Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.4 Bogotá Oct./Dec. 2014
Abdominal Obesity Increases the Risks of Colorectal Polyps
Óscar Fernando Ruiz Morales MD. (1), William Otero Regino MD. (2), Martín Alonso Gómez Zuleta MD. (3), Dennys Castro Soteldo MD. (4)
(1) Internist and Gastroenterological Fellow at the Universidad Nacional de Colombia in Bogotá, Colombia.
(2) Professor of Medicine in the Gastroenterology at the Universidad Nacional de Colombia and Gastroenterologist at the Clínica Fundadores in Bogotá, Colombia.
(3) Professor of Medicine in the Gastroenterology at the Universidad Nacional de Colombia and Gastroenterologist at the Hospital el Tunal in Bogotá, Colombia.
(4) Director of the Luis E Anderson Center for the Control of Gastrointestinal Cancer in San Cristóbal, Venezuela.
Received: 06-05-14 Accepted: 05-11-14
Abstract
Introduction: Recently several international publications have found an association between obesity and polyps and/or colorectal cancers. In our country the possibilities of these associations have not been studied. The aim of this study was to determine whether obese patients have polyps or colorectal cancers more frequently than found in the general population.
Materials and Methods: This was a cross-sectional prospective study of patients referred for total colonoscopies for screening that were conducted between March 2012 and June 2013. Three university hospitals, Clínica Fundadores and Hospital El Tunal in Bogotà, Colombia and the Centro de Control de Cancer Gastrointestinal "Luis E Anderson" in Venezuela, participated in the study. All patients were weighed and height, body mass index (kg/m2) and waist circumference in centimeters were measured. The prevalence of polyps in the population was estimated and the risk was determined by means of Odds Ratios (OR) with 95% confidence intervals (CI).
Results: 405 patients were included in the study, 68.9% of whom were women. The mean patient age was 56.1 years +/- 12.9 years. 154 (38%) had polyps, of these 113 (73%) (p = 0.01), met the criteria for abdominal obesity, and 41 (27%) (p = 0.03) did not meet these criteria. Logistic regression analysis found no relationship between increased abdominal circumference and the presence of polyps in the colon, but for every additional centimeter of waist circumference the risk of polyps increased 5.3%.
Conclusion: In the population examined, obese patients had polyps more frequently than did others, and this risk increased with the severity of obesity.
Keywords
Obesity, overweight, polyps, cancer.
INTRODUCTION
Obesity is associated with many diseases including Type 2 diabetes mellitus, hypertension, cardiovascular disease and even some cancers (1). Its prevalence is increasing everywhere in the world (1-3). In 2007, there were 523 million obese people worldwide, and more than 30% of the American population was obese (BMI> 30) (2). In 2008, there were 1.4 billion adults (age 20 or over) who were overweight (2). In 2010, 40 million children over the age of five were overweight (3). In 2007, 46.02% of the adults in Colombia were overweight and 13.71% of the country's adults were obese (4). There is growing evidence regarding health risks associated with the amount of total body fat and also with the distribution of body fat in the visceral region (5). Waist circumference is a useful independent predictor for defining cardiovascular risk as well as the risk for cancer secondary to obesity (6, 7). Visceral adiposity is a major determinant of insulin resistance which is considered to be an early and essential indicator for the development of obesity-related diseases (5).
In the last decade, many epidemiologic studies have found that as the waist/hip ratio and/or the circumference of the waist increase, the risk of developing colorectal adenomas and colon cancer (CC) also increase (5, 7-15). Adenomatous polyps are the precursors of most sporadic colorectal cancers (adenoma-carcinoma) (16). The role of adenomas in the development of colorectal cancer (CRC) was established when Vogelstein and colleagues showed that the gradual accumulation of molecular alterations such as mutations in the tumor suppressor APC pathway, followed by mutations of p53 and KRAS, leads to the transformation of the small adenomatous polyps into larger polyps with dysplasia and finally into invasive carcinomas (17). This sequence is the basis for screening to detect and remove polyps in a timely manner which can prevent the development of CRC (10, 18). CRC causes the third largest number of deaths due to cancer in the USA (20). Its five-year survival rate is 90% if detected in stage I and less than 5% when detected in stage IV (19-21). In Colombia, CRC is the fourth leading cause of cancer death for both women and men (22). CRC's development is associated with both genetic and environmental factors (1, 19) among which tobacco and alcohol consumption, low levels of physical activity, some dietary patterns such as the absence or near absence of fruit and vegetables from the diet and high fat diets, and obesity should be highlighted (23). Obesity increases the risk of death from all causes by 30%, increases the risks for cardiovascular disease by 40%, and increases the risks for all types of cancer by 10% (24). So, not only can obesity influence the incidence of cancer, but it also affects the prognosis, overall survival rate and disease-free survival rate (25). Despite this knowledge, no epidemiological studies of the association between obesity and colonic polyps (adenomas) had previously been conducted in Colombia or anywhere else in South America. We decided to perform this study to beginning filling the gap.
MATERIALS AND METHODS
This is an observational cross-sectional study for which information was collected prospectively between March 2012 and June 2013. The sample size was calculated to match the expected prevalence of polyps in the general population. Prevalence has been found to be as high as 25% at 50 years of age (26, 27, 29) and as high as 50% at 70 years (28, 29). Overall prevalence of colorectal polyps has been estimated to range from 20% to 60% in Western populations according to autopsy-based studies (30). In Colombia no data on the prevalence of colonic polyps had been collected, so we decided to perform this calculation based on an average prevalence of 30%. For a 95% confidence interval and a statistical power of 80% we obtained a sample size of 363 patients. All patients were older than 18 years and signed informed consent forms before entering the study. Study participants underwent full diagnostic colonoscopy during which cleaning was evaluated on the Boston scale (30, 31, 35). A score over two was required for each of the three segments evaluated: the splenic flexure, left colic flexure and the liver- cecum angle. A complete endoscopic visual examination of the cecum, ileocecal valve and appendicular hole was done. Minimum withdrawal time was six to ten minutes. To guarantee the reliability of endoscopic procedures, only expert gastroenterologists (WOR, MAGZ, DCS) and gastroenterology professors teachers who meet high standards of quality in these procedures (32-35) performed them. Each of the participating gastroenterologists has performed more than 10,000 colonoscopies. Patients with known histories of colon cancer, previous resection of colonic polyps, colitis of any etiology, and/or previous colon surgery were excluded. All patients filled out a questionnaire with the following information: age (completed years), gender, personal history of hypertension, type 2 diabetes mellitus and dyslipidemia and family history of colon cancer in first-degree relatives.
Immediately prior to the performance of the colonoscopy, patients were measured by nurses trained for this purpose. All measurements were metric and included height, weight, body mass index, waist circumference and chest circumference. Waist circumference was measured around a horizontal plane at the midpoint between the lower edge of the last rib and the iliac crest. Chest circumference was measured after the patient exhaled while standing shoeless with arms hanging freely as previously described and recommended (38-41). The unclothed body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). The relationship between weight and height was based on the definitions of the World Health Organization (WHO). A patient was classified as overweight when the BMI was between 25.0 and 29.9 kg/m2 and as obese when BMI was over 30.0 kg/m2 (37). Colonoscopies were performed in the usual manner following preparation with polyethylene glycol and electrolytes in divided doses previously recommended (41-48).
All colonoscopies were begun without sedation. Sedation was used selectively only when the patient did not tolerate the procedure without sedation or when requested by the referring physician. When sedation was used, propofol and remifentanil were administered by an anesthesiologist. Dosages were determined by the anesthesiologist according to the individual characteristics of each patient. Sedation was administered through a vein in the right forearm and patients' vital signs and oxygen were constantly monitored. At least two nurses trained in endoscopy assisted each procedure. The population data collected were included in virtual data tables in Google Drive (URL goo.gl/7jl81). Prior to each colonoscopy, patient eligibility was determined by internal medicine residents, gastroenterology fellows or gastroenterology assistants who had been trained and familiarized in the use of the study protocol. They initially registered demographic and risk data for each patient and later registered the results of the colonoscopy. The endoscopist did not fill out patient forms and did not always know when a patient was participating in the study. Individuals other than the endoscopists downloaded patient data from the virtual cloud on a weekly basis and entered data into other research databases for further analysis.
STATISTICAL ANALYSIS
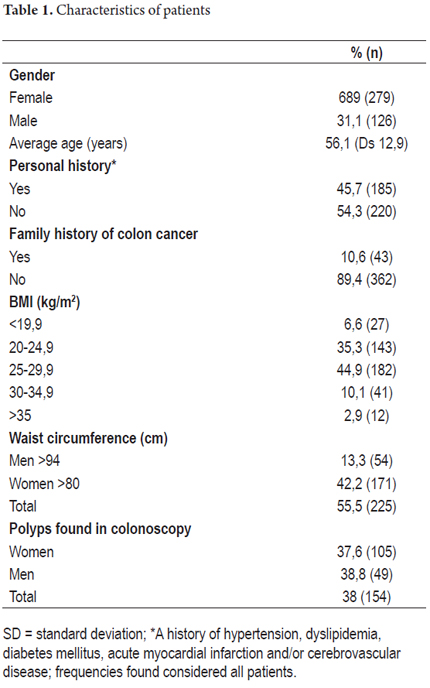
The presence of polyps found in a colonoscopy was taken to be the dependent variable. Independent variables included gender, age and medical history. Factors in patients' medical histories that were taken into account included hypertension, diabetes mellitus, dyslipidemia, and history of myocardial infarction and/or cerebrovascular disease. Initially, frequency distribution tables were produced that described the patient populations independent and dependent variables. Table 1 lists age, arithmetic means and standard deviations. Table 2 shows data screening test results. Tests applied included the chi square, Student's T, and Nova test. IBM SPSS version 21.0 was used to calculate odds ratios (OR) and 95% confidence intervals (CI). Finally a logistic regression model was constructed to simultaneously control the effect of all independent variables on the dependent variable.
RESULTS
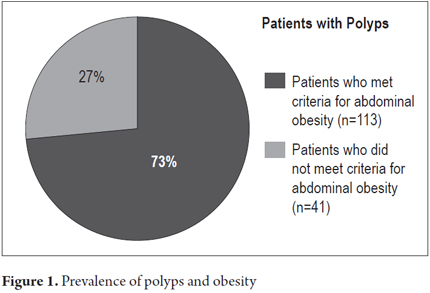
During the study period from March 2012 to June 2013, 405 patients who had undergone total diagnostic colonoscopies and who had met all inclusion criteria submitted forms for this study. The breakdown of these patients is as follows: 272 from Clínica Fundadores in Bogota, Colombia; 84 from Hospital el Tunal in Bogota, Colombia; and 49 from of the Centro de Control del Cáncer in San Cristobal, Venezuela. Of the total sample 279 (68.9%) were women, 126 (31.1%) were men, the average age was 56.1 years ± 12.9 years, 185 (45.7%) had personal medical histories of hypertension, diabetes mellitus, dyslipidemia, myocardial infarction and/or cerebrovascular disease, 43 (10.6%) had first degree family members with histories of colon cancer, 170 (41.9%) had normal BMI, 182 (44.9%) were overweight (BMI 25-29.9 kg/m2), 53 (13%) were obese (BMI> 30 kg/m2), 225 (55.5%) met the International Diabetes Federation's criteria for abdominal obesity, and 154 (38%) had polyps found during colonoscopy (Table 1). Of this last group, 113 (73%) (P = 0.01), met criteria for abdominal obesity and 41 (27%) (p = 0.03) did not meet criteria for abdominal obesity. The OR was 3.42 with a 95% CI of 2.21 - 5.29. The association between the presence of polyps and abdominal obesity is statistically significant (Figure 1): 225 patients (55.5%) of the total sample had abdominal obesity and of these 100 (44.4% of the obese patients) had polyps. In contrast, of the 180 patients (44.5% of the total sample) who did not have abdominal obesity, only 54 (30% of these patients) presented polyps.
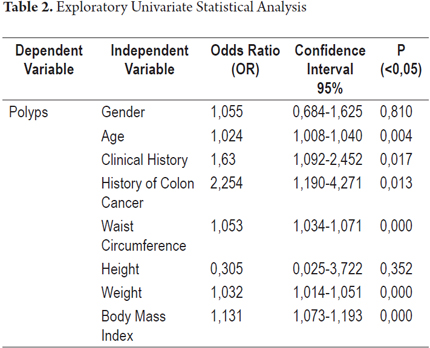
Subsequent to performing exploratory tests in the univariate analysis, no statistically significant associations were found for the independent variables of gender (OR = 1.055, 95% CI: 0.684 to 1.625; P = 0.810) and height (OR = 0.305, 95% CI: 0.025 to 3.722: P = 0.352). This indicates an absence of any association between these variables and the presence of polyps in the diagnostic colonoscopy.
Mild to moderate statistical significances were found in descending order for the following independent variables (Table 2):
Family history of colon cancer in first degree relatives (OR = 2.254, 95% CI: 1.190 to 4.271; P = 0.013)
Personal medical history of hypertension, diabetes mellitus, dyslipidemia, myocardial infarction and/or cerebrovascular disease (OR = 1.63, 95% CI: 1.092 to 2.452; P = 0.017)
Body mass index (BMI) (OR = 1.131, 95% CI: 1.073 to 1.193; P = 0.000)
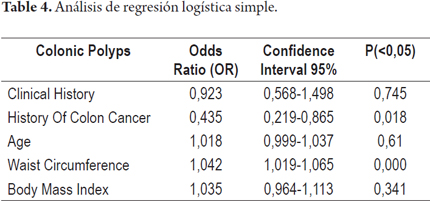
Waist circumference (OR = 1.053, 95% CI: 1.034 to 1.071; P = 0.000)
Weight (OR = 1.032, 95% CI: 1.014 to 1.051; P = 0.000)
Age (OR = 1.024, 95% CI: 1.008 to 1.040; P = 0.004)
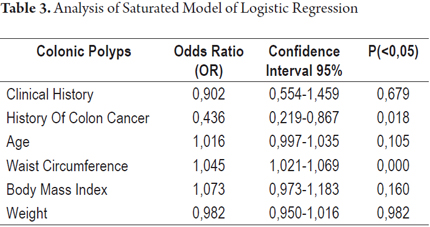
Because these data indicate associations between the presence of polyps in the diagnostic colonoscopy and these variables, logistic regression was used to explore their contributions to an explanation of polyps found in diagnostic colonoscopy. The main effects of each variable when all other variables considered in the model were simultaneously adjusted were analyzed. Two tests of the goodness of fit showed no differences between the logistic model and the observed data (Table 3). Waist circumference was found to be the only variable whose simultaneous adjustment to all other variables was statistically significant. This indicates an association between the presence of polyps and increased waist circumference (OR 1.053, 95% CI: 1.034-1.071; P = 0.000).
A second logistic regression model with characteristics similar to the initial model but without the variable of weight was run. Weight was omitted because it is involved in defining the body mass index. The behavior of the variables was the same as in the first model in which only the waist circumference variable had statistical significance (Table 4).
DISCUSSION
The rate of detection of polyps by colonoscopic screening in this study was 38% (37.6% for women and 37.8% for men). This is slightly higher than what is recommended in the current standards of quality for colonoscopy which estimate a rate of detection of more than 15% for women and more than 25% for men (41-47). A statistically significant association between abdominal obesity and the presence of polyps was found: of the 154 (38%) patients who had polyps, 113 (73%) met criteria for abdominal obesity (P = 0.01). Similarly, 225 (55.5%) of the patients with abdominal obesity had polyps while only 30% of the patients who were not obese had polyps (p <0.01). In the latter group, the prevalence of polyps was higher than the expected rate of 25% for patients in the general over 55 year of age population (26, 27, 29, 42-45).
Based on these findings, analyses of both saturated and simple logistic regressions confirmed significant statistical associations between abdominal obesity and the prevalence of polyps. For every centimeter of waist circumference above the limits defined for abdominal obesity, the risk of polyps increased 5.3% (OR 1.053, 95% CI: 1.034 to 1.071; P = 0.000). This association between abdominal obesity and the presence of colonic polyps is similar to that found in other latitudes. A recent study of 126 obese men (BMI> 30 kg/m2) undergoing screening colonoscopy found that obese men were 6.5 times more likely to have three or more colon polyps than men of the same age with normal body mass indices (49). That study also found that those with waist circumferences greater than 114 cm were almost 5 times more likely to have three or more polyps than men with smaller abdominal circumference (less than 96 cm). A Japanese study of 727 women found that a waist circumference of over 80 cm increased the risks of colon polyps and the risks of CRC (50).
A Korean study also found for each per centimeter of abdominal circumference above the defined limit for obesity of 90 cm the risk of polyps increased (OR = 1.42; 95% CI: 1.06 to 1.90) (46). Our results are also comparable with another recently published Japanese study which found that for every cm above the normal value of the index of abdominal circumference the risk of polyps significantly increased for women (OR = 1.3, 95% CI: 1.05 to 1.64, p = 0.01) but not for men (51). A b association of obesity with risks for both colorectal adenomas and colorectal carcinoma has also been demonstrated in a study from the United States which compared the highest versus with the lowest quartile (OR = 2.38, 95% CI: 1.45 to 3.92) (52).
A meta-analysis of cohort studies the lowest quartile to the highest quartile of waist circumferences has found that the combined RR for colon cancer was 1.68 (95% CI: 1.36 to 2.08) for men and 1.48 (95% CI: 1.19 to 1.84) for women (53). Although the biological plausibility of this association is unclear at present, some biological characteristics of the adipocytes should be considered. In addition to the metabolic properties for storage of glucose and triglycerides, these highly differentiated cells synthesize adiponectin which may induce inflammation (1.54 to 56) and cellular proliferation as the result of insulin derived growth factor (1, 56, 57). Leptin, another product of these cells, has been associated with tumor development (58). Leptin is pro-angiogenic, can increase the growth rate of endothelial cells, and can suppress apoptosis through a Bcl-2 dependent mechanism. Leptin can act as a mitogenic factor in the transformation and migration of many different cell types (59, 60).
In conclusion, this study found a statistically significant association between abdominal obesity represented by waist circumference and the presence of colonic polyps in the study population. This association has also been found in studies in several other countries. The impact of obesity on the colonic mucosa should be taken into consideration, and obese patients should undergo screening colonoscopy before the age of 50. Nevertheless, additional studies are needed to determine the impact of this recommendation.
Conflicts of interest
None. The costs of this research were borne entirely by the researchers.
REFERENCES
1. Guffey CR, Fan D, Singh UP, et al. Linking obesity to colorectal cancer: recent insights into plausible biological mechanisms. Curr Opin Clin Nutr Metab Care 2013; 16: 595-600. [ Links ]
2. James WP. The epidemiology of obesity: the size of the problem. J Intern Med 2008; 4: 336-352. [ Links ]
3. Burke CA. Colonic complications of obesity. Gastroenterol Clin North Am 2010; 1: 47-55. [ Links ]
4. Rodríguez J, Ruiz F, Peñaloza E, et al. Encuesta Nacional de Salud 2007. Resultados nacionales. Bogotá, Colombia: Ministerio de la Protección Social; 2009. [ Links ]
5. Kim Y, Kim Y, Lee S. An association between colonic adenoma and abdominal obesity: a cross-sectional study. BMC Gastroenterol 2009; 9: 4-10. [ Links ]
6. Yamaji T, Iwasaki M, Sasazuki S, et al. Visceral fat volume and the prevalence of colorectal adenoma. Am J Epidemiol 2009; 170: 1502-11. [ Links ]
7. Nguyen DM, El-Serag HB. The epidemiology of obesity. Gastroenterol Clin North Am 2010; 39: 1-7. [ Links ]
8. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity-related health risk. Am J Clin Nutr 2004; 79: 379-84. [ Links ]
9. Wise LA, Rosenberg L, Palmer JR, et al. Anthropometric risk factors for colorectal polyps in African-American women. Obesity 2008; 16: 859-868. [ Links ]
10. Liu CS, Hsu HS, Li CI, et al. Central obesity and atherogenic dyslipidemia in metabolic syndrome are associated with increased risk for colorectal adenoma in a Chinese population. BMC Gastroenterol 2010; 27: 10:51. [ Links ]
11. Kim YJ, Lee KM, Chung WC, et al. Association between measures of obesity and colorectal adenoma Chin Med J (Engl) 2011; 124: 3711-5. [ Links ]
12. Otake S, Takeda H, Suzuki Y, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res 2005; 11: 3642-3646. [ Links ]
13. Yamamoto S, Nakagawa T, Matsushita Y, et al. Visceral fat area and markers of insulin resistance in relation to colorectal neoplasia. Diabetes Care 2010; 33: 184-9. [ Links ]
14. Larsson SC, Rutegård J, Bergkvist L, et al. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer 2006; 42: 2590-7. [ Links ]
15. Erarslan E, Turkay C, Koktener A, et al. Association of visceral fat accumulation and adiponectin levels with colorectal neoplasia. Dig Dis Sci 2009; 54: 862-868. [ Links ]
16. MacInnis RJ, English DR, Hopper JL, et al. Body size and composition and colon cancer risk in men. Cancer Epidemiol Biomarkers Prev 2004; 13: 553-559. [ Links ]
17. Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988; 319: 525-32. [ Links ]
18. Bujanda L, Cosme A, Gil I, et al. Malignant colorectal polyps. World J Gastroenterol 2010; 16: 3103-3111. [ Links ]
19. Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225-249. [ Links ]
20. National Cancer Institute. Surveillance Epidemiology and End Results. (2011). Colorectal Cancer Facts and Figures. Revisado julio 25, 2013, desde: http://www. cancer.org/Research/CancerFactsFigures/colorectal-cancer-facts–figures-2011-2013. [ Links ]
21. Rivero M, Castro B, Fernández Gil PL. Pólipos y poliposis cólica. Medicine 2012; 11: 431-6 [ Links ]
22. Piñeros M, Hernández G, Bray F. Increasing mortality rates of common malignancies in Colombia. Cancer 2004; 101: 2285-92. [ Links ]
23. Perea MD, Castaño-Vinyals G, Altzibar JM, et al. Cancer screening practices and associated lifestyles in population controls of the Spanish multi-case control study. Gac Sanit 2012; 26: 301-10 [ Links ]
24. Teucher B, Rohrmann S, Kaaks R. Obesity: focus on all-cause mortality and cancer. Maturitas 2010; 65: 112-6. [ Links ]
25. Giovannucci E, Michaud D. The role of obesity and related metabolic disturbances in cancers of the colon, prostate, and pancreas. Gastroenterology 2007; 132: 2208-25. [ Links ]
26. Kristjansdottir S, Jonasson JG, Cariglia N, et al. Colonic adenomas found via colonoscopy: yield and risk factors for high-grade dysplasia. Digestion 2010; 82: 252-7. [ Links ]
27. Soultati A, Alexopoulou A, Dourakis SP, Colonic polyps as incidental findings. European Journal of Internal Medicine 2010; 21: 574-80. [ Links ]
28. Williams AR, Balasooriya BA, Day DW. Polyps and cancer of the large bowel: a necropsy study in Liverpool. Gut 1982; 23: 835-42. [ Links ]
29. Bacchiddu S, Álvarez-Urturri AC, Bessa Caserras J. Pólipos colorrectales. FMC 2012; 19: 472-80. [ Links ]
30. Calderwood AH, Jacobson BC. Comprehensive validation of the Boston bowel preparation scale. Gastrointest Endosc 2010; 72: 686-92. [ Links ]
31. Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: A valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009; 69(3 Pt 2): 620-5. [ Links ]
32. Gonzalez-Huix Llado F, Figa Francesch M, Huertas Nadal C. Essential quality criteria in the indication and performance of colonoscopy. Gastroenterol Hepatol 2010; 33: 33-42. [ Links ]
33. Morán Sánchez S, Torrella E, EP Delgado, et al. Colonoscopy quality assessment. Rev Esp Enferm Dig 2009; 101: 107-12. [ Links ]
34. Jover R, Herráiz M, Alarcón O, et al. Clinical practice Guidelines: quality of colonoscopy in colorectal cancer screening. Endoscopy 2012; 44: 444-51. [ Links ]
35. Lorenzo-Zúñiga V, Moreno de Vega V, Boix J. Preparación para colonoscopia: tipos de productos y escalas de limpieza. Rev Esp enferm dig 2012; 104: 426-431. [ Links ]
36. Rex DK, Lehman GA, Ulbright TM, et al. Colonic neoplasia in asymptomatic persons with negative fecal occult blood tests: influence of age, gender, and family history. Am J Gastroenterol 1993; 88: 825-31. [ Links ]
37. World Health Organization. Obesity 2012. Disponible en: http://www.who.int/mediacentre/factsheets/fs311/en/ Acceso julio 12, 2013. [ Links ]
38. Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 2012; 35: 2402-11. [ Links ]
39. Alberti KG, Zimmet P, Shaw J, for the IDF Epidemiology Task Force Consensus Group. The metabolic syndrome: a new worldwide definition. Lancet 2005; 366: 1059-72. [ Links ]
40. Alegría Ezquerra E, Castellano Vázquez JM, Alegría Barrero A. Obesity, metabolic syndrome and diabetes: cardiovascular implications and therapy. Rev Esp Cardiol 2008; 61: 752-64. [ Links ]
41. Calderwood AH, Jacobson BC. Colonoscopy Quality Metrics and Implementation. Gastroenterol Clin N Am 2013; 42: 599-618. [ Links ]
42. Schoenfeld PS, Cohen J. MD Quality indicators for colorectal cancer screening for colonoscopy. Techniques in Gastrointestinal Endoscopy 2013; 15: 59-68. [ Links ]
43. Lee RH, Tang RS, Muthusamy VR, et al. Quality of colonoscopy withdrawal technique and variability in adenoma detection rates (with videos). Gastrointest Endosc 2011; 74: 128-34. [ Links ]
44. Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143: 844-57. [ Links ]
45. Klein S, Allison DB, Heymsfield SB, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping Americas health: Association for Weight Management and Obesity Prevention; NAASO, the Obesity Society; the American Society for Nutrition; and the American Diabetes Association. Diabetes Care 2007; 30: 1647-52. [ Links ]
46. Lorenzo-Zúñiga V, Moreno-de-Vega V, Boix J. Preparation for colonoscopy: types of scales and cleaning products. Rev Esp Enferm Dig 2012; 104: 426-31. [ Links ]
47. Puckett J, Soop M. Optimizing colonoscopy preparation: the role of dosage, timing and diet. Curr Opin Clin Nutr Metab Care 2012; 15: 499-504. [ Links ]
48. Hazewinkel Y, Dekker E. Colonoscopy: basic principles and novel techniques. Nat Rev Gastroenterol Hepatol 2011; 8: 554-64 [ Links ]
49. Comstock SS, Hortos K, Kovan B et al. Adipokines and obesity are associated with colorectal polyps in adult males: a cross-sectional study. PLoS One 2014; 17: e85939. [ Links ]
50. Kaneko R, Sato Y, An Y, et al. Clinico-epidemiologic study of the metabolic syndrome and lifestyle factors associated with the risk of colon adenoma and adenocarcinoma. Asian Pac J Cancer Prev 2010; 11: 975-83. [ Links ]
51. Kaneko R, Nakazaki N, Tagawa T et al. A new index of abdominal obesity which effectively predicts risk of colon tumor development in female Japanese. Asian Pac J Cancer Prev 2014; 15: 1005-10. [ Links ]
52. Thompson CL, Berger NA, Chak A, et al. Racial differences in measures of obesity and risk of colon adenoma. Obesity 2012; 20: 673-7. [ Links ]
53. Dai Z, Xu YC, Niu L. Obesity and colorectal cancer risk: a meta-analysis of cohort studies. World J Gastroenterol 2007; 13: 4199-206. [ Links ]
54. Otake S, Takeda H, Suzuki Y, et al. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res 2005; 11: 3642-3646. [ Links ]
55. Kono S, Handa K, Hayabuchi H, et al. Obesity, weight gain and risk of colon adenomas in Japanese men. Jpn J Cancer Res 1999; 90: 805-811. [ Links ]
56. Schoen RE, Weissfeld JL, Kuller LH, et al. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterol 2005; 129: 464-475. [ Links ]
57. Gerson LB. Impact of obesity on endoscopy. Gastrointest Endosc 2009; 70: 758-62. [ Links ]
58. P. Somasundar P. McFadden DW, Hileman SM et al. Leptin is a growth factor in cancer. J Surg Res 2004; 116: 337-349. [ Links ]
59. Drew JE. Molecular mechanisms linking adipokines to obesity-related colon cancer: focus on leptin. Proc Nutr Soc 2012; 71: 175-80. [ Links ]
60. Aleksandrova K, Boeing H, Jenab M, et al. Leptin and soluble leptin receptor in risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition cohort. Cancer Res 2012; 72: 5328-37. [ Links ]











 text in
text in