Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.3 Bogotá Sept. 2014
Efficacy of Eradicating Helicobacter Pylori for Prevention of Gastric Cancer: Systematic Review and Meta-Analysis
David B. Páramo Hernández MD. (1), Iván Arturo Rodríguez Sabogal MD. (2), Jose Daniel Toledo Arenas MD. MSc. (3)
(1) Gastroenterologist and Clinical Epidemiologist at the Hospital Santa Clara and the Universidad El Bosque in Bogotá, Colombia paramo.david@gmail.com
(2) Internist and Clinical Epidemiologist at the Hospital Santa Clara and the Universidad El Bosque and the Clinica Reina Sofía in Bogotá, Colombia.
(3) Psychiatrist and Epidemiologist, Masters Degree in Controlled Clinical Experiments and Professor of Epidemiology at the Universidad El Bosque in Bogotá, Colombia.
Received: 25-11-13 Accepted: 21-07-14
Abstract
The aim of this systematic review and meta-analysis is to determine the efficacy of eradicating Helicobacter pylori for prevention of gastric cancer.
We conducted a literature review using major databases including PUBMED, EMBASE, CINAHL (EBSCO), Google Scholar, LILACS, Cochrane, ProQuest Dissertations and Theses. Seven experiments were selected out of the 3,934 references obtained by applying our inclusion and exclusion criteria. All seven were randomized controlled trials. The quality of the studies was assessed with the Cochrane assessment of risk of bias. Statistical analysis was performed with REVMAN 5.2.
Out of a total of 5,552 subjects, 55 (2.41%) of the 2,278 patients who had had H. pylori eradicated developed gastric cancer, but 96 (4.22%) of the 2,272 patients who had not had the bacteria eradicated developed gastric cancer (RR: 0.57, CI = 0.42 to 0.79). Follow-up time ranged from 3 to 15 years. The analysis of heterogeneity (Chi square) had a non-significant p value (p = 0.48) confirming the absence of heterogeneity and allowing the meta-analysis. Funnel Plot analysis was used to discard publication bias, and the sensitivity analysis showed no significant changes.
In conclusion, this study suggests that eradication of H. pylori reduces the risk of gastric cancer, particularly in high-risk populations with medium quality evidence. We recommend the practice of using eradicate of H. pylori as a preventive measure.
Keywords
Helicobacter pylori eradication, gastric cancer.
INTRODUCTION
Gastric cancer is one of the leading causes of mortality from malignant neoplasias in Colombia) and in the world (1-4). Helicobacter pylori has been one of the most important risk factors recognized in the genesis of gastric cancer (5-7).
H. pylori is classified by the IARC (International Agency for Research on Cancer) as a biological carcinogen from the Group 1. This group of carcinogens has a proven association between the agent and the neoplasia involved (8). Consequently, eradication of H. pylori has been proposed as a strategy for primary chemoprevention to reduce the incidence of gastric cancer (1, 9-12).
Mortality rates for gastric cancer in Colombia have had a tendency to remain stable despite improvements in the living conditions of the population, better access to health care services and specialized services of endoscopy and gastroenterology. After nearly 25 years of eradication treatment for Helicobacter pylori in a population that receives medical services, the mortality rate from 2000 to 2009 has been stable at around 10 deaths per 100,000 people (1, 2). Although evidence shows that H. pylori is a carcinogen and that chronic infection leads to gastric cancer in susceptible individuals, the development of studies showing that the eradication of the bacteria reduces the incidence of neoplasia has been challenged. Various statistical models estimate that to demonstrate this hypothesis a study with a sample of about 17,625 middle-aged individuals is needed including at least 10 years of follow up. This has not been possible from the logistical, methodological and financial points of view (9). Most studies are not randomized, have design flaws, do not have enough individuals in the sample to prove this causality, have monitoring periods that are too short, and/or have significant losses of sample members during follow-up (13).
The objective of this review and meta-analysis of Randomized Controlled Trials is to determine the efficacy of H. pylori eradication to prevent development of gastric cancer.
METHODS
Once the research protocol was designed a systematic review of the literature was conducted using databases such as: PubMed / Medline, Academic Google, EMBASE, LILACS, Cochrane Collaboration and ProQuest Dissertations and Theses. The following standard search terms were used: Stomach neoplasms, Eradication and Helicobacter pylori with the Boolean operator AND to limit the search to humans and randomized controlled trials. Publications up to December 31, 2012 were reviewed. Next, the two main authors independently conducted an initial selection of studies using titles and abstracts to determine which studies to include and which to discarding. Subsequently, a set of mutually agreed upon studies were chosen by the investigators and inclusion and exclusion criteria were used to obtain a sample of studies upon which Cochrane Collaboration tool for assessing risk of bias was applied using RevMan 5.2 (The English version was used because it was not available in Spanish) (14). The protocol was not registered in the Cochrane Collaboration nor is it available online.
Inclusion and exclusion criteria
Randomized controlled trials completed or published up to December 31, 2012 that met the inclusion criteria were selected. To be included a study had to be a randomized controlled trial with or without bias which studied individuals between 16 and 89 years of age who were of any race, gender, education level and socioeconomic status, and who had received H. pylori eradication treatment with corroborated elimination of the microorganism. Patients must have been evaluated for the development of gastric cancer following eradication of H. pylori, and results must have been reported together with measures of association such as risk ratios and confidence interval or reported in a way that allows those measure to be calculated.
Poorly referenced studies such as randomized controlled clinical cases (cohort studies, case-control studies, metaanalyses, systematic reviews, management guides, and case series). Articles in languages which could not be translated by the authors, articles with incomplete texts, and articles which were not found to be relevant after reading the abstract were also excluded.
Quality assessment of studies
Two authors independently assessed the quality of studies and a third author settled the differences. The Cochrane Collaboration tool for assessing risk of bias was used (14). Each study was assessed for various types of biases and each type of bias was rated High Risk, Low Risk or Unclear Risk according to the criteria specified for applying and qualifying each case. The following types of bias were assessed: sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; data dropouts and incomplete outcome data; selective outcome reporting, and other biases (14).
Data Analysis and Synthesis
The analysis and evaluation of each of the selected clinical studies were performed with Review Manager (RevMan) [Computer program]. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration (English version).
Identification data from each study were entered and later the risk of bias assessment was performed to obtain the risk of bias graph and risk of bias summary. Then the same software was used to test for heterogeneity using the Chi-square and I-square tests. Publication bias was evaluated with funnel plots.
The Mantel-Haenszel method with a fixed effects model of analysis was then used to combine results. The Risk Ratio (RR) was taken as a measure of treatment effects, and the data obtained was presented in a table and a forest plot. To analyze sensitivity, the metaanalysis was repeated after selectively removing each of the studies. Changes in the direction and magnitude of the RR and the confidence interval were then measured. Finally, the Number Needed to Treat (NNT) was calculated as a measure of the impact of the intervention to allow generalization of the results.
RESULTS
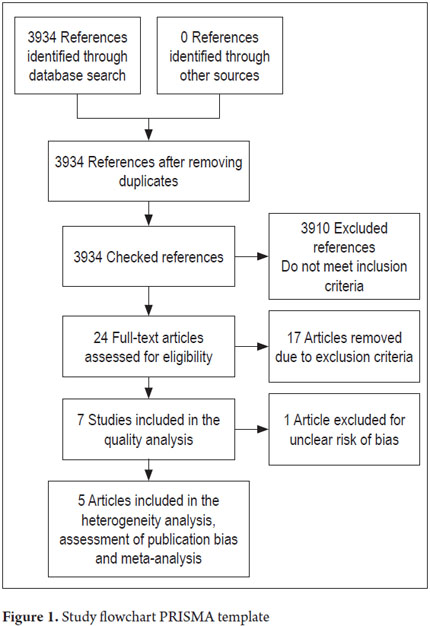
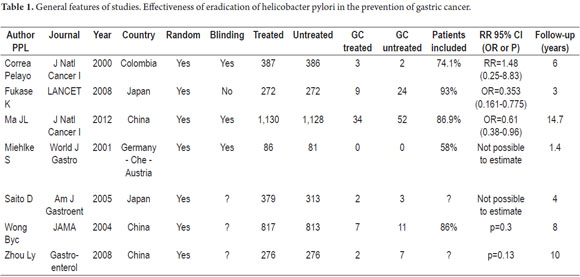
A total of 3,934 references were found. After applying the inclusion and exclusion criteria, seven randomized controlled trials were obtained (Figure 1). Their main characteristics are listed in Tables 1 and 2. They included a total of 5,757 patients: 2,882 in the event group and 2,885 in the control group. It should be noted that only data from the most recently published study by Zhou and collaborators were included (20).
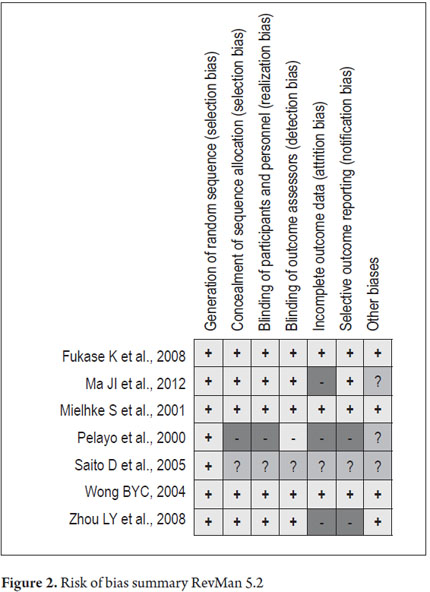
The graph summarizing risk of bias is presented in Figure 2. It is evident that the 2005 study of D. Saito et al. does not allow assessment of many potential biases due to lack of information in the publication. The 2000 study by Pelayo et al. features four high risk, and the 2004 study by Zhou et al. 2004 also has high risk of bias. The other studies generally have low risks of bias.
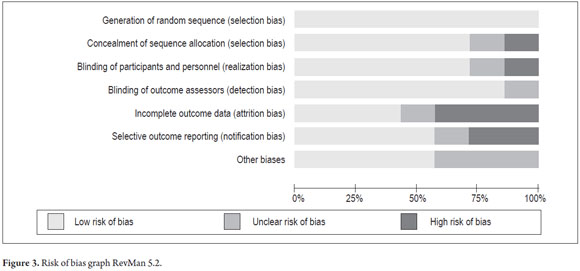
The risk of bias graph of RevMan 5.2 (Figure 3) allows a global summary analyzing the effects of each of the biases evaluated on all studies. The greatest risk of bias is from attrition given by the results for incomplete data. The next largest risk is from reporting bias due to selective reporting of results.
We have seven randomized controlled trials (RCTs): Pelayo et al. in 2000, Milhke et al. in 2001, Wong et al. in 2004, Saito D et al. in 2005, Fukase K et al. 2008, Zhou LY et al. in 2008 and a recent study by Ma Jl in 2012. The Ma Jl study has the largest number of samples, so its analysis carries the most weight. Given the unclear risk of bias in 6 of the 7 items of assessment in the study of Saito D et al. from 2005, it was decided to exclude this study.
It was not possible to estimate heterogeneity with the data published in the study of Milhke et al. 2001 because there was no event group (group of patients with gastric cancer), so this analysis was only performed with the remaining five studies.
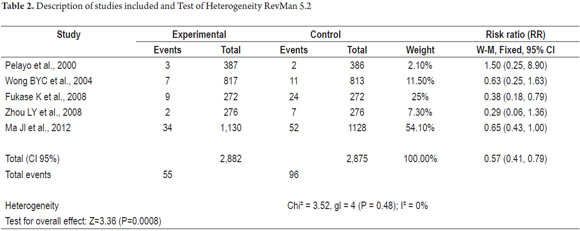
A Chi² of 3.52 was obtained with a p = 0.48 value which was not significant showing that there is no heterogeneity among the studies thus permitting combination of results (metaanalysis). To corroborate this finding the I² test was performed (% of variability in the estimated effect due to heterogeneity rather than to simple error). The result was 0% which means that the effect of heterogeneity may not be important (Table 1).
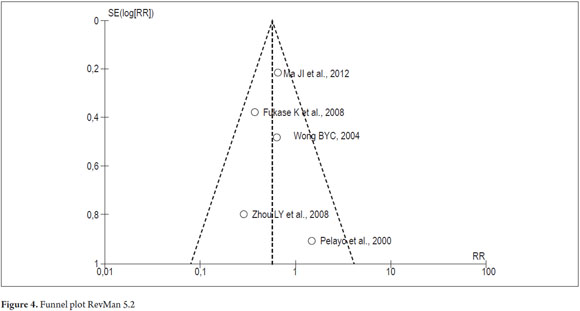
Publication bias was assessed by visual inspection of the funnel plot which showed that it maintained the points of symmetry which indicates an absence of publication bias (Figure 4).
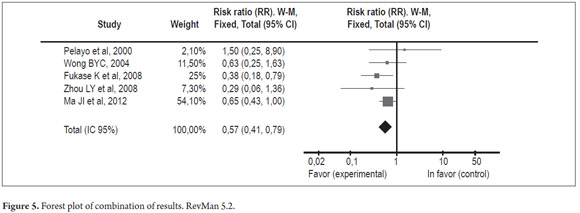
The results were combined in the metaanalysis. The statistical summaries (RR) for each of the studies showed no significant confidence intervals (values over one) except in the study of Fukase in which RR = 0.38 (CI 95%: 0.18-0.79). The study with the greatest weight in the sample was that by Ma Jl et al. in 2012 which accounted for 54.1% of the observations.
A comparison between H. pylori eradication and no intervention found an RR of 0.57 (CI 95%: 0.41 to 0.79) which is statistically significant and which favors intervention as a preventive measure (Figure 5). The direction of the combined summary measure (Rhombus) is in favor of intervention (eradication of H. pylori) as a protective measure against development of gastric cancer and has a significant confidence interval.
The sensitivity analysis showed no change in the direction of the effect or its magnitude and retains statistical significance. Loss of statistical significance occurred only in when the study by Fukase was eliminated, but the direction of RR was preserved.
The Number Needed to Treat (NNT) was calculated as 68 (CI95%: 42-158). In other words, for every 68 people who received eradication treatment for Helicobacter pylori, one case of gastric cancer would be prevented. For public health this represents an important intervention although it is not possible to determine its cost effectiveness since the studies did not analyze this and thus neither this review nor this metaanalysis can do so.
Finally, the Cochrane Collaboration recently introduced quality assessment of the evidence through the GRADE approach. This approach defines the quality of the set of evidence as the degree to which it is possible to have confidence that the estimate of the effect or association will be close to the quantity of interest.
Using GRADE profiler which is available and which is compatible with RevMan 5.2 in the Cochrane Collaboration, the quality of evidence was measured as MODERATE due to the presence of uncertainty given by the use of studies with small numbers of patients, small numbers of events and the presence of wide confidence intervals.
DISCUSSION
This systematic review and metaanalysis aim to assess the efficacy of H. pylori eradication for the prevention of gastric cancer. Our analysis of five clinical trials in which 5,757 subjects were grouped has shown that this intervention reduces the risk of gastric cancer. The relative risk 0.57 (CI = 0.42- 0.79) suggests that eradication is an effective measure for reducing the risk of gastric cancer and is statistically significant.
Our findings for size and direction of effect concur with those in the systematic review and the one metaanalysis found that evaluated this question, published by Fuccio and colleagues in the Annals of Internal Medicine in 2009 (23, 24). However, our work only included randomized controlled trials that examined the incidence of gastric cancer after eradication of bacteria as the final outcome. Unlike Fuccio, we were able to include the experience of Ma Jl and collaborators (22) that was published in 2012 with a follow-up time of 15 years and a considerable number of patients. Fuccio et al. include more experiences in their review, but these have the effect on elimination of pre-neoplastic lesions as the final outcome (24).
Everything in the medical literatures reviews regarding the most suitable moment for intervention within the steps of gastric carcinogenesis suggests that interventions, such as eradication, in the early stages of the illness are probably best for preventing gastric cancer and causing pre-neoplastic lesions to regress (9, 11, 12, 25-27). This topic was not considered in our review.
While the intervention suggests that chemoprevention (eradication) is beneficial and effective for cancer prevention, the impact can be modest (58%), and there may be controversy over its real effectiveness. Nevertheless, it is the basis for important public health proposals around the world, particularly in countries affected by gastric cancer (28), that use H. pylori eradication therapies for prevention of the disease. In addition, major international consensuses have included this concept as sufficient evidence to recommend eradication therapy for this specific purpose (29, 30).
Other issues of great importance for public health which was not included in these studies are recognized etiological factors such as diet (salt, nitrite and nitrates intake) (9, 11, 31-35).
Study limitations includes the small number of clinical trials, the fact that they were performed only in regions of high prevalence of the disease, and variances in study follow-up times. There were also some particular conditions in individual studies such as the study by Fukase and colleagues (21) which included patients with early resected gastric carcinoma who were followed up to detect de novo carcinomas. The study by Ma and colleagues (22) with 15 years follow ups of 2,258 subjects had great weight in our study sample: it accounted for 60.4% of all patients and had a relative risk 0.65 (CI = 0.43-1.0) in favor of risk reduction with intervention. When performing the sensitivity analysis, the weight of this study was very significant within the results obtained, but its exclusion did not alter the results.
Our review shows that the additional randomized controlled trials probably will not change existing ideas. They would also encounter logistical, methodological and financial difficulties, have very long-term follow-up times, and face a major difficulty since there are important ethical objections to inclusion of controls or placebo groups (24).
On the other hand, future challenges include:
The development of effective therapeutic frameworks for the eradication of H. pylori The necessity of evaluating many disease parameters including eradication rates, rates of reinfection and recurrent infections by H. pylori, and percentages of resistance to different antibiotics
Analysis of pathogenic strains and the genetic polymorphisms inherent in study populations (36-40)
Investigating inherent gastric neoplasms which appear in patients in whom the bacterium has been eradicated (41)
And, of course, to the development of an anti-Helicobacter vaccine.
CONCLUSIONS
From the practical point of view, we believe that while the impact on prevention is statistically and clinically significant. H. pylori eradication therapy can be recommended for prevention of gastric cancer, particularly in countries with high risks of gastric neoplasia. Nevertheless, the size of the effect is modest and the quality of evidence moderate. As for implications for future research, we consider that the findings of this review are consistent and that the outlook is unlikely to change with new studies. The door to new etiopathogenetic research and research into prevention of the disease is open.
Conflict of Interest Declaration
All three authors declare that they have no conflicts of interest related to the development and publication of this research.
REFERENCES
1. Otero W. Cáncer gástrico en Colombia: un diagnóstico tardío que amerita el compromiso del Estado. Revista Colombiana de Gastroenterología. 2008; 4(23): 302-304. [ Links ]
2. Instituto Nacional de Cancerología E.S.E e Instituto Geográfico Agustín Codazzi. Atlas de mortalidad por cáncer en Colombia. Tercera edición. Bogotá: Imprenta Nacional; 2011. [ Links ]
3. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71-96. [ Links ]
4. GLOBOCAN 2008 (IARC)(Internet): International Agency of Research on Cancer, WHO, Section of Cancer Information, Citado (12/6/2013). Disponible desde: http://www-dep.iarc.fr . [ Links ]
5. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984; 8390(1): 1311-1314. [ Links ]
6. Yamada T, Searle JG, Ahnen D. Helicobacter pylori in peptic ulcer disease. JAMA 1994; 272(1): 65-69. [ Links ]
7. Uemura N, Okamoto S, Yamamoto S. Helicobacter pylori infection and the development of gastric cancer. NEJM 2001; 345(11): 784-789. [ Links ]
8. International Agency for Research on Cancer, Schistosomes, Liver Flukes and Helicobacter pylori, vol. 61 of IARC Monographs on the Evaluation of Carcinogenic Risks to Human. IARC, Lyons, France, 1994. [ Links ]
9. Correa P, Piazuelo MB, Camargo MC. The future of gastric cancer prevention. Gastric Cancer 2004; 7: 9-16. [ Links ]
10. Ando T, Goto Y, Ishiguro K, et al. The interaction of host genetic factors and Helicobacter pylori infection. Inflammopharmacology 2007; 15: 10-4. [ Links ]
11. Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet 1975; 2: 58-60. [ Links ]
12. Correa P. Human gastric carcinogenesis: a multistep and multifactorial process. First American Cancer Society Award Lecture on cancer epidemiology and prevention. Cancer Res 1992; 52: 6735-40. [ Links ]
13. Sun TT, Wang JL, Fang JY. Quality of RCTs exploring Helicobacter pylori eradication for the prevention of gastric cancer and preneoplastic lesions. Expert Rev Anticancer Ther 2011; 11(10): 1509-1519. [ Links ]
14. Evaluación del riesgo de sesgos en los estudios incluidos. Manual Cochrane de revisiones sistemáticas de intervenciones. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0(updated March 2011). The Cochrane Collaboration, 2011; 197-255. Disponible desde www.cochrane-handbook.org. [ Links ]
15. Correa P, Fontham ET, Bravo JC, Bravo LE, Ruiz B, Zarama G, et al. Chemoprevention of gastric dysplasia: randomized trial of antioxidant supplements and anti-helicobacter pylori therapy. J Natl Cancer Inst 2000; 92(23): 1881-1888. [ Links ]
16. Miehlke S, Kirsch C, Dragosics B, Gschwantler M, Oberhuber G, Antos D, et al. Helicobacter pylori and gastric cancer: current status of the Austrain Czech German gastric cancer prevention trial (PRISMA Study). World J Gastroenterol 2001; 7(2): 243-247. [ Links ]
17. Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 2004; 291(2): 187-194. [ Links ]
18. Saito D, Boku N, Fujioka T, Fukuda Y. Impact of H. pylori eradication on gastric cancer prevention: endoscopic results of the Japanese Intervention Trial (JITHP-Study). A randomized multi-center trial.(Abstract). Gastroenterology 2005; 128: A4. [ Links ]
19. Zhou L, Sung JJ, Lin S, Jin Z, Ding S, Huang X, et al. A five-year follow-up study on the pathological changes of gastric mucosa after H. pylori eradication. Chin Med J (Engl) 2003; 116(1): 11-14. [ Links ]
20. Zhou L. Ten-year follow-up study on the incidence of gastric cancer and the pathological changes of gastric mucosa after H. pylori eradication in China(Abstract). Gastroenterology 2008; 134: A233. [ Links ]
21. Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008; 372(9636): 392-397. [ Links ]
22. Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012; 104(6): 488-492. [ Links ]
23. Fuccio L, Zagari RM, Minardi ME, Bazzoli F. Systematic review: Helicobacter pylori eradication for the prevention of gastric cancer. Aliment Pharmacol Ther 2007; 25(2): 133-141. [ Links ]
24. Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, et al. Meta-analysis: can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med 2009; 151(2): 121-128. [ Links ]
25. Ito M, Haruma K, Kamada T, Mihara M, Kim S, Kitadai Y, et al. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: a 5-year prospective study of patients with atrophic gastritis. Aliment Pharmacol Ther 2002; 16(8): 1449-1456. [ Links ]
26. Kodama M, Murakami K, Okimoto T, Sato R, Uchida M, Abe T, et al. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J Gastroenterol 2012; 47(4): 394-403. [ Links ]
27. Leung WK, Lin SR, Ching JY, To KF, Ng EK, Chan FK, et al. Factors predicting progression of gastric intestinal metaplasia: results of a randomised trial on Helicobacter pylori eradication. Gut 2004; 53(9): 1244-1249. [ Links ]
28. Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer 2013; 132(6): 1272-1276. [ Links ]
29. Malfertheiner P, Megraud F, OMorain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012; 61(5): 646-664. [ Links ]
30. Fock KM, Talley N, Moayyedi P, Hunt R, Azuma T, Sugano K, et al; Asia-Pacific Gastric Cancer Consensus Conference. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol 2008; 23: 351-65. [ Links ]
31. Correa P, Haenszel W, Cuello C, Tannenbaum S, Archer M. A model for gastric cancer epidemiology. Lancet 1975; 2: 58-60. [ Links ]
32. Correa P, Houghton J. Carcinogenesis of Helicobacter pylori. Gastroenterology 2007; 133(2): 659-672. [ Links ]
33. Gaddy JA, Radin JN, Loh JT, Zhang F, Washington MK, Peek RM,Jr, et al. High Dietary Salt Intake Exacerbates Helicobacter pylori-Induced Gastric Carcinogenesis. Infect Immun 2013; 81(6): 2258-2267. [ Links ]
34. Gonzalez CA, Lopez-Carrillo L. Helicobacter pylori, nutrition and smoking interactions: their impact in gastric carcinogenesis. Scand J Gastroenterol 2010; 45(1): 6-14. [ Links ]
35. Kato S, Tsukamoto T, Mizoshita T, Tanaka H, Kumagai T, Ota H, et al. High salt diets dose-dependently promote gastric chemical carcinogenesis in Helicobacter pylori-infected Mongolian gerbils associated with a shift in mucin production from glandular to surface mucous cells. Int J Cancer 2006; 119(7): 1558-1566. [ Links ]
36. García-González MA, Lanas A, Quintero E, et al. Gastric cancer susceptibility is not linked to pro-and anti-inflammatory cytokine gene polymorphisms in whites: a nationwide multicenter study in Spain. Am J Gastroenterol 2007; 102: 1878-92. [ Links ]
37. Hurme M, Lahdenpohja N, Santtila S. Gene polymorphisms of interleukins 1 and 10 in infectious and autoimmune diseases. Ann Med 1998; 30: 469-73. [ Links ]
38. Machado JC, Pharoah P, Sousa S, et al. Interleukin 1B and interleukin 1RN polymorphisms are associated with increased risk of gastric carcinoma. Gastroenterology 2001; 121: 823-9. [ Links ]
39. Figueiredo C, Machado JC, Pharoah P, et al. Helicobacter pylori and interleukin 1 genotyping: an opportunity to identify high-risk individuals for gastric carcinoma. J Natl Cancer Inst 2002; 94: 1680-7. [ Links ]
40. El-Omar EM, Rabkin CS, Gammon MD, et al. Increased risk of noncardia gastric cancer associated with proinflammatory cytokine gene polymorphisms. Gastroenterology 2003; 124: 1193-201. [ Links ]
41. Iijima K, Abe Y, Koike T, Uno K, Endo H, Hatta W, et al. Gastric cancers emerging after H. pylori eradication arise exclusively from non-acid-secreting areas. Tohoku J Exp Med 2012; 226(1): 45-53. [ Links ]











 text in
text in