Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.4 Bogotá out./dez. 2014
Hepatitis C Infections among Individuals who Received Transfusions prior to 1994 in Antioquia, Colombia
Johanna C. Arroyave O. (1), Claudia Álvarez F. (2), Gonzalo Correa A. (2), Norman Balcázar M. (3), María Patricia Arbeláez M. (4), María Cristina Navas N. (2)
(1) Research and Biomedical Innovation Group at the Instituto Tecnológico Metropolitano and Gastrohepatology Group in the Faculty of Medicine at the Universidad de Antioquia in Medellín, Colombia.
(2) Gastrohepatology Group in the Faculty of Medicine at the Universidad de Antioquia in Medellín, Colombia.
(3) Molecular Genetics Group at the Universidad de Antioquia in Medellín, Antioquia.
(4) Epidemiology Group in the Faculty of Public Health at the Universidad de Antioquia in Medellín, Antioquia.
Research Financed by Merck Sharp & Dohme Colombia and Project Sustainability of the Office of the Vice Rector of Research of the Universidad de Antioquia
This project was presented at the 10th International Meeting on Hepatitis C Virus and Related Viruses in Kyoto, Japan December 2-6, 2003
Received: 08-05-14 Accepted: 05-11-14
Abstract
Introduction: Infection with the hepatitis C virus is a public health problem. According to the World Health Organization there are an estimated 184 million cases of HCV infection around the world. The main risk factor in developing countries is transfusion of blood components. In 1993, Colombian regulations began requiring serological screening by blood banks for infectious agents including HCV. Nevertheless, data about HCV infections in the population transfused before this date is limited.
Objective: The objective of this study is to describe the frequency of HCV infection in the population of individuals transfused before 1994 in Antioquia.
Materials and Methods: A total of 166 individuals transfused before 1994 agreed to participate in the study. ELISA tests for antibodies to HCV were performed on these patients' serum samples. Samples that were positive were tested for the presence of the viral genome by RT-PCR of non-coding region 5.
Results and Conclusions: The frequency of anti-HCV antibodies in study population was 6.6% (11/166) while the HCV genome was present in seven of these eleven individuals. Genotype 1 was identified in four of the samples. No associations of different risk factors for transfusion in individuals with markers of HCV infection were found. This study provides data on the epidemiology of HCV infection in Colombia.
Keywords
Hepatitis C virus, ELISA, genotype, transfusion of blood components.
INTRODUCTION
HCV infections are a public health problem, with more than 184 million infected individuals which corresponds to about 3% of the world's population (1). Chronic infections occur in between 60% and 80% of patients who may be at risk of terminal liver disease such as liver cirrhosis and/or hepatocellular carcinoma (HCC) (2).
Globally, between 25% and 30% of HCC cases are associated with HCV infections. However, this frequency varies depending on geographic region. In Europe, the USA and Japan, HCV infections are a risk factor in 50% to 70% of HCC cases (3-6). In Argentina and Brazil, HCV infections are associated with approximately 30% of HCC cases (7,8).
An estimated 6.8 to 8.9 million adults in Latin American countries test positive for antibodies to HCV (anti-HCV). Most studies in populations of Central and South America have shown that the main risk factor is transfusion of blood components. The exceptions are countries in the southern cone such as Argentina and Brazil. Recently, increased number of cases associated with intravenous use of psychoactive drugs have been observed in these countries. This is similar to reports from Europe and the USA (9).
Colombian Ministry of Health Decree 1571 of August 1993 regulated screening of blood components for different markers of infection including anti-HCV, the marker for HCV infection. A 1995 study of information provided by the 172 blood banks in the country found that 1% of the 367,891 units of blood analyzed were reactive for anti-HCV (10). A 1997 analysis of blood banks in Valle del Cauca showed that 0.98% of blood donors in the city of Cali were seropositive for anti-HCV and that only 0.47% of the donors from the rest of the department were seropositive (11). In the most recent report of national indicators report, HCV seropositivity was 0.49% in 746,059 units of blood analyzed from blood banks throughout the country (12).
This study is a small step in piecing together a picture of pre-regulation infections rates and the limited number of studies of the state of HCV infection in the transfused population prior to regulation. This study retrospectively describes the main clinical and demographic characteristics of individuals transfused in Antioquia before 1994 and uses serological and molecular markers to analyze the frequency of HCV infections.
MATERIALS AND METHODS
Population Sample
This is a retrospective study of individuals in Antioquia who attested to having at least one blood component transfusion before 1994. An active search of hospital databases hospitals was combined with radio announcements and campaigns of scheduled visits to companies from February to September 2003. After obtaining voluntary written informed consent from the 166 participants, they filled out a questionnaire on demographics, reasons for transfusions and risk factors. A whole blood sample was obtained from each participant by venipuncture of the forearm under aseptic conditions. Serum samples were stored at -70°C until processing.
Characterization of HCV
Serology
Serum samples were analyzed in duplicate by third generation ELISA (BIOMERIEUX UBI HCV EIA) to detect total anti-HCV antibodies. The test was conducted according to the manufacturer's recommendations and had a sensitivity of 98.6% and a specificity of 99.8%. A sample was considered positive tests of both aliquots were reactive according to the ratios between optical density and the cutoff value.
Molecular detection of HCV
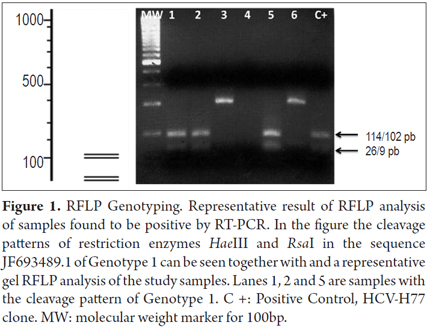
Acid guanidinium thiocyanate-phenol-chloroform extraction (AGPC) was used to extract RNA from serum samples that were reactive for total anti-HVC antibodies. Nested real time polymerase chain reaction (Nested RT PCR) was used to amplify the 5'utr noncoding region according to the protocol reported by Chan 1992 which uses the combination of HCV940, HCV211, HCV939 and HCV20914 primers. Amplified products were visualized by electrophoresis in 2% agarose gel and stained with ethidium bromide to confirm the presence of the expected fragment of 251pb. The trial control consisted of amplification the infectious clone genotype 1a HCV-h77 (kindly donated by Dr. Charles Rice of the Rockefeller University in New York).
Genotyping using Restriction Fragment Length Polymorphism (RFLP)Analysis
To describe the viral genotype in the samples analyzed, polymorphisms in the products of amplification and restriction were analyzed with HaeIII, RsaI, MvaI y HinfI enzymes for 2 hours at 37° C. The RFLP restriction pattern was identified by 4% agarose gel electrophoresis as published previously (15).
STATISTICAL ANALYSIS
Quantitative variables were expressed as medians and interquartile ranges while qualitative variables were expressed as absolute numbers and percentages. The chi-square test was used to compare qualitative variables, and the Mann-Whitney U test was used to compare quantitative variables. A p value of less than 0.05 was assumed to be statistically significant. SPSS 10.0 was used for these analyses.
RESULTS
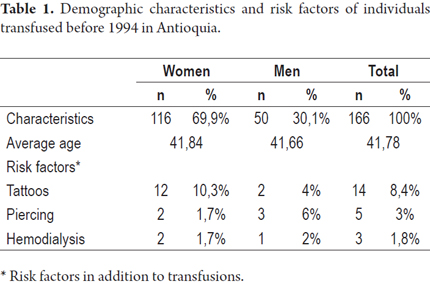
A total of 166 individuals who had been transfused before 1994 voluntarily participated in the study. Women made up 69.9% (116/166) of the study population and men made up the other 30.1% (50/166). The average age of the study population was 42 years (interquartile range 36-49, range 10-75 years). The demographic characteristics of the individuals included in the study are summarized in Table 1.
Other risk factors including hemodialysis (1.8%), tattoos (8.4%) and piercings (3%) were present in small percentages of the population studied and were also determined. None of the study subjects stated that they had used psychoactive drugs intravenously.
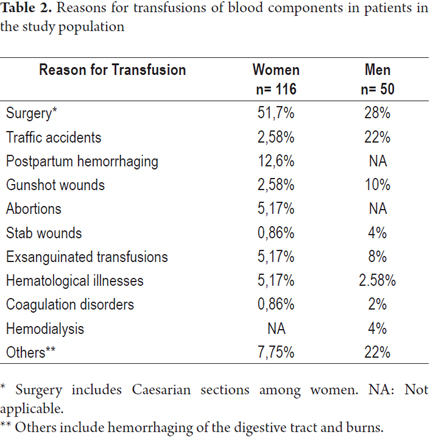
Surgery, traffic accidents, gunshot wounds and exsanguinated transfusion were the four most frequent indications for transfusions in the male population. Among the women in the study, surgery (including cesarean section), postpartum hemorrhaging, abortions and exsanguinated transfusions were the most common reasons for transfusions (Table 2). Most patients (83.1%) were transfused in hospitals in Medellín. The rest were transfused in hospitals and municipalities in the surrounding department of Antioquia with the exception of 3 patients who had received transfusions in hospitals in other departments (1.8%).
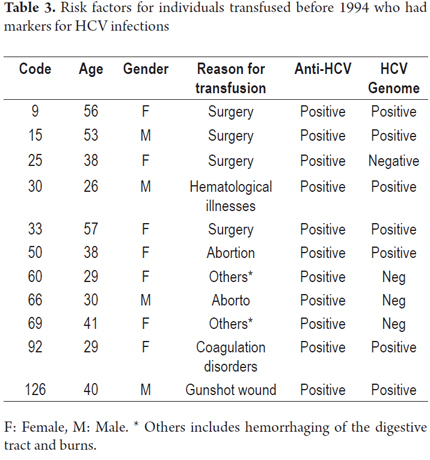
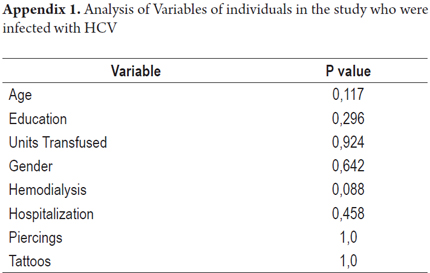
According to the results of serological tests, 11 (6.6%) samples tested positive by ELISA for anti-HCV. The viral genome was detected in 7 of the 11 (63%) specimens that had tested positive. This is a 63.6% HCV genome detection rate from the samples with anti-HCV markers (Table 3). Most of the individuals who presented HCV infection markers showed no additional transfusion risk factors. The analysis showed no statistical significance for any of the variables analyzed in the individuals with HCV infections (Appendix 1).
To assess liver functions of HCV positive individuals, serum transaminase levels were determined for five of the seven subjects who tested positive for the HCV genome. In four of them, values above the reference values were found as expected in chronic HCV infections. There were no other biochemical or histopathological tests available to determine the extent of liver compromise.
Analysis of the cleavage pattern with restriction enzymes (RFLP) allowed the identification of genotype 1 in four samples (57.1%). A computer simulation with NebCutter V2.0 was used to compare the restriction patterns to one from a previously published Colombian genotype 1 sequence (genbank no.: jf693489.1). A cleavage site at position 216 was identified for the HaeIII enzyme while two cleavage sites, at positions 102 and 225, were identified for the RsaI enzyme. In Figure 1 shows the cleavage slit pattern for these two enzymes and a gel representative of the pattern of the samples tested. A comparison of the pattern obtained in the samples with the pattern obtained through the computer simulation and patterns reported previously allowed determination of genotype 1. It was not possible to analyze the other three samples because of insufficient sample quantity.
DISCUSSION
HCV is found throughout the world. It is estimated that about 3% of the world's population is infected (16). In Latin America, the average prevalence is estimated to be 1.23%, although prevalence varies among countries (17).
In Colombia, HCV prevalence in the general population is not known, although it has been estimated that there are between 400,000 and 500,000 who are infected with the virus (18). Some studies have been conducted in groups with risk factors and among blood donors. According to one recent study from 2007 to 2010, the prevalence of anti-HCV was 0.6% in 54,499 blood donors at a blood bank in Medellin (19).
In this study, the frequency of HCV infections was determined in a population of individuals who received transfusions of blood products before 1994. The reasons for transfusions and risk factors associated with HCV in addition to transfusions were studied. A total of 11/166 (6.6%) of the samples tested had anti-HCV antibodies. It was possible to confirm the presence of the viral genome in 7/11 samples through RT-PCR. These results are similar to reports published about patients who had received multiple transfusions in other countries in the region including Honduras, Uruguay, Brazil and Argentina. Those studies described prevalences of anti-HCV ranging from 7% to 16.7% (19-22).
A study by Beltrán et al. of a population of 500 patients who had received multiple transfusions at four institutions in Medellín and Bogotá found the prevalence of HCV infections to be 9% measured by the presence of the anti-HCV marker (23). That study showed that the risk of HCV infections decreased three-fold between 1993 and 1995 in the study population, and that the risk decreased more than 90% after 1995 when screening began to cover 99% of the units of blood in Colombia. That study demonstrated the impacts on transfusions and medicine of the public health care policies of requiring screening of 100% of the units of blood, of improvements in the selection of donors, and of increasing the number of voluntary donors (23).
The frequency of HCV infections has also been explored among Colombian patients undergoing hemodialysis, among users of psychoactive drugs, among patients infected with human immunodeficiency virus (HIV), and among patients with chronic liver diseases.
The first is a prospective study conducted in four dialysis units in Cali in 2007and 2008. It included 999 hemodialysis patients and found a prevalence of 2.9%. One important finding of that study was that 41.3% of the HCV positive patients had histories of transfusions (24).
The second study was conducted in rehabilitation centers and a prison in the city of Bucaramanga in 2009. In that study, there were no cases of anti-HCV reported in 259 samples from individuals who reported using illegal drugs. Most said that they had used drugs orally or nasally (98%) and only a small percentage reported intravenous drug use (4.2%) (25).
Similar results were described in patients with HIV infections among whom the frequency of anti-HCV was 0.8%. The low frequency described in this population was attributed to the absence of risk factors such as transfusions and intravenous drug use (26).
HCV infections among patients with chronic liver disease has also been reported in a prospective study at a fourth level hospital in Medellin during the period from 2005 to 2007. A 6.9% frequency of anti-HCV antibodies was demonstrated in 131 patients who had been diagnosed with liver cirrhosis and/or hepatocellular carcinoma (27).
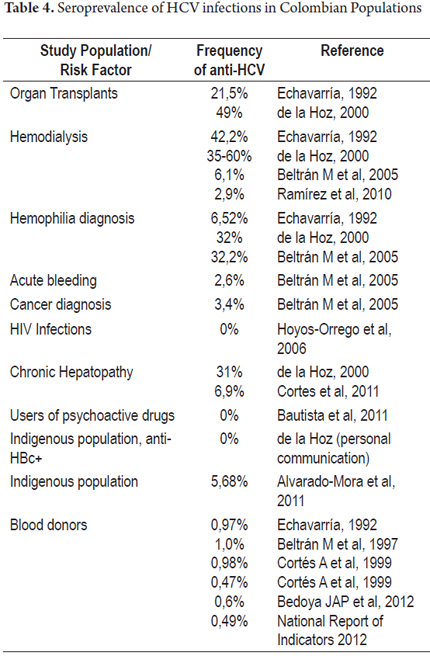
Thus, seroprevalence found in studies conducted after 2005 in populations with risk factors in Colombia ranges from 0% to 9%. These results contrast with those published by Alvarado-Mora et al. in a sample of 176 residents, aged 12 to 72 years, of the rural and urban areas of indigenous communities in the department of Amazonas. The frequency of anti-HCV in the study population was 5.68% (10/176) (28). This finding is striking and contrasts with another study conducted recently by De la Hoz et al. of 176 samples from women from indigenous communities in the same geographic area as the study by Alvarado Mora et al. The frequency of anti-HCV in that population was 0%. It should be noted that the 176 samples exhibited anti-HBV, the Hepatitis B virus marker (personal communication). As mentioned above, the main risk factor for HCV infection in Colombia has been blood transfusions, and indigenous communities are less exposed to this risk for cultural reasons. This does not coincide with the results of Alvarado-Mora et al. It also contrasts with the different studies of the Colombian population with risk factors for HCV (Table 4). The study of Alvarado et al. also evaluated samples from health workers, sex workers and/or members of displaced populations in San Andrés, Chocó and Magdalena. Seropositivity data reported for each region are 0.66%, 3.68% and 3.87% respectively, but their published the data are not broken down by risk group (28).
Studies published in Colombia to date, along with the results of this study, suggest that the prevalence of HCV in Colombia varies according to risk group analyzed. If the results from studies since 2005, are taken into account, prevalences in groups with risk factors such as transfusions, hemodialysis and chronic liver disease varies from 4.2% to 9% which coincides with published data from other countries in the region (9, 29).
Seven (63%) out of 11 samples with anti-HCV antibodies were amplified to characterize the viral genotype of the HCV genome. In addition, Genotype 1 was identified in four of these samples by RFLP analysis. To date published studies using molecular characterization have shown that Genotype 1 is the most prevalent in the country. Only the presence of HCV Genotype 1 has been described among patients with liver cirrhosis and hepatocellular carcinoma (27).
A study of 35 HCV sequences obtained from blood donors Bogotá identified HCV Subgenotype 1b in 82.8% of samples and Subgenotype 1a in 5.7% of the samples. The Subgenotypes 2a, 2b and 3a were also identified (30). In the study cited above of patients who had received multiple transfusions, twelve HCV sequences were characterized. Eight of these were characterized as Subgenotype 1b (66.7%), two were confirmed as Subgenotype 1a (16.7%), and one sample each was confirmed as Subgenotype 2b and 3a (31). Seven of the eight patients with HCV Subgenotype 1b had received their first transfusion before 1986. This finding agrees with the estimate made by Alvarado-Mora et al. who postulated that the introduction of HCV (subgenotype 1b) into Bogotá, Colombia occurred around 1950. The incidence of the disease grew exponentially between 1970 and 1990, probably as the result of exsanguinated transfusions (30).
The results of this study contribute to the knowledge of the epidemiology of HCV infections in Colombia among individuals who were transfused before 1994. The frequency of infection with HCV Genotype 1 described in this population is similar to those reported in other countries of the region. Nevertheless, for better management of cases and identification of candidates for treatment, further studies should be performed among individuals who received transfusions before 1996.
Acknowledgements
The authors thank Merck Sharp & Dohme Colombia and the vice-rector for research at the University of Antioquia (sustainability project) for funding for the completion of this work.
REFERENCES
1. Who IN, States M. Global policy report on the prevention and control of viral hepatitis in WHO Member States 2013. [ Links ]
2. Hajarizadeh B, Grebely J, Dore G. Epidemiology and natural history of HCV infection. Nat Rev Gastroenterol Hepatol 2013; 10: 553-62. [ Links ]
3. Perz JF, Armstrong GL, Farrington L, Hutin Y, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol 2006; 45: 529-38. [ Links ]
4. De Martel C, et al. Global burden of cancers attributable to infections in 2008: a review and synthetic analysis. Lancet Oncol 2012; 13: 607-15. [ Links ]
5. Tornesello ML, et al. Mutations in TP53, CTNNB1 and PIK3CA genes in hepatocellular carcinoma associated with hepatitis B and hepatitis C virus infections. Genomics 2013; 102: 74-83. [ Links ]
6. Aghemo A, Colombo M. Hepatocellular carcinoma in chronic hepatitis C: from bench to bedside. Semin. Immunopathol 2013; 35: 111-20. [ Links ]
7. Castello G, Scala S, Palmieri G, Curley S, Izzo F. HCV-related hepatocellular carcinoma: From chronic inflammation to cancer. Clin Immunol 2010; 134: 237-50. [ Links ]
8. Fassio E, et al. Etiology of hepatocellular carcinoma in Latin America: a prospective, multicenter, international study. Ann Hepatol 2010; 9: 63-9. [ Links ]
9. Kershenobich D, et al. Trends and projections of hepatitis C virus epidemiology in Latin America. Liver Int 2011; 31(Suppl. 2): 18-29. [ Links ]
10. Beltrán M, Raad J, Ayala M, Ching R. Tamizaje de enfermedades infecciosas en bancos de sangre, Colombia, 1995. Biomédica 1997; 17: 137-142. [ Links ]
11. Cortés AB, Beltrán M, Olaya B, Hernández M. Riesgo de enfermedades infecciosas transmitidas por transfusión en el Valle del Cauca, Colombia. Colomb Med 1999; 30: 13-18. [ Links ]
12. Informe nacional de Indicadores. Red Nacional de Bancos de Sangre y Servicios Transfusionales. Instituto Nacional de Salud. 2012. [ Links ]
13. Chomczynski P, Sacchi N. Single-Step Method of RNA Isolation by Acid Guanidinium Extraction. Anal. Biochem 1987; 162: 156-159. [ Links ]
14. Chan SW, et al. Analysis of a new hepatitis C virus type and its phylogenetic relationship to existing variants. J. Gen. Virol 1992; 73: 1131-41. [ Links ]
15. Davidson F, et al. Survey of major genotypes and subtypes of hepatitis C virus using RFLP of sequences amplified from the 5 non-coding region. J Gen Virol 1995; 76: 1197-204. [ Links ]
16. Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global Epidemiology of hepatitis C Virus infection: New estimates of age-specific antibody to hepatitis C virus seroprevalence. Hepatology 2013; 57: 1333-1342. [ Links ]
17. Méndez-Sánchez N, Gutiérrez-Grobe Y, Kobashi-Margáin RA. Epidemiology of HCV infection in Latin America. Ann Hepatol 2010; 9: 27-29. [ Links ]
18. De la Hoz F. Epidemiología de la hepatitis C en Latinoamérica y Colombia. Biomédica 2000; 20: 66-72. [ Links ]
19. Vinelli E, Lorenzana I. Transfusion-transmitted infections inmulti-transfused patients in Honduras. J Clin Virol 2005; 34 (Suppl. 2): S53-60. [ Links ]
20. López L, et al. Risk factors for hepatitis B and C in multi-transfused patients in Uruguay. J Clin Virol 2005; 34 (Suppl. 2): S69-74. [ Links ]
21. Paula EV, et al. Transfusion-transmitted infections among multi-transfused patients in Brazil. J Clin Virol 2005; 34 (Suppl. 2): 27-32. [ Links ]
22. Remesar M, Gamba C, Kuperman S, Marcos M A. Antibodies to hepatitis C and other viral markers in multi-transfused patients from Argentina. J Clin Virol 2005; 34 (Suppl. 2): 20-26. [ Links ]
23. Beltrán, M. et al. Hepatitis C virus seroprevalence in multi-transfused patients in Colombia. J. Clin. Virol 2005; 34 (Suppl. 2): 33-38. [ Links ]
24. Ramírez R, et al. Prevalencia de anticuerpos contra el virus de hepatitis C en unidades de diálisis de Cali-Colombia Prevalence of anti-HCV antibodies among patients on dialysis in Cali-Colombia. Rev Col Gastroengerología 2010; 14-18. [ Links ]
25. Amorocho HB, Zorelly B, Moreno J. Ausencia de infección por virus de la hepatitis C en usuarios de drogas ilícitas en la ciudad de. Rev Col Gastroengerología 2011; 15-20. [ Links ]
26. Hoyos-Orrego A, Massaro-Ceballos M, Ospina-Ospina M, Gómez-Builes C, Vanegas-arroyave N. Serological markers and risk factors for hepatitis b and c viruses in patients infected with human immunodeficiency virus. Rev Inst Med trop S Paulo 2006; 48: 321-326. [ Links ]
27. Cortes-Mancera F, et al. Etiology and Viral Genotype in Patients with End-Stage Liver Diseases admitted to a Hepatology Unit in Colombia. Hepat Res Treat 2011; 363205. [ Links ]
28. Alvarado-Mora MV, et al. Hepatitis B (HBV), hepatitis C (HCV) and hepatitis delta (HDV) viruses in the Colombian population--how is the epidemiological situation? www.plosone.org. 2011. [ Links ]
29. Szabo SM, et al. The epidemiologic burden of hepatitis C virus infection in Latin America. Ann Hepatol 2012; 11: 623-35. [ Links ]
30. Alvarado-Mora MV, Gomes-Gouve MS. Molecular Characterization , Distribution, and Dynamics of Hepatitis C Virus Genotypes in Blood Donors in Colombia. J Med Virol 2010; 1898: 1889-1898. [ Links ]
31. Di Filippo D, et al. Molecular characterization of hepatitis c virus in multi-transfused Colombian patients. Virol J 2012; 9: 242. [ Links ]











 texto em
texto em