Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.4 Bogotá Oct./Dec. 2014
Diagnostic and Therapeutic Approaches for First Visits of Patients with Crohn's Disease
Juan Ricardo Márquez Velásquez MD. (1)
(1) Coloproctologist at the Instituto de Coloproctología S.A.S. of Clínica Las Américas in Medellín, Colombia. Mail: juanmarquezv@gmail.com
Received: 12-02-14 Accepted: 05-11-14
Abstract
Crohn's disease (CD) is the fundamental protagonist of so-called Inflammatory Bowel Disease (IBD). This is a term by which several entities are known. Its origin is multifactorial. It is characterized by chronic and recurrent inflammation with different degrees of involvement of the gastrointestinal tract, but also with potential commitment of other organs.
In the last decade there has been a renewed interest in these entities due to a boom in innovative medicines. Despite these medications, CD and IBD remain incurable. The increasing incidence of CD in our country requires us both scientifically and morally to develop basic, practical guidelines with checklists adapted to our environment for the diagnosis and management of CD through the first consultations of patients.
Keywords
Crohn's disease, inflammatory bowel disease, checklist, vaccination, tuberculosis.
INTRODUCTION
Crohn's disease (CD) is a type of what is called Inflammatory Bowel Disease (IBD) which is a severe immune response of the intestinal mucosa to an environmental stimulus caused by enteric pathogens in a genetic and immunologically susceptible individual. This leads to a chronic, progressive, destructive and uncontrolled inflammatory response of the bowel's mucosa.
It is characterized by transmural fistulizing compromise which can affect the entire digestive tract and the perianal region. Its main symptom is diarrhea, with or without bleeding, that is associated with heterogeneous manifestations with periods of exacerbation and remission. These manifestations include abdominal pain, weight loss, anemia, fever, anorexia, asthenia and, in some cases, serious complications such as intestinal obstruction and fistulas. Fistulas, mostly in the perianal region, occur in 15% of the patients, are often complex, do not heal and develop abscesses. In 10% of these cases they are rectovaginal (1, 2). There can also be extraintestinal manifestations that are especially common in colonic CD (3).
With the development and modernization of a society, IBD initially starts with an increase in ulcerative colitis (UC) which stabilizes at a certain number of affected patients. After an interval of about 10 years, CD appears and the number of people affected increases until more are affected by CD than by UC (4). Today, IBD occupies one of the first places among gastrointestinal pathologies in developed countries in terms of incidence and prevalence. Figures vary between 4-7/ 100,000 people/year and 120-200 /100,000 patients for UC, and 8-12/100,000 people/year and 50-200/100,000 people/year for CD. There are approximately 1.4 million people in the USA and 2.2 million in Europe who suffer from these diseases (3-6).
It is likely that the increased incidence of these diseases is due to a set of environmental and economic factors including:
Improved sanitary conditions
Diets that are richer in fat and carbohydrates, lower in fiber with additions of chemical and preservatives.
Declining incidence of childhood diseases due to widespread use of broad-spectrum antibiotics, poly-immunization, and improved sanitation systems (water, sewage, etc.)
Many other changes including the decline in household size, increasingly sedentary lifestyles, obesity and environmental pollution (4).
There exist worrisome data that motivated me to write this article. The IMPACT study, a collaborative descriptive study involving 24 countries European Union countries (7), surveyed 4,900 IBD patients. It found that most patients were diagnosed in time (1-3 years) but 18% waited for more than five years. A significant majority of 64%, needed emergency medical care before their diagnoses. The hospitalization rate of 85% within the most recent five years is extremely high. Half of the people with IBD had active disease when they answered. Seventy-four percent had requested sick leave from their jobs during the previous year because of IBD. Of these, 26% were off work for more than 25 days (7).
Colombia is no stranger to these issues. A retrospective descriptive study performed in two hospitals in Bogota between 1968 and 1990 (8), suggested there are 9 cases of UC for each case of CD. Almost twenty years later, an observational descriptive study performed at the Pablo Tobon Uribe Hospital in Medellin found only 4.9 UC cases per case of CD (9). The incidence of diseases have increased in Colombia, but the incidence of CD has grown faster so that it is closing the gap in the proportions of the two diseases.
Based on the literature and the existing guidelines, I will put forth a clear practical course, adjusted to our environment and needs. It will contain the basic steps for diagnosis and initial monitoring of patients with CD as well as the therapeutic approach. However, I will not go into details, as it is not the purpose of this article. At the end, the reader will find a checklist which may be freely incorporated into any clinical history form.
THE FIRST EVALUATION
The first consultation of a patient with suspected IBD or with a confirmed diagnosis of CD is a special time but also a challenge for both the clinician and the patient. Apart from building empathy which is a critical ingredient in any doctor-patient relationship, this consultation is particularly important due to the implications of this type of diagnosis.
Patients may be referred to a gastroenterologist with or without prior diagnoses. The patient often has a torpid clinical history with active disease although the kind of illness is completely unknown. Instead, the clinical history is often just a series of symptoms without any clear indication of when they began, although the patient often knows her/his diagnosis or believes he/she knows it through various sources. The most easily available and widely used today is, of course, the internet. The patient will have gone through countless tests, been poly-medicated, been hospitalized on more than one occasion, and may have even undergone surgery. Still worse, the patient will have been managed by several physicians but never with a clearly targeted therapeutic road map. At the end, the patient will have an uncertain but certainly grim prognosis. Whatever type of patient is seated in front of us, the common denominators when she or he arrives to this "long-awaited" first consultation with the specialist are a great deal of uncertainty, high expectations, and great terror.
Empathy between doctor and patient, and doctor and family, is essential. The rush of the professional and the short times imposed by health care organizations can derail a proper doctor-patient relationship. This makes the subsequent physical examination and treatment more difficult. This is even truer if the patient faces surgery for which trust in the physician plays an essential factor in the patient's acceptance, recovery and reintegration into daily life.
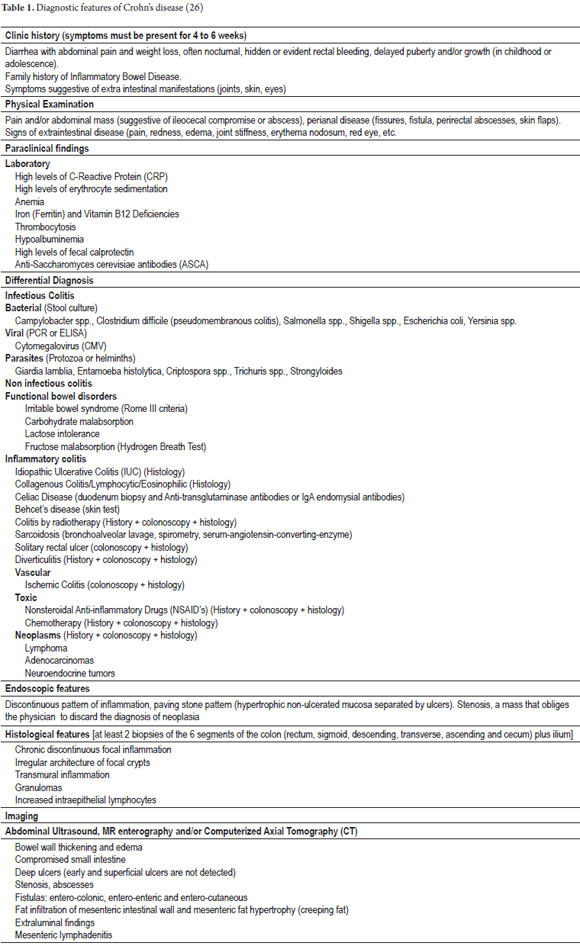
During this first consultation the specialist should try to confirm the degree of inflammatory activity in order to diagnose the disease. She should try to obtain clear information from previous tests and treatments with the goal of designing a therapeutic schedule to finally offer the patient objective information about treatment and prognosis with precise short, medium and long-term goals (Table 1) (10).
Paso 1
Taking the Patient's Clinical History
Important information that should always be asked for includes: Gender, race, age at time of disease onset, location, extent and duration of disease, family history of IBD, vaccination history, gynecological and obstetric history (last date of menstruation), type of treatment received up until that moment, and complications.
It may be important to use a health related of quality of life (HRQOL) questionnaire to obtain objective information about the patient's current quality of life, work history, family history and socioeconomic history. This should always be done AFTER you have fully established the diagnosis. Since this questionnaire should be specifically designed for IBD, it will not be valid for any other case.
The short form of the Inflammatory Bowel Disease Questionnaire (IBDQ) consists of 10 questions. Basically, it is used to determine the impact of the disease on the quality of life of patients (23). With this simple and short exercise we can quickly stage the severity, aggressiveness and prognosis of the disease for the patient in question. All scores are reported on a 7-point scale (1 = poor HRQOL, 7 = optimal HRQOL) (Table 2) (30).
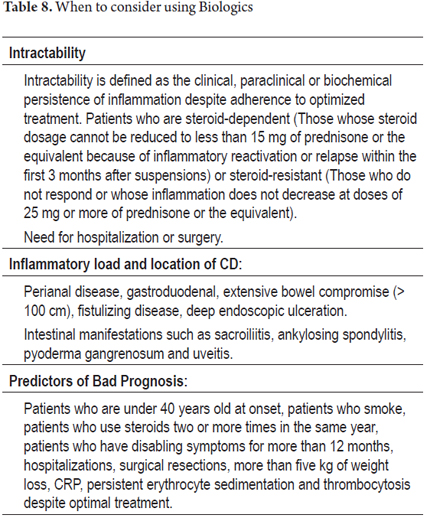
There are data that, if found the medical record of a patient, mean that that patient is in a high state of high risk and has a poor prognosis from the beginning. Such patients will probably need early treatment with biologics. These data are: under 40 years of age at disease onset, smoking, perianal or gastroduodenal disease, extensive bowel compromise (> 100 cm), fistulizing disease, deep endoscopic ulceration, steroid use, hospitalizations, surgery, weight loss, and extraintestinal manifestations such as sacroiliitis, ankylosing spondylitis, pyoderma gangrenosum and uveitis.
Step 2
Evaluation of inflammatory activity
We should stage every patient according to the state of her or his disease, its location and the type of compromise (inflammatory, stenosing, fistulizing). Ileocolonoscopy with biopsies is the baseline test for diagnosis and subsequent management of the disease.
The risk of fistulizing disease is 20% to 40%. The clinical course of fistulas varies and depends on their location and complexity. Perianal fistulas heal with the most difficulty (11).
Symptoms, laboratory tests (including measurement of inflammatory markers), serology and imaging studies help us establish the degree and extent of inflammatory involvement. However, there is no "gold standard" test (12).
Extraintestinal manifestations are the most common in CD especially when it affects the colon. (11) They occur in 21% to 36% of patients with IBD and can be divided into 3 groups:
1. Disorders that affect the skin (pyoderma gangrenosum and erythema nodosum), eyes (uveitis, iritis, episcleritis and scleritis), joints (most frequent extraintestinal manifestations including rheumatic disorders such as peripheral and axial arthropathy, ankylosing spondylitis, and bone metabolism disorders) and mouth. They usually occur in patients with colonic CD. Activity of their intestinal disease is parallel to the extraintestinal disorder.
2. Secondary complications of IBD and its extensions include kidney stones, obstructive uropathy, malabsorption and cholelithiasis. They are more common in patients with CD than among those with UC.
3. Other potential extraintestinal manifestations that cannot be characterized into the first two groups include osteoporosis, liver diseases, amyloidosis, vascular, hematologic, pulmonary, cardiac and neurological complications.
Severity Indices
For the first clinical assessment we rely on the Montreal classification which divides patients by age at diagnosis (A), location of the bowel segments involved (L); and behavior and severity of inflammation (B) (13).
Montreal classification
Age at diagnosis (A) |
Location (L) |
Behavior (B) |
A1: less than 16 years |
L1: Ileal |
B1: Non-stricturing, non-penetrating (inflammatory) |
A2: between 17 y 40 years |
L2: Colonic |
B2: Stricturing |
A3: more than 40 years |
L3: Ileal-colonic |
B3: Penetrating (Fistulizing) |
L4: Isolated upper digestive |
P: perianal disease |
Crohn's Disease Activity Index
The Crohn's Disease Activity Index (CDAI) is used in clinical studies and rarely in everyday clinical practice (14). Nevertheless, this is the scoring system upon which all scientific articles are based when they draw conclusions and make recommendations. It has been designed to measure the effectiveness of medical therapy, clinical monitoring and assessment of the severity of the disease.
Calculation of the CDAI can classify disease activity according to the value obtained as in this list (a CDAI calculator can be obtained online at www.ibdjohn.com/cdai/):
1. Response: 100 point decrease in CDAI
2. Referral: CDAI <150 points
3. Mild activity: CDAI 151-219 points
4. Moderate activity: CDAI 220-450 points
5. Serious activity: CDAI > 450.
Harvey-Bradshaw Index (HBI)
The Harvey-Bradshaw index is also known as the Simplified Index and as the Modified CDAI. The Harvey-Bradshaw index is the most practical and easy to use index and is often used in clinical practice. It is based on subjective and clinical variables. Only 5 CDAI variables are used: anti-diarrheal medication, hematocrit values and weight variation are not included, and this index only evaluates parameters during the preceding 24 hours. It is easy and fast and allows for out-patient examinations. It is less certain because a patient's condition can change from one day to the next. Nevertheless, it has demonstrated an excellent correlation with the CDAI. It is useful in clinical practice and for clinical studies, as demonstrated by its application in PRECISE 1 and 2. It has the same disadvantages and limitations as does the CDAI (32).
1. General well being |
0 = well in general 1 = mild compromise 2 = moderate 3 = bad 4 = terrible |
2. Abdominal pain |
0= ausente 1= leve 2= moderado 3= severo |
3. Number of liquid or soft stools per day |
(1 point per stool) |
4. Abdominal mass |
0= none 1= dubious 2= definite |
5. Complications (1 point per item) |
Arthralgia, uveitis, erythema nodosum, aphthous ulcers, pyoderma gangrenosum, anal fissure, new fistula, abscess |
TOTAL SCORE (add scores of questions 1-5) HBI: |
Remission <5 Mild disease 5-7 Moderate disease 8-16 Severe disease >16 |
Adapted from Harvey RF, Bradshaw JM. 1980 (32)
Bio-chemical markers (bio-markers)
C-reactive protein (CRP) is a sensitive acute-phase reactant whose levels increase in cases of inflammation, infection or tissue injury. It is an accurate marker for CD. Its levels rise when there is active inflammation, and it correlates well with the CDAI (15). A low CRP level may indicate that CD is either idle or in a quiescent stage. However, this is only partly true because up to 50% of patients with active CD do not show high levels of CRP despite having documented active inflammation.
Fecal calprotectin is a protein located in the cytosol of macrophages and neutrophils which are released in situations of cell damage. It is detected in the feces for periods of up to one week at room temperature. It is a marker that correlates well with the clinical, endoscopic and histological activity indices of the disease. It can assess the efficacy of treatment as well as discriminate degrees of inflammatory activity from absent (<50 ug/g), to moderate (50-150 ug/g), to severe (> 150 ug/g). When levels of fecal calprotectin above 250 ug/g are found, it indicates that the disease is associated with severe inflammation of the mucosa (16, 28).
In any case, ileocolonoscopy is the test of choice to determine the degree of mucosal inflammation.
Serology
Although a series of markers, antimicrobial peptides and antibodies have been studied to differentiate between IUC and CD as well as to define their prognoses, none of them are sufficiently sensitive or specific to be used alone. Anti-saccharomyces cerevisiae antibodies (ASCA) have a sensitivity of 60% for CD but may be positive in up to 15% of IUC cases. Perinuclear antineutrophil cytoplasmic autoantibodies (pANCAs) have a sensitivity between 40% and 60% for IUC.
A sensitivity of 50% and a specificity of 92% to 97% for CD has been established for both combinations pANCA-/ASCAS+ and pANCA+/ASCAS- while a sensitivity of 45% and a specificity of 81% to 98% has been established for these combinations for diagnosis of IUC (27).
Nucleotide-binding oligomerization domain containing 2 (NOD2) has been associated with CD in young people with extensive fibrostenosing disease. ASCA is associated with aggressive disease which requires surgery. Anti-OmpC is associated with fibrostenosis, perforation and need for surgery in the small intestine (10).
Step 3
History of Vaccinations and Administration of Required Vaccines
The use of medications such as steroids, immunosuppressants and biologicals favor the occurrence of opportunistic infections that may be preventable with vaccination.
Live virus vaccines are contraindicated in patients who have one of the conditions below that defines them as immunosuppressed:
1. Treatment with glucocorticoids: more than 20 mg/day of prednisone or the equivalent or up to 2 mg/kg/day for more than 2 weeks and/or in the three months after treatment with glucocorticoids.
2. Treatment with an effective dose of 6-MP/AZA and/or in the three months after treatment with 6-MP/AZA.
3. Treatment with methotrexate or in the three months after treatment with methotrexate.
4. Treatment with inhibitors of tumor necrosis factor or in the three months after treatment with tumor necrosis factor inhibitors.
5. Severe protein-calorie malnutrition.
A history of the patient's vaccinations should be taken during the first consultation. In due time, the patient's antibody titers for mumps, measles and rubella (MMR), varicella, and hepatitis A and B should be checked (31).
Approach based on serological results for Hepatitis B
Transaminases |
AgHBs |
AntiHBc |
AntiHBs |
Behavior |
Normal |
- |
- |
- |
Vaccinate |
Normal |
- |
+ |
+ |
Immune due to natural infection |
Normal |
- |
+ |
- |
Hidden infection Risk 5%-10% |
Normal |
- |
- |
+ |
Vaccinated |
Normal |
+ |
+ |
- |
Chronic carrier* |
Abnormal |
+ |
+ |
- |
Chronic Hepatitis* |
* Hepatology evaluation vs. infectious disease evaluation
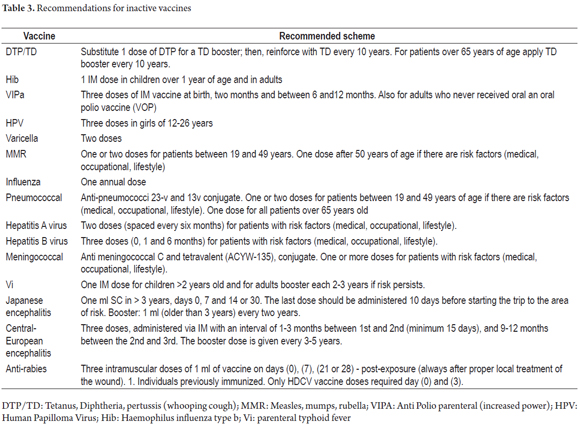
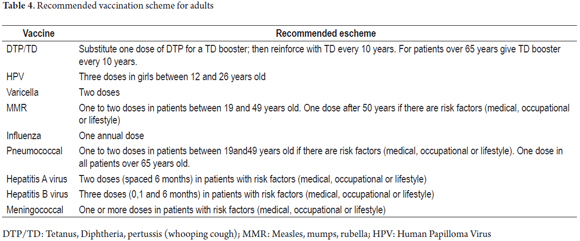
According to the Advisory Committee on Immunization Practices (ACIP) (17), once patients have been documented to have CD, vaccines with one of the following characteristics should be administered: inactivated vaccines or polysaccharides or toxoids which can be applied without safety problems although the immune response is lower than in the general population. Vaccines indicated for these patients are listed in Table 3, but the recommended adult scheme is outlined in Table 4.
Contraindicated vaccines are made with live, attenuated microorganisms that include:
- Triple Viral
- Varicella
- Rotavirus
- Oral cholera
- Yellow fever
- Oral typhoid fever (Ty21a)
- Oral polio vaccines
If biological or immunosuppressive therapy is not going to start within one month, live attenuated vaccines (MMR, varicella and herpes zoster) can be applied. Infants of mothers with IBD who received biologics during pregnancy will have some level of these medications for six months after delivery. The only live virus vaccine that should be administered during this period is that for rotavirus and for this reason live virus vaccines are contraindicated (25). Application of attenuated vaccines requires at least three months after administration of immunosuppressants ends.
Step 4
Preparation for therapy
The ultimate goal of IBD therapy is anti-inflammatory and immunosuppressive treatment aimed at the immune response that causes tissue damage. Azathioprine (AZA) should be administered at a dose of 2.5 mg/kg with a complete blood count follow-up eight days later. The mean corpuscular volume (MCV) is the most important indicator in this follow because there is no Thiopurine Methyl Transferase (TPMT) available in Colombia. Additional CBCs should be checked at 15 days, one month and then every third month thereafter.
Special care should be taken with non-melanoma skin cancer and cervical dysplasia associated with human papillomavirus (HPV), especially for patients receiving azathioprine. The daily use of sunscreen and annual reviews by a dermatologist and a gynecologist are recommended (10).
Tests should include blood iron levels, vitamin B12 and vitamin D. Because patients who take prednisone at doses greater than 7.5 mg for more than six weeks, even if they are not continuous, begin to have osteopenia, patients who chronically receive steroids should be tested with bone densitometry and should be considered for supplemental calcium and vitamin D.
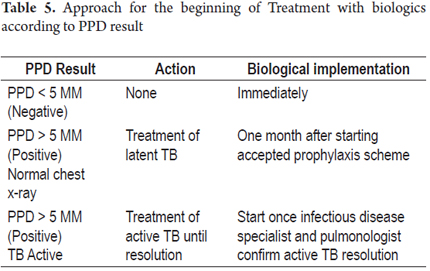
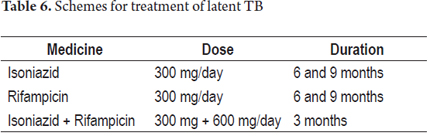
Any patient for whom treatment with biologics is suggested needs to have a chest x-ray and PPD (purified protein derivative) in our environment or quantiferon® due to the risk -between 4 and 90 times- of reactivation of TB with this class of medicines. These tests decrease the occurrence of this entity in 90% of the time because it can be detected early in its latent vs. active states (24). Below there are shown two practical schemes for both test interpretation (PPD) and treatment according to the result of PPD (Tables 5 and 6) (31).
Therapy with tumor necrosis factor inhibitors can begin one month after starting prophylaxis with any of the previously proposed schemes. Regardless of previous serological status, any patient receiving this therapy has a higher susceptibility to TB infection and development of any form of symptoms (pulmonary, extra-pulmonary, atypical, miliary, etc.). Therefore, special care must be taken in monitoring during treatment and for up to 6 months after administration of these medicines ends. Finally, prior to treatment it is mandatory for patients who have suffered from TB in the past, or whose test and symptoms suggest that they have, to be tested by pulmonology and infectious diseases. Therapy can only start after a favorable opinion by these specialists.
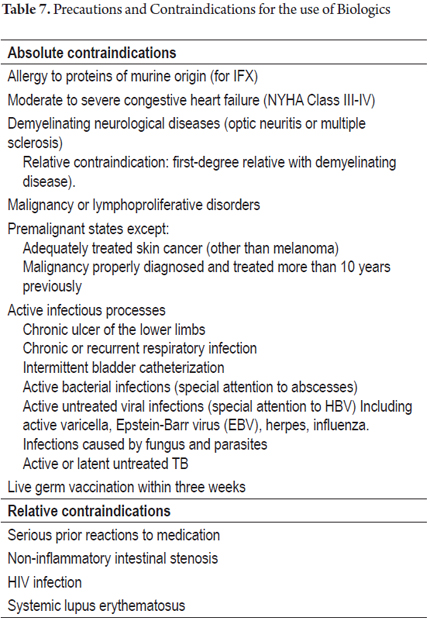
There are certain parameters and precautions to be followed for patients receiving immunomodulation or immunosuppressive therapy. There are a series of absolute and relative contraindications for the use of biologics that are listed in Table 7 (29).
Although it is not part of the assessment during the patient's appointment, I would like to briefly outline the diagnostic criteria for histological tests and imaging so that physicians will be clear about what should be analyzed when reviewing clinical tests that the patient might bring, and what tests and type of images need to be requested.
ENDOSCOPIC FEATURES
Whenever a diagnosis of CD is suspected, a colonoscopy with ileal intubation should be performed (18) due to the high frequency of ileal compromise. Endoscopically, asymmetric mucosa is found with two types of ulcers: ulcers that are linear, serpentine and deep with transmural involvement, and discontinuous ulcers interspersed between normal mucosa (18). The second pattern has aphthoid ulcerations of 1mm to 2 mm in diameter that appear on the lymphoid follicles. The compromise is segmental and may be accompanied by stenosis (19). Nevertheless, it should always be kept in mind that this disease can affect any part of the digestive tract.
Before the development of video capsule endoscopy (VCE), sectional ileocolonoscopy and cross sectional CT imaging of the small intestine were required. Still, VCE should be reserved for cases in which ileocolonoscopy plus radiological images of the small intestine are inconclusive but in which there is a high degree of suspicion of CD (18). A normal VCE has a high negative predictive value for the diagnosis of active CD of the small intestine.
HISTOPATHOLOGIC FEATURES
The earliest type of lesion that occurs as a result of CD is focal infiltration of a crypt by epithelial neutrophils. Distortion and discontinuous inflammation of the cryptic architecture, focal cryptitis, and non-caseating epithelioid granulomas are found. They may affect any intestinal layer, lymph nodes, the mesentery, the peritoneum and even the liver. CD is mainly characterized by skip lesions ulcers spaced between normal mucosa clearly marked with transmural compromise. This affects the entire intestinal wall making it thick and fibrotic with a narrowed opening. It also thickens the mesenteric fat. This gives the impression that the fat wants to catch the intestine by surrounding it, a phenomenon known as "creeping fat". Histological differentiation between UC and CD is difficult in as many as 15% of cases is impossible (20). This is called unclassified colitis. It becomes possible to reach a final conclusion in these cases only when a surgical specimen is extracted and analyzed.
IMAGING FEATURES
The availability of multiplane imaging modalities such as MRI and CT scans allows full evaluation of the intestinal wall (especially of the small intestine) and of extraluminal structures. The contribution of these images is essential for initial diagnosis as well as for monitoring and detection of complications such as stenosis, fistulas and abscesses (21, 22). The choice between an MRI and a CT scan depends on experience and local availability.
Transabdominal ultrasound has several advantages for IBD assessment. They include availability on short notice, absence of ionizing radiation, possibility of direct evaluation of morphology (thickening of the wall) and clinical signs like localized pain. In experienced hands the sensitivity of ultrasound for examining CD has been reported to be as high as 80% to 90% for small intestine compromises and for diagnosis of stenosis (21).
Magnetic resonance imaging (MRI) and computed tomography (CT) complement conventional x-ray imaging such as an intestinal transit study. While a transit study provides a more detailed picture of the intestinal mucosa, multi-cut imaging allows a full assessment of the intestinal wall and of extraluminal structures. That is why multi-cut imaging has been replacing other types of imaging. CT scans are used to detect complications such as abscesses and stenosis outside of the intestinal wall, especially in cases of CD. They show specificity greater than 90% for detecting inflamed areas while sensitivity varies between 70-80% because very small changes of the mucosa cannot be detected (22).
MRI has a diagnostic yield similar to that of CT scans but with the advantage that MRI does not use ionizing radiation to assess areas of stenosis and establish the degree of compromise of the disease (22).
In summary, these imaging modalities are promising for initial diagnoses involving the small intestine, for detecting extraluminal compromises in lymph nodes and mesenteric fat, for identifying the degree and extent of colonic inflammation, and for detection of complications.
TREATMENT
The goal of treatment is to control symptoms in order to induce remission, but more importantly to ensure that state of clinical remission. This means the total absence of symptoms, free from steroids, negative biomarkers, with healed mucosa healing and without extraluminal lesions. The ultimate goal of all of this is to induce a deep state of sustained remission which thereby improves the patient's quality of life and the length of the patient's life by reducing or preventing complications in the short, medium and long term (work absenteeism, hospitalization, surgery, colorectal cancer, etc.).
Current therapy should be structured according to the clinical presentation, the extent and the severity of the disease and should also take into account previous responses to treatments and the presence of any complications.
There are some very important considerations that must be kept in mind from the moment conventional therapy starts, and which must be constantly reviewed as the treatment progresses:
1. From the beginning identify patients with poor prognoses (Table 8)
2. Start immunomodulators early and optimize them at the maximum standard dose (2.5 mg/kg Aza or 1.5 mg/kg of 6-MP) without extending their use if complete remission is not achieved on time (10-12 weeks).
3. Reassess the patient in the required periods according to the medication they are receiving.
a. Prednisone: 2 to 4 weeks
b. Azathioprine: 10 to 12 weeks
c. Salicylates: 12 weeks
4. Avoid using steroids (never underestimate their toxicity) two or more times in the same year.
5. Follow IBD control paradigms:
a. Clinical remission
b. Steroid-free
c. Negative biomarkers
d. Mucosal healing
e. Absence of extraluminal lesions
CONCLUSION
Although CD is the least frequent form of IBD in our environment, it has epidemiologically higher growth rates and has high rates of morbidity and high socio-economic costs. Morally and academically, these circumstances force us to join our efforts to clear the paths in order to achieve a combined objective of practical check lists for earlier diagnosis with better therapeutic approaches. All of these will result in lower rates of morbidity and lower costs which will be important contributions to the development and welfare of our specialty, of society and of our patients who are ultimately our reason for existence. (Annex 1)
REFERENCES
1. Sands, B. Histopathology in inflammatory bowel disease. Gastroenterology 2000; 118: S68-S82. [ Links ]
2. Sicilia B, Vicente R, Gomollon F. Enfermedad de Crohn y colitis ulcerosa: discusión de la epidemiologia clínica. Acta Gastroenterol Latinoam 2009; 39: 135-145. [ Links ]
3. Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011; 140: 1785-1794. [ Links ]
4. Shanahan F, Bernstein CN. The evolving epidemiology of inflammatory bowel disease. Curr Opin Gastroenterol 2009; 25: 1-5. [ Links ]
5. Moum B, Ekbom A. Epidemiology of inflammatory bowel disease – methodological considerations. Digest Liver Dis 2002; 34: 364-369. [ Links ]
6. Loftus CG, Loftus EV Jr., Harmsen WS, et al. Update on the incidence and prevalence of Crohns disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis 2007; 13: 254-61. [ Links ]
7. Wilson B, et al. Presentado en el 7th Congress of ECCO, Feb 16-18, 2012 Barcelona, Spain. Poster 406. [ Links ]
8. Arguello M, Archila PE, Sierra F, et al. Enfermedad inflamatoria intestinal. Rev Col Gastroenterol 1991; 6(4): 237-272. [ Links ]
9. Juliao F, Ruiz MH, Florez JF, et al. Fenotipo e historia natural de la enfermedad inflamatoria intestinal en un centro de referencia en Medellín-Colombia. Rev Col Gastroenterol 2010; 25(3): 240-251. [ Links ]
10. Di Palma JA, Farraye FA. Crohns disease: the first visit. Gastroenterology and Hepatology 2011; 7(3): 163-169. [ Links ]
11. Vatn M H. Natural history and complications of IBD. Curr Gastroenterol Rep 2009; 11(6): 481-7. [ Links ]
12. Lichtenstein GR, Hanauer SB, Sandborn WJ, et al. Practice parameters committee of the American college of gastroenterology . Management of crohn´s disease in adults. Am J Gastroenterol 2009; 104: 465-483. [ Links ]
13. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a working party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol 2005; 19(Suppl. A): 5-36. [ Links ]
14. Sandborn WJ, Feagan BG, Hanauer SB, Lochs H, Lofberg R, Modigliani R, Present DH, Rutgeerts P, Scholmerich J, Stange EF, Sutherland LR. Review of Activity Indices and Efficacy Endpoints for Clinical Trials of Medical Therapy in Adults with Crohn´s Disease. Gastroenterology 2002; 122: 512-530. [ Links ]
15. Vermeire S. Serologic markers in the diagnosis and management of IBD. Gastroenterol Hepatol. 2007; 3:424-426. [ Links ]
16. Schoepner AM, Beglinger C, Starumann A, et al. Fecal calprotectine correlates more closely with the Simple Endoscopic Score for Crohn´s disease (SES-CD) than CRP, blood leukocytes and the CDAI. Am J Gastroenterol 2010; 105: 162-169. [ Links ]
17. Advisory Committe on Inmunization Practices. Recommended adult inmunization Schedule 2010. Ann Intern Med 2010; 152: 36-39. [ Links ]
18. Bourreille A, et al. Role of small -bowel endoscopy in IBD: international OMED -ECCO consensus. Endoscopy 2009; 41: 618 -637. [ Links ]
19. Nikolaus S, Schreiber S. Diagnostics of inflammatory Bowel Disease. Gastroenterology 2007; 133: 1670. [ Links ]
20. Jenkins D, Balsitis M, Gallivan S, et al. Guidelines for the initial biopsy diagnosis of suspected chronic idiopathic inflammatory bowel disease. The British Society of Gastroenterology initiative. J Clin Pathol 1997; 50: 93-105. [ Links ]
21. Horsthuis K, Bipat S, Bennink R, Stoker J. Inflammatory Bowel Disease with US, MR, SCintigraphy, and CT: Metaanalysis of Prospectives Studies. Radiology 2008; 24 (1): 64-79 [ Links ]
22. Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohns disease. Inflamm Bowel Dis 2011; 17(5): 1073-80. [ Links ]
23. Jowet SL, Seal CJ, Barton JR, et al. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol 2001; 96: 2921-2928. [ Links ]
24. Wasan SK, Baker SE, Skolnik PR, et al. A practical guide to vaccinating the inflammatory bowel disease patient. Am J Gastroenterol 2010; 105: 1231-1238. [ Links ]
25. Advisory Commmitte on Inmunization Practices, Recommended adult immunization schedule: United States 2010. Ann Inter Med 2010; 152: 36-39. [ Links ]
26. Laass MW, Roggenbuck D, Conrad K. Diagnosis and classification of Crohns disease, Autoimmun Rev (2014), online. [ Links ]
27. Bossuyt X. Serologic markers in inflammatory bowel disease. Clin Chem 2006; 52: 171-81. [ Links ]
28. Smith LA, Gaya DR. Utility of fecal calprotectine analysis in adult inflammatory bowel disease. World J Gastroenterol 2012; 18 (46): 6782-6789. [ Links ]
29. Cabriadaa J, Vera I, Domènechc E, et al. Recomendaciones del Grupo Español de Trabajo en Enfermedad de Crohn y Colitis Ulcerosa (GETECCU) sobre el uso de fármacos antifactor de necrosis tumoral en la enfermedad inflamatoria intestinal (2013). Gastroenterol Hepatol 2013; 36(3): 127-146. [ Links ]
30. Irvine EJ, Zhou Q, Thompson AK. The Short Inflammatory Bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT Investigators. Canadian Crohns Relapse Prevention Trial. Am J Gastroenterol 1996; 91(8): 1571-8. [ Links ]
31. Rahier JF, Ben-Horin S, Chowers Y, et al. European evidence-based Consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. Journal of Crohn and Colitis 2009. [ Links ]
32. Harvey RF, Bradshaw JM. A simple index of Crohn disease activity. Lancet 1980; 315(8167): 514. [ Links ]











 text in
text in