacter pylori: Tratamiento actual. Un importante reto en gastroenterología. Rev Col Gastroenterol 2009; 24: 279-92. [ Links ]
13. Blaser MJ. Hypotheses on the pathogenesis and natural history of Helicobacter pylori-induced inflammation. Gastroenterology 1992; 102: 720-7. [ Links ]
14. Malfertheiner P, Megraud F, OMorain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012; 61: 646-64. [ Links ]
15. Qu XH, Huang XL, Xiong P, Zhu CY, Huang YL, Lu LG, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol 2010; 16: 886-96. [ Links ]
16. Lahner E, Persechino S, Annibale B. Micronutrients (Other than iron) and Helicobacter pylori infection: a systematic review. Helicobacter 2012; 17: 1-15. [ Links ]
17. Arnold DM, Bernotas A, Nazi I, Stasi R, Kuwana M, Liu Y, et al. Platelet count response to H. pylori treatment in patients with immune thrombocytopenic purpura with and without H. pylori infection: a systematic review. Haematologica 2009; 94: 850-6. [ Links ]
18. Jeon CY, Haan MN, Cheng C, Clayton ER, Mayeda ER, Miller JW, et al. Helicobacter pylori infection is associated with an increased rate of diabetes. Diabetes Care 2012; 35: 520-5. [ Links ]
19. Roubaud-Baudron C, Krolak-Salmon P, Quadrio I, Mégraud F, Salles N. Impact of chronic Helicobacter pylori infection on Alzheimers disease: preliminary results. Neurobiol Aging 2012; 33: 1009.e11-9. [ Links ]
20. Kountouras J, Tsolaki M, Gavalas E, Boziki M, Zavos C, Karatzoglou P, et al. Relationship between Helicobacter pylori infection and Alzheimer disease. Neurology 2006; 66: 938-40. [ Links ]
21. Jafarzadeh A, Esmaeeli-Nadimi A, Nemati M, Tahmasbi M, Ahmadi P. Serum concentrations of Helicobacter pylori IgG and the virulence factor CagA in patients with ischaemic heart disease. East Mediterr Health J 2010; 16: 1039-44. [ Links ]
22. Ki MR, Goo MJ, Park JK, Hong IH, Ji AR, Han SY, et al. Helicobacter pylori accelerates hepatic fibrosis by sensitizing transforming growth factor-β1-induced inflammatory signaling. Lab Invest 2010; 90: 1507-16. [ Links ]
23. Papagiannakis P, Michalopoulos C, Papalexi F, Dalampoura D, Diamantidis MD. The role of Helicobacter pylori infection in hematological disorders. Eur J Intern Med 2013. [ Links ]
24. Meucci G, Tatarella M, Vecchi M, Ranzi ML, Biguzzi E, Beccari G, et al. High prevalence of Helicobacter pylori infection in patients with colonic adenomas and carcinomas. J Clin Gastroenterol 1997; 25: 605-7. [ Links ]
25. Siddheshwar RK, Muhammad KB, Gray JC, Kelly SB. Seroprevalence of Helicobacter pylori in patients with colorectal polyps and colorectal carcinoma. Am J Gastroenterol 2001; 96: 84-8. [ Links ]
26. Breuer-Katschinski B, Nemes K, Marr A, Rump B, Leiendecker B, Breuer N, et al. Helicobacter pylori and the risk of colonic adenomas. Colorectal Adenoma Study Group. Digestion 1999; 60: 210-5. [ Links ]
27. Fireman Z, Trost L, Kopelman Y, Segal A, Sternberg A. Helicobacter pylori: seroprevalence and colorectal cancer. Isr Med Assoc J 2000; 2: 6-9. [ Links ]
28. Limburg PJ, Stolzenberg-Solomon RZ, Colbert LH, Perez-Perez GI, Blaser MJ, Taylor PR, et al. Helicobacter pylori seropositivity and colorectal cancer risk: a prospective study of male smokers. Cancer Epidemiol Biomarkers Prev 2002; 11: 1095-9. [ Links ]
29. Aydin A, Karasu Z, Zeytinoglu A, Kumanlioglu K, Ozacar T. Colorectal adenomateous polyps and Helicobacter pylori infection. Am J Gastroenterol 1999; 94: 1121-2. [ Links ]
30. Moss SF, Neugut AI, Garbowski GC, Wang S, Treat MR, Forde KA. Helicobacter pylori seroprevalence and colorectal neoplasia: evidence against an association. J Natl Cancer Inst 1995; 87: 762-3. [ Links ]
31. Strofilas A, Lagoudianakis EE, Seretis C, Pappas A, Koronakis N, Keramidaris D, et al. Association of Helicobacter pylori infection and colon cancer. J Clin Med Res 2012; 4: 172-6. [ Links ]
32. Zumkeller N, Brenner H, Zwahlen M, Rothenbacher D. Helicobacter pylori infection and colorectal cancer risk: a meta-analysis. Helicobacter 2006; 11: 75-80. [ Links ]
33. Zhao YS, Wang F, Chang D, Han B, You DY. Meta-analysis of different test indicators: Helicobacter pylori infection and the risk of colorectal cancer. Int J Colorectal Dis 2008; 23: 875-82. [ Links ]
34. Sonnenberg A, Genta RM. Helicobacter pylori is a risk factor for colonic neoplasms. Am J Gastroenterol 2013; 108: 208-15. [ Links ]
35. Toulaymat M, Marconi S, Garb J, Otis C, Nash S. Endoscopic biopsy pathology of Helicobacter pylori gastritis. Comparison of bacterial detection by immunohistochemistry and Genta stain. Arch Pathol Lab Med 1999; 123: 778-81. [ Links ]
36. Riba A, Ingeneri T, Strand C. Improved histologic identification of Helicobacter pylori by immunohistochemistry using a new Novocastra monoclonal antibody. Lab Med 2011; 42: 35-9. [ Links ]
37. Plummer M. Helicobacter pylori and colonic neoplasms. Am J Gastroenterol 2013; 108: 216-7. [ Links ]
38. Humans IWGotEoCRt. Biological agents. Volume 100 B. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum 2012; 100: 1-441. [ Links ]
39. Hartwich A, Konturek SJ, Pierzchalski P, Zuchowicz M, Labza H, Konturek PC, et al. Helicobacter pylori infection, gastrin, cyclooxygenase-2, and apoptosis in colorectal cancer. Int J Colorectal Dis 2001; 16: 202-10. [ Links ]
40. Shmuely H, Passaro D, Figer A, Niv Y, Pitlik S, Samra Z, et al. Relationship between Helicobacter pylori CagA status and colorectal cancer. Am J Gastroenterol 2001; 96: 3406-10. [ Links ]
41. Georgopoulos SD, Polymeros D, Triantafyllou K, Spiliadi C, Mentis A, Karamanolis DG, et al. Hypergastrinemia is associated with increased risk of distal colon adenomas. Digestion 2006; 74: 42-6. [ Links ]
42. Mulholland G, Ardill JE, Fillmore D, Chittajallu RS, Fullarton GM, McColl KE. Helicobacter pylori related hypergastrinaemia is the result of a selective increase in gastrin 17. Gut 1993; 34: 757-61. [ Links ]
43. Graham DY, Go MF, Lew GM, Genta RM, Rehfeld JF. Helicobacter pylori infection and exaggerated gastrin release. Effects of inflammation and progastrin processing. Scand J Gastroenterol 1993; 28: 690-4. [ Links ]
44. Prewett EJ, Smith JT, Nwokolo CU, Hudson M, Sawyerr AM, Pounder RE. Eradication of Helicobacter pylori abolishes 24-hour hypergastrinaemia: a prospective study in healthy subjects. Aliment Pharmacol Ther 1991; 5: 283-90. [ Links ]
45. Kim JH, Park HJ, Cho JS, Lee KS, Lee SI, Park IS, et al. Relationship of CagA to serum gastrin concentrations and antral G, D cell densities in Helicobacter pylori infection. Yonsei Med J 1999; 40: 301-6. [ Links ]
46. Parente F, Imbesi V, Maconi G, Cucino C, Sangaletti O, Vago L, et al. Influence of bacterial CagA status on gastritis, gastric function indices, and pattern of symptoms in H. pylori-positive dyspeptic patients. Am J Gastroenterol 1998; 93:1073-9. [ Links ]
47. Lehmann FS, Schiller N, Cover T, Hatch R, Seensalu R, Kato K, et al. H. pylori stimulates gastrin release from canine antral cells in primary culture. Am J Physiol 1998; 274: G992-6. [ Links ]
48. Konturek SJ, Konturek PC, Hartwich A, Hahn EG. Helicobacter pylori infection and gastrin and cyclooxygenase expression in gastric and colorectal malignancies. Regul Pept 2000; 93: 13-9. [ Links ]
49. Ryberg B, Axelson J, Håkanson R, Sundler F, Mattsson H. Trophic effects of continuous infusion of [Leu15]-gastrin-17 in the rat. Gastroenterology 1990; 98: 33-8. [ Links ]
50. Håkanson R, Sundler F. Trophic effects of gastrin. Scand J Gastroenterol Suppl 1991; 180: 130-6. [ Links ]
51. Thorburn CM, Friedman GD, Dickinson CJ, Vogelman JH, Orentreich N, Parsonnet J. Gastrin and colorectal cancer: a prospective study. Gastroenterology 1998; 115: 275-80. [ Links ]
52. Singh P, Dai B, Wu H, Owlia A. Role of autocrine and endocrine gastrin-like peptides in colonic carcinogenesis. Curr Opin Gastroenterol 2000; 16: 68-77. [ Links ]
53. Sobhani I, Lehy T, Laurent-Puig P, Cadiot G, Ruszniewski P, Mignon M. Chronic endogenous hypergastrinemia in humans: evidence for a mitogenic effect on the colonic mucosa. Gastroenterology 1993; 105: 22-30. [ Links ]
54. Renga M, Brandi G, Paganelli GM, Calabrese C, Papa S, Tosti A, et al. Rectal cell proliferation and colon cancer risk in patients with hypergastrinaemia. Gut 1997; 41: 330-2. [ Links ]
55. Chu M, Rehfeld JF, Borch K. Effects of gastric fundectomy and antrectomy on the colonic mucosa in the hamster. Digestion 1992; 53: 28-34. [ Links ]
56. Sirinek KR, Levine BA, Moyer MP. Pentagastrin stimulates in vitro growth of normal and malignant human colon epithelial cells. Am J Surg 1985; 149: 35-9. [ Links ]
57. Smith AM, Watson SA. Gastrin and gastrin receptor activation: an early event in the adenoma-carcinoma sequence. Gut 2000; 47: 820-4. [ Links ]
58. Hoosein NM, Kiener PA, Curry RC, Brattain MG. Evidence for autocrine growth stimulation of cultured colon tumor cells by a gastrin/cholecystokinin-like peptide. Exp Cell Res 1990; 186: 15-21. [ Links ]
59. Chao C, Hellmich MR. Gastrin, inflammation, and carcinogenesis. Curr Opin Endocrinol Diabetes Obes 2010; 17: 33-9. [ Links ]
60. Fournier DB, Gordon GB. COX-2 and colon cancer: potential targets for chemoprevention. J Cell Biochem Suppl 2000; 34: 97-102. [ Links ]
61. Fosslien E. Molecular pathology of cyclooxygenase-2 in neoplasia. Ann Clin Lab Sci 2000; 30: 3-21. [ Links ]
62. Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, et al. Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 1997; 99: 2254-9. [ Links ]
63. Cuzick J, Otto F, Baron JA, Brown PH, Burn J, Greenwald P, et al. Aspirin and non-steroidal anti-inflammatory drugs for cancer prevention: an international consensus statement. Lancet Oncol 2009; 10: 501-7. [ Links ]
64. Thun MJ. Aspirin and gastrointestinal cancer. Adv Exp Med Biol. 1997;400A:395-402. [ Links ]
65. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 1998; 93: 705-16. [ Links ]
66. Jones M, Helliwell P, Pritchard C, Tharakan J, Mathew J. Helicobacter pylori in colorectal neoplasms: is there an aetiological relationship? World J Surg Oncol 2007; 5: 51. [ Links ]
67. Soylu A, Ozkara S, Alis H, Dolay K, Kalayci M, Yasar N, et al. Immunohistochemical testing for Helicobacter Pylori existence in neoplasms of the colon. BMC Gastroenterol 2008; 8: 35. [ Links ]
68. Cavallo P, Cianciulli A, Mitolo V, Panaro MA. Lipopolysaccharide (LPS) of helicobacter modulates cellular DNA repair systems in intestinal cells. Clin Exp Med 2011; 11: 171-9. [ Links ]
69. Ohshima H, Bartsch H. Chronic infections and inflammatory processes as cancer risk factors: possible role of nitric oxide in carcinogenesis. Mutat Res 1994; 305: 253-64. [ Links ]
70. Chin MP, Schauer DB, Deen WM. Prediction of nitric oxide concentrations in colonic crypts during inflammation. Nitric Oxide 2008; 19: 266-75. [ Links ]
71. Török NJ, Higuchi H, Bronk S, Gores GJ. Nitric oxide inhibits apoptosis downstream of cytochrome C release by nitrosylating caspase 9. Cancer Res 2002; 62: 1648-53. [ Links ]
72. Jaiswal M, LaRusso NF, Burgart LJ, Gores GJ. Inflammatory cytokines induce DNA damage and inhibit DNA repair in cholangiocarcinoma cells by a nitric oxide-dependent mechanism. Cancer Res 2000; 60: 184-90. [ Links ]
73. Peek RM, Miller GG, Tham KT, Perez-Perez GI, Zhao X, Atherton JC, et al. Heightened inflammatory response and cytokine expression in vivo to cagA+ Helicobacter pylori strains. Lab Invest 1995; 73: 760-70. [ Links ]











 text in
text in