Servicios Personalizados
Revista
Articulo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Accesos
Accesos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en
SciELO
Similares en
SciELO -
 Similares en Google
Similares en Google
Compartir
Revista colombiana de Gastroenterología
versión impresa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.4 Bogotá oct./dic. 2014
Evaluation of the Efficacy of Argon Plasma Treatment of Barrett's Esophagus with Dysplasia: Prospective Follow-up Evaluation not less than one year
Rodrigo Castaño MD. (1), Oscar Álvarez MD. (2), Amy Piñeres MD. (3), Mario H Ruiz MD. (4), Andrés Rojas MD. (5), Alejandra Álvarez (6), Luis Miguel Ruiz (6), David Restrepo (7), Víctor Calvo (8)
(1) Gastrointestinal Surgeon and Endoscopist, Chief of the Graduate General Surgery Program at Universidad Pontificia Bolivariana, Member of the Gastrohepatology group at the Universidad de Antioquia and the Instituto de Cancerología Clínica las Américas in Medellín, Colombia. rcastanoll@hotmail.com
(2) Radiologist, Internist and Gastroenterologist, Professor at the San Antonio Campus of the University of Texas, Director of Gastroenterology at the Veterans' Administration Hospital of Texas Valley Coastal Bend in Harlingen, Texas.
(3) General Surgeon at the Clínica Bolivariana in Medellín, Colombia.
(4) General Surgeon at the Hospital Pablo Tobón Uribe in Medellín, Colombia.
(5) General Surgeon at the Instituto de Cancerología-Clínica las Américas in Medellín, Colombia.
(6) Medical Student in the Faculty of Medicine of the Universidad Pontificia Bolivariana in Medellín, Colombia.
(7) Medical Student in the Faculty of Medicine of the Universidad CES in Medellín, Colombia.
(8) Statistician in Medellín, Colombia.
Received: 31-03-14 Accepted: 05-11-14
Abstract
Introduction: Barrett's esophagus (BE) with dysplasia has a recognized potential for malignancy. Neither acid suppression nor anti-reflux surgery produce consistent or complete regression of metaplasia or dysplasia in the epithelium. Endoscopic argon plasma ablation (APC) offers the possibility of eradication, but factors that may influence the outcome of therapy have not been consistently evaluated.
Objective: The objective of this study was to evaluate the efficacy of APC for eradication of BE with dysplasia and to evaluate the factors that influence the immediate outcome and results after one year of follow up.
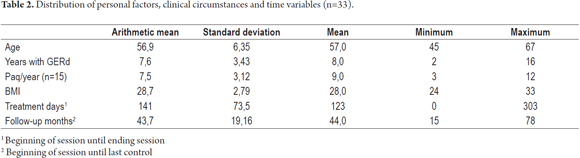
Methods: The study population included thirty-three patients suffering from BE with dysplasia: 19 men (58%), mean age of 56.9 ± 6.35 years (range 45-69 years) and an average length of EB 4.1 cm (range 2 to 8 cm.). All patients had undergone APC at intervals of 4 to 6 weeks to eradicate Barrett's esophagus. Patients also received double doses of proton pump inhibitors (PPIs). Patients were endoscopically monitored at 3, 6 and 12 months and thereafter every year with four-quadrant biopsies of treated areas.
Results: Ablation of BE and dysplasia was achieved in all patients after a median of 2 sessions of APC (1-4 sessions). Recurrence of BE and dysplasia is associated with longer initial lengths affected by BE and larger circumferences of its circular or proximal margin, despite treatment with additional APC sessions (no significant differences). Documented recurrences of Barrett's esophagus occurred in seven patients (21.2%), documented recurrences of dysplasia occurred in three patients (9.1%). Two of these were low grade and one was high grade. The most frequent complication was chest pain which was experienced by six patients (18.2%), four had stenoses that required endoscopic dilatation (12.1%), and one patient's (3%) bleeding required sclerotherapy during the procedure. The average follow-up time was 43.7 ± 19 months. No esophageal carcinoma developed nor were there any deaths related to the disease or therapy.
Conclusions: Treatment with APC is safe and effective ablative therapy for short segments of BE with dysplasia. Post treatment recurrences of BE and dysplasia are common especially among patients with long segments of BE.
Keywords
Barrett's esophagus, gastroesophageal reflux, argon plasma, dysplasia in the esophagus.
INTRODUCTION
Barrett´s esophagus (BE), also known as Barrett´s epithelium, is a premalignant condition that is secondary to chronic gastroesophageal reflux (1). Recent prospective studies using endoscopic monitoring estimate that the incidence of esophageal adenocarcinoma associated with BE has decreased and is approximately 1:300 patients per year (2, 3). Nevertheless, the incidence of esophageal adenocarcinoma has increased by as much as sixfold over the last 30 years (4). Serious risk factors regarding esophageal adenocarcinoma include long duration of BE (5), masculine gender (6), the presence of dysplasia or hiatal hernia (7, 8), alcohol (9) and/or tobacco consumption (10), obesity and possibly duodenal gastroesophageal reflux (11, 12). On the other hand, consumption of vegetables, folic acid and antioxidants protects against BE (13).
The current handling of BE includes endoscopic monitoring although this continues to be a debatable measure due to the morbidity and mortality associated with esophagectomies used to treat high grade dysplasia and early adenocarcinomas (3). There is also a lack of conclusive evidence about its cost-effectiveness (14). The focus of medical therapy is to stop gastroesophageal reflux using powerful agents that reduce acid secretion such as proton pump inhibitors (PPIs) (15). Surgery is also recommended for the control of reflux, particularly for patients who are intolerant to drugs or whose response to treatment with drugs is inadequate (16). Unfortunately, none of these approaches have been shown to induce the regression of BE in a complete or consistent way (17, 18).
Consequently, new thermo-ablation techniques continue to be considered in the context of suppression of gastric acid secretion (19). One of these techniques is argon plasma coagulation (APC) which consists of administration of a high frequency electrical current to tissues by way of a flow of ionized argon gas (plasma). This technique does not involve contact of a catheter with any tissue. The thickness of BE is 0.6 mm which is within the limits of APC therapy (20, 21). APC is less costly, more portable, and safer than treatments such as laser and photodynamic therapy (22, 23). Coagulation with APC is widely available, permits the treatment of large areas, has low operation costs, is effective and safe, and is easy to use in the hands of expert endoscopists. The rate of removal of the metaplastic epithelium is quite encouraging, though these results should be interpreted with caution due to the meagerness of the available information series and the short follow up period (24, 25).
The final goal of this therapy is to uproot the tissue with intestinal metaplasia and reduce the malignant potential related to the presence of BE. A limited number of studies have reported on the efficiency of therapy with APC for the ablative handling of BE with dysplasia or cancer in situ (26-30). Although some studies suggest application in non-dysplastic BE (31-37), its benefit in many others is not clear (22, 38-45). APC has even been used in combination with surgery (46-51). Nevertheless, the monitoring of these patients has been brief and the resulting long term stability of the neo-squamous epithelium is uncertain (52).
The objective of this study is to evaluate the efficiency of APC as an ablative therapy for BE with mild or severe dysplasia. The study was conducted among various third level institutions in Medellin. It investigated the factors that influence the outcomes of therapy and the stability of the neo-squamous epithelium after at least one year of monitoring.
PATIENTS AND METHODS
Type of study
This is a descriptive observational study of a series of cases in which patients diagnosed with BE with mild or severe dysplasia were treated with APC (endoscopic ablation) and followed-up at two third level endoscopy services in Medellin, in the period between January 2006 and December 2011.
Diagnosis of Barrett's esophagus
All patients had columnar epithelia in the esophagus (intestinal metaplasia diagnosed histologically) above the gastroesophageal junction. This was endoscopically determined as tissue that extended for more than a centimeter above the most proximal folds of the stomach. BE was measured according to the Prague classification criteria, so the length of circumferential BE (CB) and the distance of the proximal margin of BE were both measured. Initially, a lavage with a mucolytic agent (N- acetyl cysteine) was done to achieve better fixation of the methylene blue dye. With the advent of digital chromoendoscopy (NBI or FICE) this procedure was suppressed. Biopsy samples were taken from the four quadrants at 1 to 2 cm intervals for histological confirmation of BE prior to ablation. The concomitant presence of high or low grade dysplasia and the presence of nodulations (possible dysplasia with raised lesion) were either discarded or confirmed. Biopsy samples were always taken from these patients and also during follow-ups in order to document the re-epithelialization of the squamous epithelium and to look for evidence of "buried" BE glands. All samples were evaluated by at least two pathologists with a special interest and training in gastrointestinal histopathology (JCP). None of the patients underwent endoscopic ultrasound.
Inclusion criteria
Patients with endoscopic, histopathological and clinical diagnoses of BE with dysplasia of any degree who were between the ages of 18 and 70 years of age were included.
Exclusion criteria
The following patients were excluded: those who declined to participate in the study, patients who could not be monitored through time, those with terminal illnesses, those who had undergone previous esophageal surgery, and patients with invading esophageal adenocarcinoma.
Sample Population
The sample was a non-probabilistic sample of continuous cases: patients who met the inclusion criteria of diagnosis of BE with dysplasia who were treated by endoscopic APC in participating medical centers entered the study in a consecutive manner.
Collection technique
The information was collected in a retrospective and prospective manner from the detailed registers of medical records, endoscopy reports and histopathology results of patients that met the inclusion criteria. The investigators recorded this data in a previously designed tool.
Medications
All patients were treated with PPIs, usually 20 or 30 mg of omeprazole or lansoprazole twice a day in order to reach adequate suppression of acid. For patients with persistent acid reflux, the PPI dosage was increased above these levels.
Coagulation with argon plasma
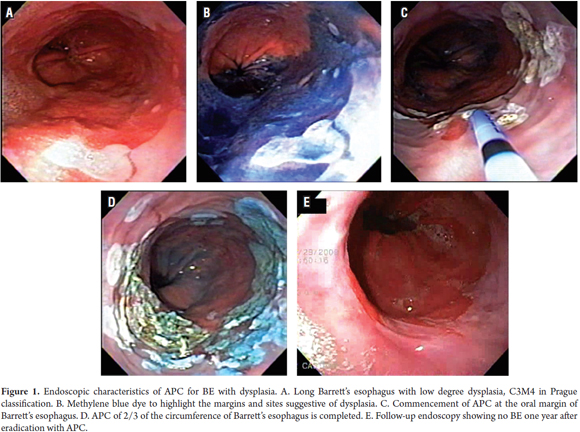
Patients were treated on an outpatient basis. In order to inhibit esophageal peristalsis, patients were sedated with a mix of 3 to 5 mg IV midazolam IV, 20-50 mg IV meperidine, and 10 to 20mg IV hyoscine hydrobromide. Pulse oximetry and blood pressure were monitored during the entire process. Ablation was performed using an Erbe APC 200 system (Erbe Medical UK Ltd, Leeds, UK) and a flexible Erbe gastrointestinal probe set at an adjusted intensity of 50 watts and a flow of 1 liter/minute. Patients were treated at monthly intervals with an aim of treating up to two thirds of the esophageal circumference per session until macroscopic clearance was achieved. Spot ablation was used for shorter segments and islands of BE. Larger areas were treated with downward (head to toe) movement of the probe to achieve complete coagulation of the damaged tissue (Figure 1).
Any symptom related to the procedure was registered by the patient. In addition all patients were contacted by telephone 24 hours after treatment to determine if any complications had developed. Persistence of BE in an area greater than 10% of the original area following two sessions of APC was defined as therapeutic failure. Failure of squamous re-epithelialization following two sessions of APC was also considered to be therapeutic failure. Patients with documentary evidence of some beneficial effect of the therapy continued in the study process for to a maximum of four sessions.
One year monitoring
The patients were trained and encouraged to continue with the same dosages of PPIs that were used during the study. They underwent check-up endoscopies at three month intervals until one year after completion of treatment. The extent of any remaining BE was documented and additional biopsies were taken according to the Seattle protocol for biopsies of one sample every two centimeters from all four quadrants.
Statistical Analysis
Absolute distributions, relative distributions and measures of central tendency, position, and dispersion were used in the descriptive analysis. The chi-square test of independence, Fisher's exact test and ratio of verisimilitude were used whenever necessary. The Kolmogorov-Smirnov test was used to establish a criteria of normality for the data. On that basis the Student's t-test was applied for the mean difference. A p value of less than 0.05 was considered to be statistically significant. The Kaplan-Meier method was used for the analysis of survival and the Log Rank (Mantel-Cox) and Breslow (Generalized Wilcoxon) were used for comparisons of data curves. SPSS Statistics version 17 was used for data entry, processing and analysis.
Informed consent and ethical issues
All patients included in the study signed an informed consent form before undergoing endoscopic APC. The study was approved by the Universidad de Antioquia Ethics Committee which evaluates the work performed by interns who apply for the title of specialist.
RESULTS
From April 2004 to September 2009, a total of 33 patients suffering from BE with dysplasia were included in this study. Ten 10 patients (30%) had severe dysplasia and 23 patients (70%) had low grade dysplasia. All patients underwent APC treatment, and all the patients continued receiving high doses of PPIs after APC treatment.
By the end of treatment all thirty-three patients had complete responses including regeneration of squamous epithelia. This was verified by the lack of endoscopic evidence of columnar epithelium segments and histological confirmation of the presence of squamous epithelia. The average monitoring time was 43.7 ± 19 months. Three patients (9.1%) required only one session each of APC. Nineteen patients (57.6%) required two sessions each, and eleven patients (33.3%) each required three sessions.
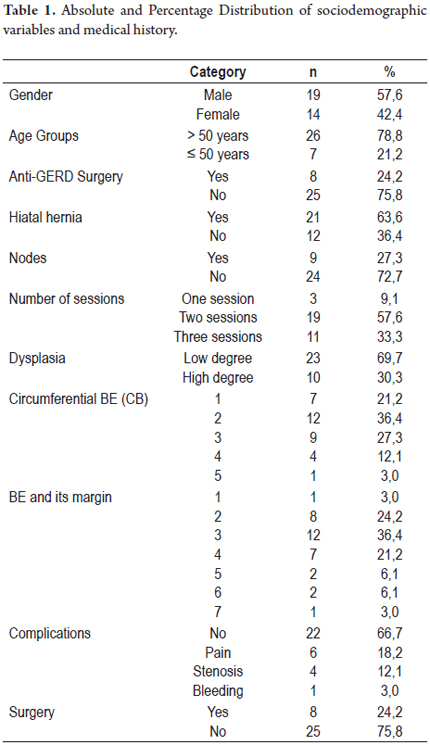
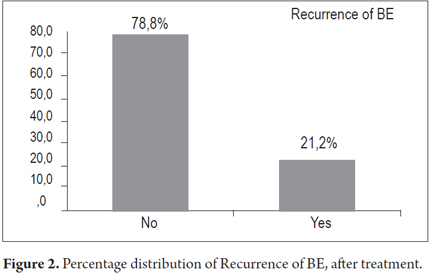
There were recurrences in 30.3% of the patients, recurrence of BE's esophagus in seven patients (21.2%) and dysplasia in three patients (9.1%). There were no cases of progression toward malignancy encountered in biopsies during monitoring period.
Complications were documented in 33.3% of the patients: pain in 6 patients (18.2%) which was either managed without drugs or with analgesics, stenoses in 4 patients (12.1%) and bleeding during the procedure in 1 patient (3%). The case of bleeding was treated through endoscopic sclerotherapy. During monitoring there were no mortalities associated with the treatment and/or the illness. The general characteristics of the group are shown in Table 1.
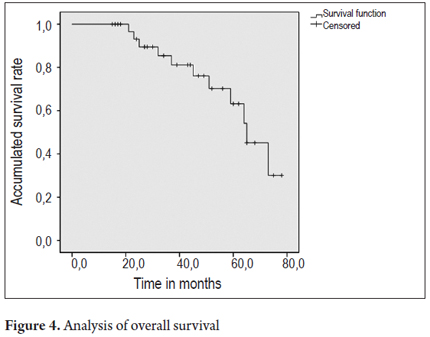
Finally, there were recurrences of BE in 7 patients and recurrences of dysplasia in 3 patients. Cancer did not develop in any of the patients treated (Figures 2 and 3).
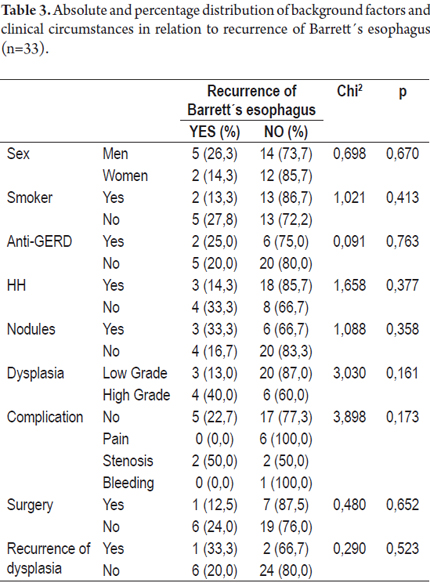
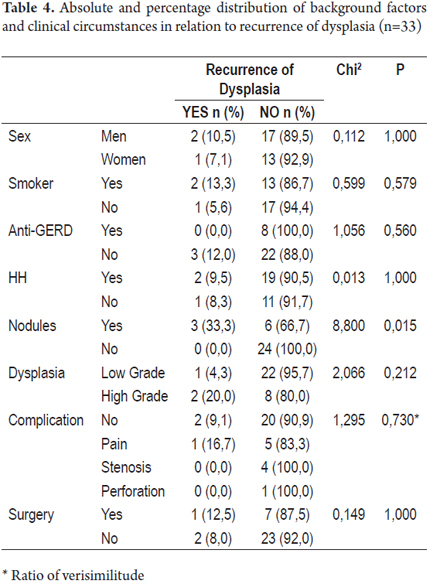
Three of the patients with low grade dysplasia (13.0%) experienced recurrences of BE after treatment while four patients with high grade dysplasia (40%) had recurrences of BE.
Of the patients with dysplasia, one patient (4.3%) presented a recurrence of low grade dysplasia after treatment and two patients who had had high grade dysplasia (20.0%) presented recurrences, one low grade and one high grade.
The average age of the patients in this study was 56.9 ± 6.35 years. Fifty percent of the patients had had GERD during the previous eight years. It is important to emphasise that the average duration of treatment from beginning to end was 141 ± 73.5 days, and the average time spent monitoring patients until their last check-up was 43.7 ± 19 months (See Table 2).
Tables 3 and 4 present absolute and percentage distributions of antecedents and clinical circumstances related to presence of recurrence of Barrett's Esophagus and/or dysplasia.
In patients who experienced recurrences of BE, the average time that they had suffered from GERD was 9.3 ± 2.8 while the average time suffering from GERD for patients who had no recurrences of BE was 7.2 ± 3.5. No statistically significant differences were found between these variables (p>0.05) as was the case for the variables of age and body mass index (BMI), days of treatment and months of monitoring (See Table 5).
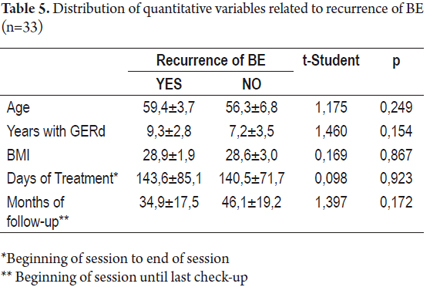
The patients that presented a relapse of Dysplasia and BE presented at least a one centimeter longer BE, both in its circumferential compromise (BC Prague classification) as well as in its proximal margin (BM Prague classification)
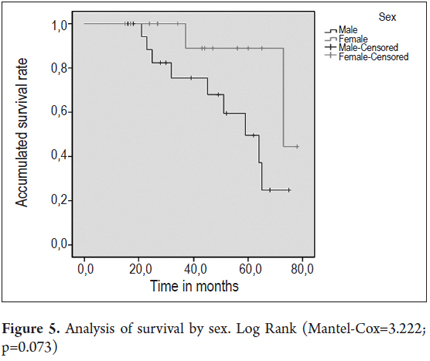
Figures 4 and 5 present survival curves for the total patient population and separately for men and for women.
DISCUSSION
Barrett's esophagus is the most serious complication observed in chronic GERD. This situation has already been assessed in our milieu by Dr. Sierra (2). As a premalignant condition BE significantly increases the risk of adenocarcinoma of the esophagus (7). In the last two decades, considerable efforts have been made in the development of endoscopic treatments. This is especially true for treatments using ablation which is currently considered to be a valid therapeutic option by medical associations that establish standards for managing BE. The standard surgical treatment of esophagectomies for patients with high grade dysplasia has a reported morbidity rate of up to 47% and a mortality rate that ranges from 1.2% to 4% in different studies. In contrast, endoscopic treatment has a reported mortality rate of only 0.04% (33).
APC induces squamous regeneration in place of BE in which the new tissue has no differences with the healthy epithelium of the esophageal lining as shown by various molecular findings (53). APC is usually well tolerated by the majority of patients, has a low rate of complications and only rare and self-limiting secondary effects. Nevertheless, there is no consensus about the wattage that should be used for APC. Both 50 watt and 70 watt treatments are in use without any clear differences in esophageal wall penetration or in post-procedure pain (54).
The first studies of APC for treating BE without dysplasia were published by Dumoulin in 1997 (55). These were followed by the studies by Mork (56) and Byrne (24) which reported 100% eradication rates for BE and dysplastic epithelium. The first APC treatment of BE with dysplasia was reported by Maass (57). The various series published to date report rates of complete eradication of BE without dysplasia that oscillate between 36% and 100% after short term monitoring. Madish shows an eradication rate of 98% for 70 patients with BE without dysplasia. The recurrence rate was 12% after an average follow-up period of 51 months (33). Ferraris documents a complete eradication rate of 97% with a 6.1% annual recurrence rate associated with laparoscopic fundoplication as a protective factor (52). In contrast, another study published by Mork reports an 84% eradication rate with a 66% recurrence rate in 25 patients with similar monitoring times (41). In our study, we found a 100% eradication rate for dysplastic BE and a 30.3% relapse rate (20.2% recurrence of BE and 9.1% recurrence of dysplasia) with an average monitoring time of 43 months. Manner used high energy 90W APC to treat 51 patients with BE without dysplasia. Nine patients (18%) presented minor complications including odynophagia, retrosternal pain, fever and odynophagia. Major complications were also documented in that study. They included hemorrhaging, stenosis, and perforation in 5 patients (10%) (58). In our experience the global rate of complications was of 33.3%. Of the total, pain accounted for 18.2%, stenoses for 12.1%, and bleeding for 3%. These numbers are quite similar to those evidenced in other reports (24, 56). Bright compared treatment of patients with BE without dysplasia endoscopic by APC to treatment of this type of patients with anti-acid secretion therapy and endoscopic monitoring (38). Recurrence of BE was evaluated after a 12 months follow-up. The ablation of metaplasia was achieved in 95% of the patients (25 of 26 patients treated). Following 12 months, 14 of the 23 patients treated with APC had at least 95% relapse, and only 9 of the 23 had no signs of recurrence of BE. None of the patients who underwent endoscopic monitoring had more than 5% relapse. The length of the Barrett´s esophagus was significantly reduced (average of 3.0 vs. 0.5 cm). A significant retraction of Barrett´s esophagus followed ablation with APC, although complete regression was achieved in less than half of the group. The same author compared APC and anti-reflux surgery. After 68 months of monitoring that study demonstrated that the neosquamous epithelia maintained stability in the majority of patients treated with APC, and dysplasia developed only in patients who underwent surgery (59).
Nevertheless, ablation for non-neoplastic BE continues to be a controversial topic. Especially controversial is its impact on the risk of the ailment´s progression toward adenocarcinoma. There are even reports of adenocarcinoma under the restored squamous epithelium subsequent to treatment (60-62). In addition, squamous carcinoma has been found within the treated area (63).
Patients with BE frequently present disorders of esophageal motility with decreased circumference of the esophageal opening in addition to decreased sensitivity and severity of symptoms. Twenty patients whose BE had been treated with APC were evaluated with manometry of the esophagus by Smythe (64) who found no evidence of substantial changes in the esophageal motility following APC. On the other hand, evaluation of sensitivity with different solutions showed a significantly smaller sensitivity to acid and alkali in the treated group. Basu´s study also showed no effects on motility in patients with BE treated with APC (65).
A recent cost study that compared various ablation therapies and endoscopic monitoring for patients with BE, BE with low grade dysplasia and BE with high grade dysplasia has demonstrated that endoscopic ablation should be the preferred strategy for patients with BE and high grade dysplasia. Ablation should also be chosen for any patient with dysplasia or even without dysplasia, but from the point of view of cost-effectiveness, endoscopic monitoring should not be necessary for effective eradication of BE (66).
Ablation of BE with APC has been compared to multiband mucosectomy by Boix (67) who suggesting that endoscopic mucosectomies have better outcomes than does APC. APC requires a greater number of sessions although it has a higher percentage of eradication of the metaplastic epithelium. These authors suggest a combination of treatment beginning with endoscopic mucosal resection with multiband ligation followed by APC when made necessary by remnant islands of BE (68). The intensity of the wattage used in the various studies ranges from 30W to 90W. Studies with lower wattages have higher rates of buried BE, only 68% regression and a high rate of recurrence (69). Pereira-Lima demonstrated a 100% re-epithelialization rate and a complete absence of "buried BE" with a wattage of 65W to 70W without significant numbers of complications (27). Shultz used a maximum wattage of 90W to achieve complete ablation of metaplastic epithelium in 98.6% of the cases (34). A recent review found that the majority of studies use 50W to 60W. The complications reported among the approximately 500 cases evaluated patients included one death due to perforation, two cases of perforation in which the patients survived, one case of subcutaneous emphysema without clinical evidence of perforation, and 7 stenoses (70). Minor complications reported include odynophagia and dysphagia with frequent retrosternal pain. The procedure may be done under conscious sedation, but general anesthesia is not recommended according to Tigges (51).
At present the greatest concern about APC is the recurrence of BE. Although any underlying metaplasia has neoplastic potential in the new squamous epithelium, some studies suggest that the potential for malignant transformation is low. The histopathological samples in our study showed subepithelial lesions in 20.2% of the cases. Molecular studies suggest that for patients with BE oncogene expression of miR-143 remains as high in the neo-squamous mucosa as in the squamous mucosa above a metaplastic segment. This in turn suggests that this mucosa is not normal in terms of the mucosa observed in individuals without Barrett´s esophagus. Expression of miR-143 could promote a pattern of Barrett´s epithelia which could have a role in the development of Barrett´s esophagus (71). The evaluation of p53 before and after APC in patients with BE without dysplasia has shown that the p53 prevalence was high in the squamous epithelium adjacent to the BE. After ablation, none of the cases with loss of p53 expression and none of the cases that were negative for p53 later became positive for p53 (72).
The long term success of APC in the treatment of BE is influenced by two factors: the wattage administered (50W) and the use of high dosages of PPIs. These result in marked differences in relapse rates during monitoring in the various published articles reviewed for this study. On the other hand, this study confirms the reports by Kahaleh and Basu that the two factors that determine swift relapses in patients with BE treated with APC are the persistence of gastroesophageal reflux and lengths of the area affected by the initial BE that are greater than 3cms (45, 69).
APC treatment of Barrett's Esophagus has many advantages: it can performed on an outpatient basis, it is of short duration, unlike photodynamic therapy it does not require a substantial change in lifestyle, it is cheap, and the equipment may be used for other procedures.
CONCLUSIONS
In our study, the effectiveness of the endoscopic APC for treatment of BE without dysplasia was 100% in terms of complete ablation of the lesion, but the relapse rate was 30.3% including a 21.2% recurrence rate for BE and a 9.1% recurrence rate for dysplasia. The average monitoring time was 43 months. During monitoring there were no cases of progression to adenocarcinoma. It was not possible to determine whether the risk factors associated with GERD and BE have an impact or relationship to subsequent recurrence following APC. One to three sessions of APC were required for complete ablation, but the majority of patients (56.7%) required two sessions. More sessions were required for patients with areas affected by BE that were longer than 3cms. These patients also had higher rates of recurrence of both BE and dysplasia. Up to 33.3% morbidity has been attributed to APC, but there have been no reports of mortalities as a consequence of the treatment. Controlled and randomized clinical trials with larger numbers of patients and longer monitoring times are still needed in order to determine the real effectiveness and recurrence rate.
Conflicts de interests
The authors have no conflicts of interest. This study was carried out with the support of the Sustainability Project of the Office of the Vice Rector of Research at the Universidad de Antioquia.
REFERENCES
1. Spechler SJ, Goyal RK. The columnar-lined esophagus, intestinal metaplasia, and Norman Barrett. Gastroenterology 1996;110: 614-21. [ Links ]
2. Sierra F. Incidencia de adenocarcinoma en esófago de Barrett, Fundación Santa Fe de Bogotá, 11 años de seguimiento. Rev Col Gastroenterol 2008; 23: 13-25. [ Links ]
3. Grant KS, Demeester SR, Kreger V, et al. Effect of Barretts esophagus surveillance on esophageal preservation, tumor stage, and survival with esophageal adenocarcinoma. J Thorac Cardiovasc Surg 2013. [ Links ]
4. Solaymani-Dodaran M, Card TR, West J. Cause-Specific Mortality of People With Barretts Esophagus Compared With the General Population: A Population-Based Cohort Study. Gastroenterology 2013. [ Links ]
5. Pohl H, Wrobel K, Bojarski C, et al. Risk factors in the development of esophageal adenocarcinoma. Am J Gastroenterol 2013; 108: 200-7. [ Links ]
6. Rubenstein JH, Morgenstern H, Appelman H, et al. Prediction of Barretts Esophagus Among Men. Am J Gastroenterol 2013. [ Links ]
7. Sikkema M, Looman CW, Steyerberg EW, et al. Predictors for neoplastic progression in patients with Barretts Esophagus: a prospective cohort study. Am J Gastroenterol 2011; 106: 1231-8. [ Links ]
8. Andrici J, Tio M, Cox MR, Eslick GD. Hiatal Hernia and the Risk of Barretts Esophagus: A Meta-Analysis. Journal of gastroenterology and hepatology 2012. [ Links ]
9. Hardikar S, Onstad L, Blount PL, Odze RD, Reid BJ, Vaughan TL. The role of tobacco, alcohol, and obesity in neoplastic progression to esophageal adenocarcinoma: a prospective study of Barretts esophagus. PloS one 2013; 8: e52192. [ Links ]
10. Cook MB, Shaheen NJ, Anderson LA, et al. Cigarette smoking increases risk of Barretts esophagus: an analysis of the Barretts and Esophageal Adenocarcinoma Consortium. Gastroenterology 2012; 142: 744-53. [ Links ]
11. El-Serag HB, Hashmi A, Garcia J, et al. Visceral abdominal obesity measured by CT scan is associated with an increased risk of Barretts oesophagus: a case-control study. Gut 2013. [ Links ]
12. Cheng P, Li JS, Gong J, Zhang LF, Chen RZ. Effects of refluxate pH values on duodenogastroesophageal reflux-induced esophageal adenocarcinoma. World J Gastroenterol 2011; 17: 3060-5. [ Links ]
13. Sun Q, Huang Q, Feng AN, et al. Columnar-lined esophagus in Chinese patients with proximal gastric carcinomas. Journal of digestive diseases 2013; 14: 22-8. [ Links ]
14. De Palma GD. Management strategies of Barretts esophagus. World J Gastroenterol 2012; 18: 6216-25. [ Links ]
15. Kastelein F, Spaander MC, Steyerberg EW, et al. Proton Pump Inhibitors Reduce the Risk of Neoplastic Progression in Patients With Barretts Esophagus. Clin Gastroenterol Hepatol 2012. [ Links ]
16. Marano S, Mattacchione S, Luongo B, Paltrinieri G, Mingarelli V, Tosato F. Barretts esophagus after laparoscopic Nissen-Rossetti fundoplication: functional evaluation. Minerva chirurgica 2011; 66: 517-25. [ Links ]
17. Bennett C, Green S, Decaestecker J, et al. Surgery versus radical endotherapies for early cancer and high-grade dysplasia in Barretts oesophagus. Cochrane Database Syst Rev 2012; 11: CD007334. [ Links ]
18. Simonka Z, Paszt A, Abraham S, et al. The effects of laparoscopic Nissen fundoplication on Barretts esophagus: long-term results. Scand J Gastroenterol 2012; 47: 13-21. [ Links ]
19. Bergman JJ, Corley DA. Barretts esophagus: who should receive ablation and how can we get the best results? Gastroenterology 2012; 143: 524-6. [ Links ]
20. Ackroyd R, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MW. Ablation treatment for Barrett oesophagus: what depth of tissue destruction is needed? Journal of clinical pathology 1999; 52: 509-12. [ Links ]
21. Watson JP, Bennett MK, Griffin SM, Matthewson K. The tissue effect of argon plasma coagulation on esophageal and gastric mucosa. Gastrointest Endosc 2000; 52: 342-5. [ Links ]
22. Hage M, Siersema PD, van Dekken H, et al. 5-aminolevulinic acid photodynamic therapy versus argon plasma coagulation for ablation of Barretts oesophagus: a randomised trial. Gut 2004; 53: 785-90. [ Links ]
23. Orth K, Stanescu A, Ruck A, Russ D, Beger HG. Photodynamic ablation and argon-plasma coagulation of premalignant and early-stage malignant lesions of the oesophagus--an alternative to surgery?. Der Chirurg; Zeitschrift fur alle Gebiete der operativen Medizen 1999; 70: 431-8. [ Links ]
24. Byrne JP, Armstrong GR, Attwood SE. Restoration of the normal squamous lining in Barretts esophagus by argon beam plasma coagulation. Am J Gastroenterol 1998; 93: 1810-5. [ Links ]
25. Van Laethem JL, Cremer M, Peny MO, Delhaye M, Deviere J. Eradication of Barretts mucosa with argon plasma coagulation and acid suppression: immediate and mid term results. Gut 1998; 43: 747-51. [ Links ]
26. Ragunath K, Krasner N, Raman VS, Haqqani MT, Phillips CJ, Cheung I. Endoscopic ablation of dysplastic Barretts oesophagus comparing argon plasma coagulation and photodynamic therapy: a randomized prospective trial assessing efficacy and cost-effectiveness. Scand J Gastroenterol 2005; 40: 750-8. [ Links ]
27. Pereira-Lima JC, Busnello JV, Saul C, et al. High power setting argon plasma coagulation for the eradication of Barretts esophagus. Am J Gastroenterol 2000;95:1661-8. [ Links ]
28. Van Laethem JL, Jagodzinski R, Peny MO, Cremer M, Deviere J. Argon plasma coagulation in the treatment of Barretts high-grade dysplasia and in situ adenocarcinoma. Endoscopy 2001; 33: 257-61. [ Links ]
29. Attwood SE, Lewis CJ, Caplin S, Hemming K, Armstrong G. Argon beam plasma coagulation as therapy for high-grade dysplasia in Barretts esophagus. Clin Gastroenterol Hepatol 2003; 1: 258-63. [ Links ]
30. May A, Gossner L, Gunter E, Stolte M, Ell C. Local treatment of early cancer in short Barretts esophagus by means of argon plasma coagulation: initial experience. Endoscopy 1999; 31: 497-500. [ Links ]
31. Nomura T, Yamashita K, Miyashita M, Tajiri T. Argon plasma coagulation in Barretts esophagus. Nihon Rinsho 2005; 63: 1458-62. [ Links ]
32. Pedrazzani C, Catalano F, Festini M, et al. Endoscopic ablation of Barretts esophagus using high power setting argon plasma coagulation: a prospective study. World J Gastroenterol 2005; 11: 1872-5. [ Links ]
33. Madisch A, Miehlke S, Bayerdorffer E, et al. Long-term follow-up after complete ablation of Barretts esophagus with argon plasma coagulation. World J Gastroenterol 2005; 11: 1182-6. [ Links ]
34. Schulz H, Miehlke S, Antos D, et al. Ablation of Barretts epithelium by endoscopic argon plasma coagulation in combination with high-dose omeprazole. Gastrointest Endosc 2000; 51: 659-63. [ Links ]
35. Morris CD, Byrne JP, Armstrong GR, Attwood SE. Prevention of the neoplastic progression of Barretts oesophagus by endoscopic argon beam plasma ablation. Br J Surg 2001; 88: 1357-62. [ Links ]
36. Kelty CJ, Ackroyd R, Brown NJ, Stephenson TJ, Stoddard CJ, Reed MW. Endoscopic ablation of Barretts oesophagus: a randomized-controlled trial of photodynamic therapy vs. argon plasma coagulation. Aliment Pharmacol Ther 2004; 20: 1289-96. [ Links ]
37. Grade AJ, Shah IA, Medlin SM, Ramirez FC. The efficacy and safety of argon plasma coagulation therapy in Barretts esophagus. Gastrointest Endosc 1999; 50: 18-22. [ Links ]
38. Bright T, Watson DI, Tam W, et al. Prospective randomized trial of argon plasma coagulation ablation versus endoscopic surveillance of Barretts esophagus in patients treated with antisecretory medication. Dig Dis Sci 2009; 54: 2606-11. [ Links ]
39. Bozymski EM. Argon plasma coagulation for non-dysplastic Barretts epithelium: a hard act to follow. Am J Gastroenterol 2007; 102: 1128-9; author reply 9-30. [ Links ]
40. Dumot JA, Greenwald BD. Argon plasma coagulation, bipolar cautery, and cryotherapy: ABCs of ablative techniques. Endoscopy 2008; 40: 1026-32. [ Links ]
41. Mork H, Al-Taie O, Berlin F, Kraus MR, Scheurlen M. High recurrence rate of Barretts epithelium during long-term follow-up after argon plasma coagulation. Scand J Gastroenterol 2007; 42: 23-7. [ Links ]
42. Sharma P, Wani S, Weston AP, et al. A randomised controlled trial of ablation of Barretts oesophagus with multipolar electrocoagulation versus argon plasma coagulation in combination with acid suppression: long term results. Gut 2006; 55: 1233-9. [ Links ]
43. Manner H, May A, Miehlke S, et al. Ablation of nonneoplastic Barretts mucosa using argon plasma coagulation with concomitant esomeprazole therapy (APBANEX): a prospective multicenter evaluation. Am J Gastroenterol 2006; 101: 1762-9. [ Links ]
44. Dulai GS, Jensen DM, Cortina G, Fontana L, Ippoliti A. Randomized trial of argon plasma coagulation vs. multipolar electrocoagulation for ablation of Barretts esophagus. Gastrointest Endosc 2005; 61: 232-40. [ Links ]
45. Kahaleh M, Van Laethem JL, Nagy N, Cremer M, Deviere J. Long-term follow-up and factors predictive of recurrence in Barretts esophagus treated by argon plasma coagulation and acid suppression. Endoscopy 2002; 34: 950-5. [ Links ]
46. Pagani M, Granelli P, Chella B, Antoniazzi L, Bonavina L, Peracchia A. Barretts esophagus: combined treatment using argon plasma coagulation and laparoscopic antireflux surgery. Dis Esophagus 2003; 16: 279-83. [ Links ]
47. Formentini A, Schwarz A, Straeter J, Stanescu A, Henne-Bruns D. Treatment of Barretts esophagus with argon plasma coagulation and antireflux surgery. A retrospective analysis. Hepatogastroenterology 2007; 54: 1991-6. [ Links ]
48. Pinotti AC, Cecconello I, Filho FM, Sakai P, Gama-Rodrigues JJ, Pinotti HW. Endoscopic ablation of Barretts esophagus using argon plasma coagulation: a prospective study after fundoplication. Dis Esophagus 2004; 17: 243-6. [ Links ]
49. Ackroyd R, Tam W, Schoeman M, Devitt PG, Watson DI. Prospective randomized controlled trial of argon plasma coagulation ablation vs. endoscopic surveillance of patients with Barretts esophagus after antireflux surgery. Gastrointest Endosc 2004; 59: 1-7. [ Links ]
50. Morino M, Rebecchi F, Giaccone C, Taraglio S, Sidoli L, Ferraris R. Endoscopic ablation of Barretts esophagus using argon plasma coagulation (APC) following surgical laparoscopic fundoplication. Surg Endosc 2003; 17: 539-42. [ Links ]
51. Tigges H, Fuchs KH, Maroske J, et al. Combination of endoscopic argon plasma coagulation and antireflux surgery for treatment of Barretts esophagus. J Gastrointest Surg 2001; 5: 251-9. [ Links ]
52. Ferraris R, Fracchia M, Foti M, et al. Barretts oesophagus: long-term follow-up after complete ablation with argon plasma coagulation and the factors that determine its recurrence. Aliment Pharmacol Ther 2007; 25: 835-40. [ Links ]
53. Hage M, Siersema PD, Vissers KJ, et al. Molecular evaluation of ablative therapy of Barretts oesophagus. J Pathol 2005; 205: 57-64. [ Links ]
54. Dotti VP, Baretta GA, Yoshii SO, Ivano FH, Ribeiro HD, Matias JE. [Endoscopic argon plasma thermo-coagulation of Barretts esophagus using different powers: histopathological and post procedure symptons analysis]. Rev Col Bras Cir 2009; 36: 110-7. [ Links ]
55. Dumoulin FL, Terjung B, Neubrand M, Scheurlen C, Fischer HP, Sauerbruch T. Treatment of Barretts esophagus by endoscopic argon plasma coagulation. Endoscopy 1997; 29: 751-3. [ Links ]
56. Mork H, Barth T, Kreipe HH, et al. Reconstitution of squamous epithelium in Barretts oesophagus with endoscopic argon plasma coagulation: a prospective study. Scand J Gastroenterol 1998; 33: 1130-4. [ Links ]
57. Maass S, Martin WR, Spiethoff A, Riemann JF. Barrett esophagus with severe dysplasia in argon beam therapy. Z Gastroenterol 1998; 36: 301-6. [ Links ]
58. Manner H. Argon plasma coagulation therapy. Curr Opin Gastroenterol 2008; 24: 612-6. [ Links ]
59. Bright T, Watson DI, Tam W, et al. Randomized trial of argon plasma coagulation versus endoscopic sur veillance for barrett esophagus after antireflux surgery: late results. Ann Surg 2007; 246: 1016-20. [ Links ]
60. Van Laethem JL, Peny MO, Salmon I, Cremer M, Deviere J. Intramucosal adenocarcinoma arising under squamous re-epithelialisation of Barretts oesophagus. Gut 2000; 46: 574-7. [ Links ]
61. Shand A, Dallal H, Palmer K, Ghosh S, MacIntyre M. Adenocarcinoma arising in columnar lined oesophagus following treatment with argon plasma coagulation. Gut 2001; 48: 580-1. [ Links ]
62. van Hillegersberg R, Haringsma J, ten Kate FJ, Tytgat GN, van Lanschot JJ. Invasive carcinoma after endoscopic ablative therapy for high-grade dysplasia in Barretts oesophagus. Digestive surgery 2003; 20: 440-4. [ Links ]
63. Allende D, Dumot J, Yerian L. Esophageal squamous cell carcinoma arising after endoscopic ablation therapy of Barretts esophagus with high-grade dysplasia. Report of a case. Dis Esophagus 2013; 26: 314-8. [ Links ]
64. Smythe A, Elghellal K, Kelty C, Mitton D, Patel K, Ackroyd R. The effect of argon plasma coagulation ablation on esophageal motility and chemoreceptor sensitivity in Barretts esophagus patients. Dis Esophagus 2010; 23: 445-50. [ Links ]
65. Basu KK, Talwar V, de Caestecker JS. Effects of low-power argon plasma coagulation thermoablation of Barretts epithelium on oesophageal motility. Eur J Gastroenterol Hepatol 2006; 18: 733-7. [ Links ]
66. Inadomi JM, Somsouk M, Madanick RD, Thomas JP, Shaheen NJ. A cost-utility analysis of ablative therapy for Barretts esophagus. Gastroenterology 2009; 136: 2101-14 e1-6. [ Links ]
67. Boix J, Lorenzo-Zuniga V, de Vega VM, Planas R. Argon plasma coagulation versus endoscopic mucosal resection in patients with dysplastic Barretts esophagus. Dig Dis Sci 2009; 54: 1808-9. [ Links ]
68. Lorenzo-Zuniga V, Boix J. Endoscopic mucosal resection and mucosa ablation with argon-plasma coagulation in a high-risk patient with Barretts oesophagus and oesophageal adenocarcinoma. Dig Liver Dis 2006; 38: 713-4. [ Links ]
69. Basu KK, Pick B, Bale R, West KP, de Caestecker JS. Efficacy and one year follow up of argon plasma coagulation therapy for ablation of Barretts oesophagus: factors determining persistence and recurrence of Barretts epithelium. Gut 2002; 51: 776-80. [ Links ]
70. Claydon PE, Ackroyd R. Argon plasma coagulation ablation of Barretts oesophagus. Scand J Gastroenterol 2005; 40: 617-28. [ Links ]
71. Dijckmeester WA, Wijnhoven BP, Watson DI, et al. MicroRNA-143 and -205 expression in neosquamous esophageal epithelium following Argon plasma ablation of Barretts esophagus. J Gastrointest Surg 2009; 13: 846-53. [ Links ]
72. Lopes CV, Pereira-Lima J, Hartmann AA. p53 immunohistochemical expression in Barretts esophagus before and after endoscopic ablation by argon plasma coagulation. Scand J Gastroenterol 2005; 40: 259-63. [ Links ]











 texto en
texto en