Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.3 Bogotá Sept. 2014
Esophageal Gastroduodenal Cryptococcosis: A Case Report
Albis Cecilia Hani MD. (1), Rómulo Vargas MD. (2), Alejandro Concha MD. (2), Valeria Atenea Costa MD. (3)
(1) Head of Gastroenterology and Digestive Endoscopy at the Hospital Universitario San Ignacio at Universidad Javeriana in Bogotá, Colombia.
(2) Professor of Gastroenterology and Digestive Endoscopy at the Hospital Universitario San Ignacio at Universidad Javeriana in Bogotá, Colombia.
(3) Resident in the Graduate Program of Gastroenterology and Digestive Endoscopy at the Hospital Universitario San Ignacio at Universidad Javeriana in Bogotá, Colombia.
Received: 03-02-14 Accepted: 21-07-14
Abstract
There are very few patients, whether they are immunocompromised or not, with their gastrointestinal tracts compromised by Cryptococcus neoformans. This is true for both local manifestations as an isolated disease and for the disseminated disease of cryptococcoma. We report a case of cryptococcosis with esophageal, gastric and duodenal compromise in a patient diagnosed with Category 3 AIDS. To our knowledge after a search of the literature, this is the only reported case in Colombia and Latin America.
Keywords
Digestive Cryptococcosis, Cryptococcus neoformans, esophageal gastrointestinal involvement, HIV/AIDS.
CASE REPORT
The patient was a 40 year old man who was born and raised in Bogotá. The patient was married and unemployed. He came to the hospital consulted with symptoms of nonradicular mechanical low back pain following one month of evolution of a painful mass in the mesentery which was gradually growing. Patient expressed a feeling of abdominal distension, but did not have any emesis, dysphagia or alterations in bowel habits.
The patient had been diagnosed in 2011with level C3 AIDS (CD4 (46 K/D), viral load 44,744 copies). The patient also had from miliary tuberculosis involving the mesenteric, peritoneal, pulmonary lymph nodes, a coinfection with hepatitis B, an unknown virological profile, oral and skin Kaposis sarcomas, perianal herpetic ulcers, and a cytomegalovirus CMV infection. The patient had drug dependencies on bazuco (a form of crack cocaine) and alcohol, and had been diagnosed with antisocial personality disorder. He smoked 30 packs of cigarettes per year. At some point prior to consultation the patient had undergone surgical laparotomy to treat abscessed lymphadenopathy.
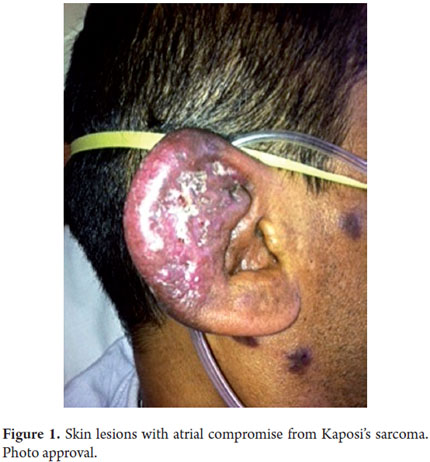
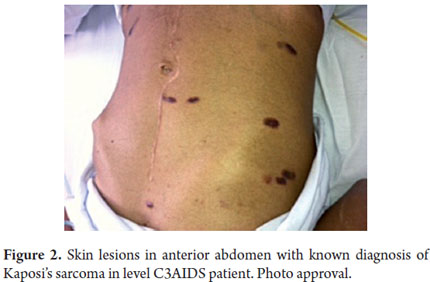
A physical examination showed: heart rate 88 beats/minute, respiratory rate - 18 breaths per minute. Arterial blood pressure 120/80. Oxygen saturation was maintained at 95% using nasal prongs. The patients mucous was dry, he presented violet colored macules on his face, thorax, abdomen and extremities, and his right ear was infected (Figures 1 and 2). His penile frenulum presented violet superficial ulceration along the balanopreputial groove. His abdomen was flat, soft and doughy. The mesentery presented well-defined edges, was very mobile, and was painful upon palpation. He had hypotrophy of his extremities.
An abdominal CAT scan showed concentric thickening of the gastric wall in the pyloric antrum region associated with multiple lymphadenopathies.
A thoracic CAT scan showed profuse and marked thickening of inter- and intralobular septa, with ground-glass opacity in this location and a disordered cobblestone pattern. In addition, many nodules, especially those located in the peripheries of both lungs, had slightly irregular contours. Enlarged lymph sizes were observed in prevascular stations, the aortalpulmonary window, the subcarinal area and in the left armpit. Vascular structures were normal.
Cultures of blood and bone marrow grew Cryptococcus neoformans.
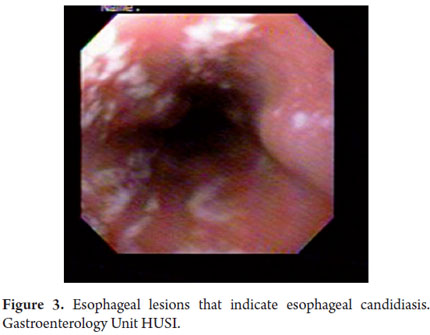
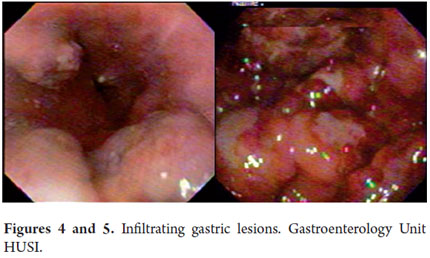
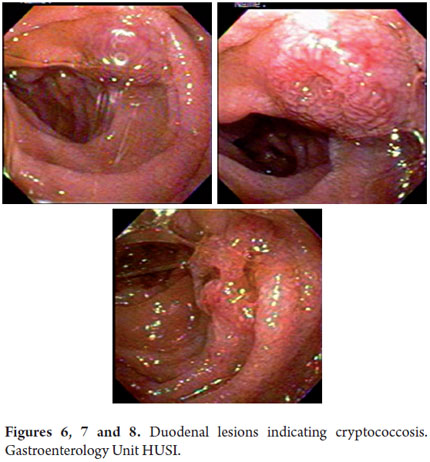
Upper gastrointestinal endoscopy revealed many whitish lesions in the middle and proximal thirds of the esophagus which had the appearance of cotton in the distal third of the esophagus (Figure 3). On the left side there was a raised purplish 1 cm lesion which was difficult to biopsy. Retroflexion in the fundus revealed two raised violet lesions with central depressions. They measured 1cm and 2 cm respectively and were located toward the greater and lesser curvatures. The gastric corpus was not distended because of insufflation, had distorted folds and a narrowed opening. A 3 cm raised lesion was found near the angular incisura. Biopsies were taken from this lesion and from the folds. The antrum was also not distended because of insufflation and had an appearance similar to that of the corpus but without any exophytic lesions (Figures 4 and 5). The pylorus was centered and appeared to be infiltrated. In the duodenal bulb, there were multiple elevated violet lesions with central depressions that measured from 6mm to 12 mm. They extended up to the second and third duodenal portions (Figures 6, 7, and 8). The initial endoscopic diagnosis was that esophageal candidiasis grade II injury still needed to be ruled out but that lesions in the esophagus, fundus, subcardial area, and duodenum could well be Kaposis sarcomas.
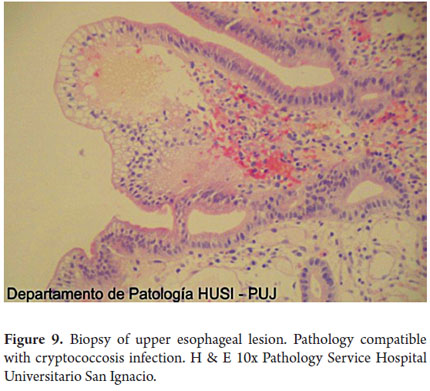
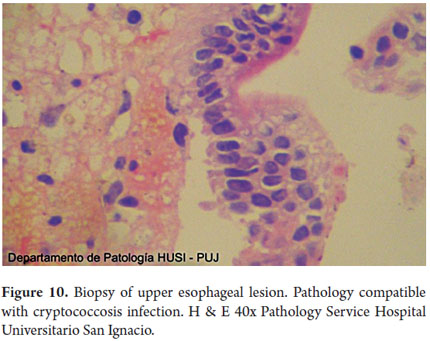
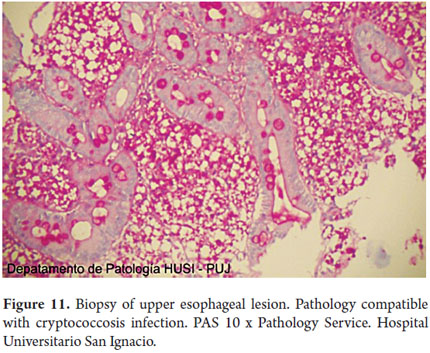
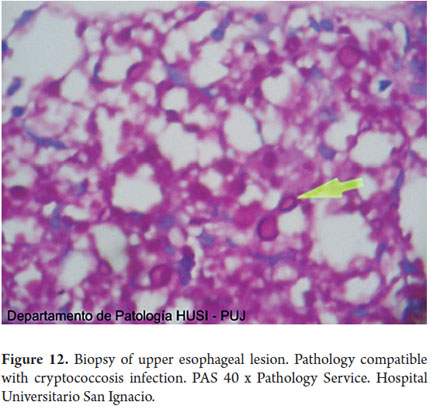
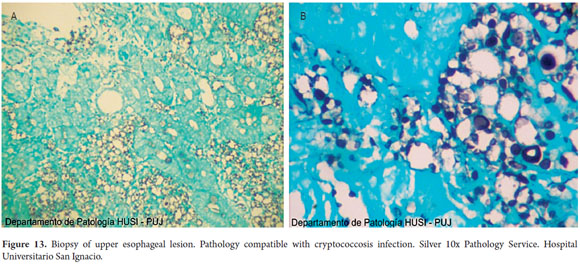
Special histochemical stains for fungi that were used on biopsies from the esophagus, stomach and duodenum showed forms compatible with cryptococcosis (Figures 9, 10, 11, 12 and 13).
On the basis of these clinical, endoscopic and pathological findings, a diagnosis of gastroesophageal cryptococcosis was made. The patient was kept in the hospital and placed on antifungal therapy consisting of amphotericin B plus 800 mg of oral fluconazole daily for two weeks. This was followed by treatment with oral fluconazole. After improvement in abdominal distention, the patient was discharged from the hospital and monitored as an outpatient. The patient has not had any additional endoscopic examinations as of the date of publication of this article.
DISCUSSION
We report a case of esophageal, gastric and duodenal cryptococcal infection in a patient with a known diagnosis of level C3AIDS in which we found disseminated cryptococcosis.
NATURE OF THE INFECTION: PREDISPOSITIONS
It has been over a century since cryptococcus was first described in 1894, but only in the last 30 years have researchers begun to understand this complex and devastating body. Moreover, despite current treatments, patients still die of cryptococcosis all over the world (1).
Although widespread use of antiretroviral therapy has decreased the incidence of cryptococcosis in developed countries, the incidence and mortality rates are high in areas where HIV is still uncontrolled, and access to antiretroviral therapy is limited. It is estimated that worldwide there are about 1 million cases of patients with cryptococcosis and HIV annually (2).
It is known that despite intensive medical care and the availability of antiretroviral therapy, the disease has high rates of mortality. Meningeal cryptococcosis, to take just one example, has a three month mortality rate of 20%. The mortality rate is estimated at 100% in the post-consultation period of two weeks for patients who do not receive antifungal therapy (2).
Cryptococcus neoformans is a ubiquitous organism in soils rich in bird droppings. The natural defense against this fungus depends primarily on cell-mediated immunity in which CD4 T lymphocytes play a central role (3).
In recent years cryptococcus neoformans has been recognized as an opportunistic and often fatal fungal infection especially for people in states of immunosuppression. Although it is primarily a disease of the central nervous system, it can infect a variety of organs (4).
Conditions that predispose patients to primary cryptococcus infections, disseminated or otherwise, include Cushings syndrome, organ transplantation, leukemia, lymphomas, cirrhosis and diabetes (3).
It is important to note that cryptococcus infections have also been reported in heterogeneous groups of patients who receive high-dose steroids, monoclonal antibodies such as Alemtuzumab and Infliximab and/or other immunosuppressants (2).
PATHOPHYSIOLOGICAL MECHANISMS
Opportunistic pathogens break the hosts line of defense while non-opportunistic pathogens induce a fundamental defect in the immune system or circumvent normal defense mechanisms to infect their host (1).
Cryptococcus neoformans preferentially infects immunocompromised individuals. CD4 and CD8 cells are necessary for effective elimination of cryptococcus in murine models. This suggests that it possesses virulence factors to evade the immune systems initial defense. Its polysaccharide capsule is cryptococcus neoformans most important and powerful virulence factor (1).
This capsule depletes complement components by binding them, but cryptococcus neoformans also produces melanin, superoxide dismutase and mannitol which are antioxidants that neutralize specific effector molecules of the innate immune system. Other virulence factors include phospholipase which destabilizes host cell membranes, urease which alters pH, and proteinases which degrade host proteins (1).
CLINICAL MANIFESTATIONS
Infection occurs through the respiratory route, and extra pulmonary manifestations occur as a result of hematogenous spread. While the central nervous system is most commonly affected in symptomatic cryptococcosis, a variety of extra-neurological locations including various parts of the gastrointestinal tract have been reported (3).
Cryptococcal infections are seen in patients with less than 200 CD4 and are manifested in the lungs, central nervous system and eyes. Pulmonary infiltrates and meningitis are the most common (4).
Cryptococcus neoformans is the most common etiological agent causing meningitis in patients who are immunosuppressed and in patients who are not immunosuppressed. During infection the fungus is an observed in the form of yeast and is only occasionally seen as hyphae (filamentous forms) or pseudohyphae (5).
GASTROINTESTINAL CRYPTOCOCCOSIS AND OTHER DIGESTIVE DISEASES
Isolated cases of gastrointestinal cryptococcosis resulting from dissemination of the fungus have been reported. Although there is now a higher incidence of the disease, attributing gastrointestinal clinical symptoms to cryptococcosis is extremely unusual (4).
In HIV patients, cryptosporidium parvum and CMV are usually the cause of gastric ulcers and inflammation, but other organisms such as toxoplasma gondi, Leishmania donovani, Treponema pallidum, mycobacterium avium complex, Bartonella henselae, and Cryptococcus neoformans have been involved (4).
HIV patients may have gastrointestinal symptoms due to opportunistic infections. These are characterized by nausea, vomiting, early satiety, anorexia, abdominal pain and hematemesis. Cytomegalovirus gastritis or gastrointestinal sarcoma may also be associated with extragastric impairment (6).
Gastrointestinal Kaposis sarcoma may be associated with human herpesvirus-8 (HHV-8), complicates skin diseases in up to 50% of patients, and mainly affects the stomach. Compromise of the small intestine and colon occurs in patients with very advanced states of immunodeficiency. Compromise of the small intestine usually produces distention, nausea, colic, and profuse diarrhea that may be associated with malabsorption and weight loss (6).
Most opportunistic infections in HIV patients occur when the CD4 count less than 200. Cytomegalovirus is the virus most commonly implicated in gastrointestinal tract compromise in these patients. Mycobacterial infections are also common, and coinfection has been seen in 40% of cases. Mycobacterial infections may present as diarrhea and/or abdominal pain (7).
One author who examined the autopsy reports of 24 patients who had been diagnosed with pulmonary or disseminated cryptococcosis found that a third of them had evidence of gastrointestinal compromise. This affected, in order of frequency, the colon, esophagus, stomach and small intestine. In all eight cases there was a predisposing condition underlying immunosuppression, and in seven of the eight cases several other extrapulmonary sites were involved (8).
GASTROINTESTINAL COMPROMISE DUE TO CRYPTOCOCCUS NEOFORMANS IS EXTREMELY RARE
No gastrointestinal compromise or disease was reported in either a series of 68 pre-mortem diagnoses of patients with cryptococcosis and AIDS or a larger series of 106 patients with AIDS and cryptococcosis (8).
Laguna et al. have documented that over 90% of HIV patients experience gastrointestinal symptoms at some point during the course of their disease. Opportunistic gastrointestinal infections are more common in the esophagus and intestine and less common in the stomach.
Another case that has been reported is that of a patient with peritonitis who underwent a laparotomy which showed jejunal perforation and was subsequently found to have cryptococcosis in examinations of biopsies. This was a case with an unusual clinical presentation for an HIV patient (7).
Cases in which the initial presentation of gastric cryptococcosis in AIDS patients was esophageal herpes have also been reported (4).
One African AIDS patient with hematochezia was diagnosed with anal ulceration due to cryptococcosis (3).
ENDOSCOPIC FINDINGS AND DIAGNOSTIC METHODS IN CASES OF DIGESTIVE CRYPTOCOCCOSIS
Lesions due to cryptococcosis and found endoscopically have presented as nodules, ulcers and plates (7), and fine yellowish granular mucosa may has also been described (8).
Gastric lesions may be evident endoscopically as inflamed polyps or nonspecific erosions. Duodenal lesions may be observed as multiple white plaques (8).
Diagnosis of gastrointestinal cryptococcosis is difficult due to the scarcity of the fungal microorganism and failure to recognize it in biopsies since it stains positive for mucin chains (4).
Blood cultures of patients with disseminated disease are positive in up to 50% of cases (9).
There are reported cases in which the initial presentation of disseminated cryptococcosis was gastroduodenal cryptococcosis. Gastrointestinal compromise due to cryptococcus neoformans has had poor results (7).
TREATMENT
The total duration of antifungal therapy for patients like ours who are immunosuppressed HIV patients with cryptococcemia or cryptococcosis with or without meningitis had been determined to be 12 months. This includes an initial 4 to 6 weeks treatment with 0.7-1.0 mg/Kg/day of intravenous amphotericin B deoxycholate plus 100 mg/kg/day of oral flucytosine (divided into four separate doses) for at least for two. This is followed by fluconazole 6 mg/Kg day (400 mg/day) for at least 8 weeks. Lipid formulations can be substituted for amphotericin B especially in patients with renal dysfunction or predisposition to it (2).
Another induction option is amphotericin B plus 800 mg fluconazole daily for 2 weeks followed by 800 mg/day of fluconazole alone for at least 8 weeks (2).
After the initial induction phase, consolidation therapy consists of 400 mg/day of fluconazole for 8 weeks. Then maintenance therapy of 200 mg/day of fluconazole or 400 mg/day of itraconazole should be completed over the course of one year (2).
Antiretroviral therapy should be initiated two to ten weeks after starting antifungal treatment. It is important to consider stopping antiretroviral if the patient has more than 100 CD4 cells per cubic millimeter of blood and an undetectable or very low viral load for three months. Restarting maintenance antiretroviral therapy should be reconsidered if the CD4 count falls below 100 (2).
Often unfairly ignored as a minor category of publication, case reports are a valuable educational resources and shared reflections of our most challenging cases. Case reports are also a great introduction for gastroenterology residents to the world of academic publishing. These cases inform, educate and promote debate. They are a humble reminder that things in medicine are not always as they seem and that diseases do not read textbooks (10).
In this particular case, it was not thought that the patient might have digestive symptoms of fungal infection due to the low incidence of these diseases in clinical practice and the attribution of endoscopic findings to other infectious and neoplastic diseases.
We consider it important to report this case because until this case there had been no cases of esophageal or gastrointestinal cryptococcosis reported in Colombia. A literature review studying cases over the course of nine years in Colombia reported 891 (95.7%) cases of neurocryptococcosis, 27 (2.9%) pulmonary forms, 5 (0.5%) cases with cutaneous lesions, two (0.2%) cases involving lymph nodes, two (0.2%) oropharyngeal lesions and one case (0.1%) of each of the following: clinical peritonitis, liver lesion, and lower limb cellulite with urinary tract infection. There were no cases of gastrointestinal compromise (11). Similarly, according to our research and knowledge, there have been no other cases reported in Latin America.
Acknowledgements
We would like to thank the pathology service at the Hospital Universitario San Ignacio.
REFERENCES
1. Shaunna M. Huston, Christopher H. Mody. Cryptococcosis: An Emerging Respiratory Mycosis. Department of Medical Science, University of Calgary, Alberta, Canada. Clinics in Chest Medicine 2009; 30(2): 253-64. [ Links ]
2. Perfect, Jhon et al. Clinical Practice Guidelines for the Management of Cryptococcal Disease: 2010. Update by the Infectious Diseases Society of America IDSA. 2010. [ Links ]
3. Chaitowitz, Mark; Marie-Leen Shaw; Mokoena, Taole R. Gastrointestinal cryptococcosis presenting as spontaneous jejunal perforation in a non-immunocompromised Host. Digestive Diseases and Sciences 2003; 48: 1196-9. [ Links ]
4. Rupashree Sundara, Lakshmi Raoa. Gastric cryptococcal infection as an initial presentation of AIDS: a rare case report). Asian Pacific Journal of Tropical Medicine 2011; 4: 79-80. [ Links ]
5. Xiaorong Lin. Cryptococcus neoformans: Morphogenesis, infection, and evolution. Review Article Infection. Genetics and Evolution 2009; 9: 401-416. [ Links ]
6. Mandell, Douglas, Bennetts. Principles and Practice of Infectious Diseases. 7th ed. 2009. [ Links ]
7. Saha S, Agarwal N, Srivastava A, Kumar A. Perforation peritonitis due to gastrointestinal cryptococcosis as an initial presentation in an AIDS patient. Singapore Med J 2008; 49(11): e305-7. [ Links ]
8. Naga Chalasani, et al. Endoscopic features of gastroduodenal cryptococcosis in AIDS. Gastrointestinal Endoscopy 1997. [ Links ]
9. T Ferry, B Ponceau, JB Gaultier, MA Piens, F Biron, S Picot, C Chidiac, D Peyramond. Disseminated cryptococcosis and histoplasmosis co-infection in a HIV-infected woman. France Journal of Infection 2005; 51(3): 173-176. [ Links ]
10. Bhavesh B Shah. "Just write it up" the art of writing a case report in Gastroenterology. Gastrointestinal Endoscopy 2009; 70(5): 977-979. [ Links ]
11. Lizarazo J, Linares M, et al (Grupo colombiano para el estudio de la criptococosis). Estudio clínico y epidemiológico de la criptococosis en Colombia: resultados de nueve años de la encuesta nacional, 1997-2005. Biomédica 2007; 27: 94-109. [ Links ]











 text in
text in