Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.4 Bogotá Oct./Dec. 2014
Clinical Issues, Serological issues and Treatment of Chronic Hepatitis C at Two Medical Centers in Bogotá, Colombia
Jhon E Prieto Ortiz MD. (1), Santiago Sánchez Pardo MD. (2), Leonardo Rojas Díaz MD. (3), Sandra Huertas Pacheco MD. (3)
(1) Gastroenterologist and Hepatologist at the Clínica Universitaria Colombia in Bogotá, Colombia.
(2) Medical Intern at the Fundación Universitaria Sanitas in Bogotá, Colombia.
(3) Pathologist and Epidemiologist at the Clínica Universitaria Colombia in Bogotá, Colombia.
Received: 11-12-13 Accepted: 05-11-14
Abstract
Introduction: Hepatitis C affects about 170 million people worldwide. The World Health Organization (WHO) has estimated global prevalence at 2%. Overall, about 40% of patients respond to dual therapy treatment for genotype. In Colombia data available for confirm a similar pattern and for describing the clinical characteristics of patients with this infection are scarce.
Methods: Medical records of patients in the Hepatology outpatient service at the Clínica Universitaria Colombia who had been diagnosed with chronic hepatitis C by one of the authors between January 1, 2010 and May 30, 2013 were retrospectively reviewed for clinical characteristics, serological characteristics and treatment responses.
Results: The medical records of 163 patients were evaluated: 62% were female, 38% were male, and their mean age was 58.2 years. The main risk factor for acquiring hepatitis C was a history of transfusions before 1992. This factor was present in 62% of the patients. The decision to start treatment was made for 77 patients (47.2%), but 86 patients (52.8%) did not start treatment. Reasons included advanced age and advanced cirrhosis which together accounted for more than 50% of these patients. Other reasons for not starting treatment were minimal disease (4.7%), minimal sign of disease plus advanced age (10.5%), spontaneous healing (14%), low probability of response (3.3%) and others (14%). Of the 62 patients for whom information about previous or recent treatments was available, 30.6% had sustained virological responses (SVR), 29.0% were classified as relapsers, 8.1% as partial responders, 19.4% had no response, and 12.9% discontinued treatment because of intolerance.
Conclusions: The most frequent antecedent of HCV in the group of patients studied a history of transfusions associated with gynecological surgery before 1992. About half of the patients were diagnosed late. Hepatitis was more likely to have been treated in these patients than in patients in other studies, but the SVR rate was similar to those found in other series. This study opens doors to the realization of other studies to more broadly define the prevalence, risk factors and treatment response variables of this entity in our country.
Keywords
Gastroenterology, hepatitis C virus, hepatitis C infection, risk factors, treatment.
THEORETICAL FRAMEWORK
Epidemiology
The hepatitis C virus (HCV) and the hepatitis B virus (HBV) are responsible for most of the chronic hepatitis in the world: a third of the world's population has been exposed to these viruses (1, 2). The number of HCV carriers is estimated at 130 to 170 million people. HCV and HBV are the leading causes of cirrhosis and liver transplantation in developed countries, and they are responsible for 1.2 million deaths a year due to complications of portal hypertension resulting from cirrhosis including bleeding from esophageal varices, ascites, encephalopathy, and hepatocellular carcinoma (3-6).
The World Health Organization (WHO) estimates the prevalence of hepatitis C prevalence at approximately 2% of the world's population. Most cases are in in Asia (92 million) and Africa (28 million). The WHO's estimate for Europe is about 9 million and its estimate for the Americas is about 12 million (2).
Data from the Centers for Disease Control (CDC) in the United States show a fall in the incidence of acute hepatitis C from 230,000 per year in the 1980's to 9,000 cases per year in recent records. The current incidence in the USA is 0.3 per 100,000 people and the current prevalence is between 1.0% and 1.9% (6-8). Between 1999 and 2002, prevalence in the USA was 1.6% which means that about 4.1 million people had antibodies against the virus (anti-HCV) and 80% of them were viremic (8).
Although Latin American data vary by country, the overall prevalence is between 1% and 2%. In the second quarter of 2011, there were 23 cases of hepatitis C reported in Colombia which is an incidence of 0.5 per 100,000 inhabitants and a prevalence 0.97% (9, 10).
Hepatitis C
The hepatitis C virus is an RNA virus that is the only member of the genus Hepacivirus in the Flaviviridae family. The disease infects only humans and chimpanzees. Each virus has a diameter of about 60 nm. They bind to the surfaces of hepatocytes and enter those cells through endocytosis. The viral RNA contains approximately 9,600 nucleotides and encodes a polyprotein precursor of about 3,000 amino acids. Cytosolic recognition of viral products induces the production of proinflammatory cytokines such as interferon leading to recruitment of signaling complexes to activate transcription factors. Subsequent expression of interferon-β and interferon regulatory factor 3 (IRF-3) induces the innate immune response and maturation of the adaptive immune system to control the infection (11, 12). Six genotypes and more than 100 subtypes have been described, but approximately 60% to 80% of all infections are caused by genotype 1 (subtypes 1a and 1b) and genotype two. Other genotypes are common in areas such as Egypt (genotype 4), South Africa (genotype 5) and South Asia (genotype 6) (10, 13). Genotyping is not only useful for epidemiological studies but for clinical management as well since it can predict the likelihood of response to treatment and the optimal duration of treatment (24, 25). In Colombia, the most common genotype is genotype 1 according to several studies. A study by Arias et al. published in 2010 found genotype 1 in 95% of the 284 patients studied (14-16).
Risk Factors
The most important risk factors for acquiring the infection that are described in the international literature are intravenous drug use, blood transfusions in general before 1992, transfusions of blood products for hemophiliacs before 1987, and hemodialysis. Maternal-fetal transmission is very rare and is associated with co-infection with HIV-1 (8). Other factors include low socioeconomic status, a large number of sexual partners (over 20), tattoos, dental procedures, endoscopic procedures, and accidents among health professionals (8-10, 41).
Natural History of Infection
For most of its progression to cirrhosis, HCV presents no symptoms or manifestations which occur only after the liver's condition is really insufficient (17, 18). Acute (HCV) infections represent approximately 15% of all cases. Of these only 25% to 30% of patients are symptomatic. The manifestations are the same as those of any viral hepatitis, except for fulminant hepatitis (10, 19). Chronic hepatitis C develops in up to 85% of the patients who acquire the infection. Five to twenty-five percent of them develop hepatocellular carcinoma after having been carriers for over 20 years. Only 15% to 35% of patients heal spontaneously within six months after the primary infection (3, 6, 7, 41). Progress of the disease to fibrosis or cirrhosis is related to factors such as age at infection (before or after 40 years of age), duration of infection (over 20 years), male gender, alcohol consumption greater than 50g/day, coinfection with other viruses such as Hepatitis B or HIV, the source of infection, the immune competence of the host, virus-specific factors such as genotype, and viral load (20, 21).
Treatment
Classic studies such as those by Manns and Fried demonstrated the usefulness of treatment of patients with chronic hepatitis C to achieve a sustained viral response (SVR). This is defined as achievement of undetectable levels of viral RNA in the blood in the six months after completion of treatment. Treatment lasts for 48 weeks for genotype 1 and for 24 weeks for genotype 2. The average responses are 40% and 80%, respectively. SVR also depends on the genotype, the viral load, the degree of fibrosis, the characteristics of the population and adherence to treatment (22-26). Until 2011, treatment for chronic infections was a combination of pegylated interferon and ribavirin for both genotypes (47). Now, treatment for genotype 1 uses new protease inhibitors such as Boceprevir and Telaprevir in conjunction with traditional therapy. Response-guided therapy has managed to shorten the duration of treatment and increase SVR rates to close to 75% (27-33, 41, 47). Relapsers are patients with negative viral loads at the end of treatment who become positive within the first 6 months of follow-up. They achieve SVRs of up to 80% during their second treatment period and are the group which largest displays the best response among partial responders (Patients with decreases of more than log 2 at twelve weeks, but whose viral load is positive at the 24th week). About 50% of patients achieve SVR while approximately 30% are null responders (less than 2 log drop at week 12) (28, 29, 40, 41). Other promising therapeutic agents include viral protein inhibitors such as HCV core protein NS4B of viral entry, host targets such as cyclophilin A, the miR122 protein and two new drugs, sofosbuvir and simeprevir which have been approved by FDA in the USA for treatment of chronic hepatitis C (34, 35).
Liver Biopsy
A liver biopsy is necessary for people infected with genotype 1 since the degree of fibrosis predicts response, defines the patient's prognosis, and defines treatment for patients without cirrhosis (36-38). A biopsy may be unnecessary for people infected with HCV genotypes 2 and 3 since over 80% of them achieve SVR (36, 38-44).
Justification
To date, there have been no published studies from Colombia that provide clinical and treatment information about patients with chronic hepatitis C. For this reason, we have developed this estimation of the frequency of established risk factors and clinical characteristics and description of the treatment of a group of these patients.
Objective
This study describes the clinical and serological characteristics as well as treatment responses of 163 adult patients with chronic hepatitis C from the hepatology service at the Clínica Universitaria Colombia and an outpatient department of hepatology in Bogota during the study period.
Methodology
This is a retrospective review of medical records of patients with chronic hepatitis C who were diagnosed during consultation or who had already been diagnosed within this hospital and who were followed-up in the Hepatology outpatient service of the Clínica Universitaria Colombia. Follow-ups were conducted by the authors during the period from January 1, 2010 to May 30, 2013. Data were tabulated and descriptive statistics were calculated with Excel and SPSS) and expressed as text, tables and graphs.
Design, Patient Population and Definition of Variables
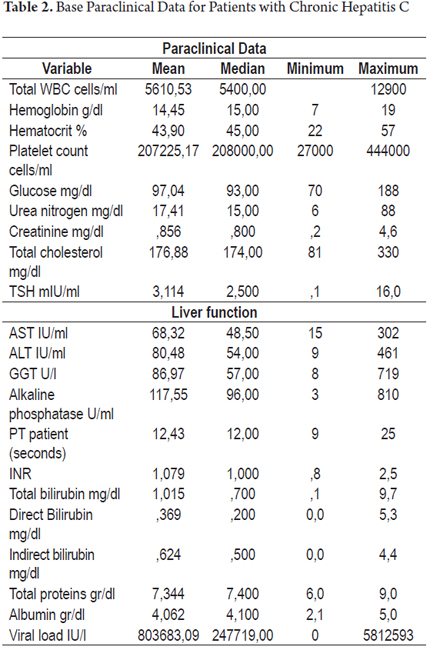
This is a retrospective study (clinical) based on the medical records of adult patients diagnosed with chronic hepatitis C who were seen in the outpatient clinic of the Clínica Universitaria Colombia. Only patients who had positive viral loads or who had been treated for hepatitis C were included. Other information that was collected included demographic characteristics (gender and age), reason for referral of patients for evaluation by the hepatology clinic, risk factors for infection with hepatitis C, and data from physical examinations and laboratory tests (including genotype and viral subtype when available). Additional aspects of treatment were reviewed and patients were classified according to the characteristics that made them candidates for treatment (or for not treatment), whether they had previously received treatment, their responses to previous treatments, and any management of adverse effects (if available). For patients who had had liver biopsies staging of the disease was also recorded using the Metavir classification (Tables 1 and 2).
Statistical Analysis
Each of the variables studied was descriptively analyzed. Means and standard deviations were calculated for continuous variables, and proportions were calculated for categorical variables. The information was analyzed using descriptive statistics (frequency measurements) and presented in text, tables and/or graphs using Excel and SPSS.
Ethical Considerations
This is a retrospective study which uses data from the medical records of patients. For this reason therefore it is considered to be "safe" according to the classification established by Resolution 8430 of 1993 from the Colombian Ministry of Health. This takes into account that no changes were made in data about any intervention or biological, physiological, psychological or social variables of individual participants. The study was conducted in accordance with the principles stated in the Eighteenth World Medical Assembly Declaration (Helsinki, 1964). Identification data and diagnoses of patients were not recorded in publications, and the researchers handled all data from medical records with complete confidentiality.
RESULTS
Patients' General Characteristics
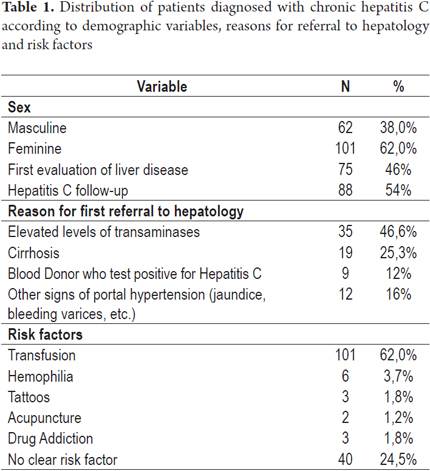
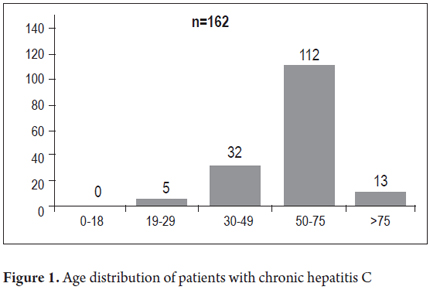
Of the 163 patients included in the study 101 patients (62%) were women and 62 (38%) were men. The average patient age was 58.2 years (21 to 94 years) (Figure 1). Seventy-five (46%) were new patients and 88 (54%) had already been diagnosed with Hepatitis C and were being treated or monitored. The main reasons for referrals of new patients to the hepatology clinic were elevated transaminases (35 patients, 46.7%), cirrhosis (19 patients, 25.3%), blood donors who tested positive for antibodies to hepatitis C (9 patients, 12%), and other signs of portal hypertension (12 patients, 16%). The most common risk factor for acquiring hepatitis C in our series was a history of blood transfusions before 1992 which was demonstrated in 101 patients (62%). The reasons for these transfusions were gynecological surgery (45 patients, 44.6%), trauma related surgery (12 patients, 11.9%), orthopedic surgery (9 patients, 8.9%), gastrointestinal ulcer surgery (11 patients, 10.9%), and other types of surgery including cardiovascular surgery and tonsillectomies (24 patients, 23.8%). Other risk factors found were hemophilia (3.7%), tattoos (1.8%), acupuncture (1.2%) and drug addiction (1.8%).
Physical Examination and Staging
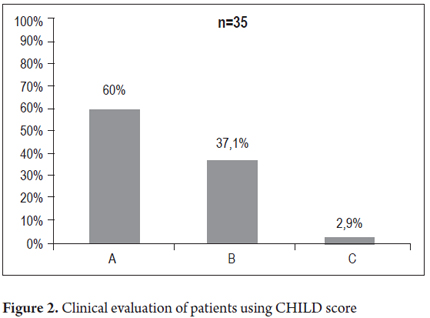
Sixty-one of the patients (37.4%) had clinical signs of chronic liver disease and 102 patients (62.6%) had normal physical examinations. Of the 61 patients with abnormal physical examinations, jaundice was found in 6 patients (3.7%), ascites in 27 patients (16.6%), increased liver consistency suggestive of cirrhosis in 56 patients (34.4%), palpable spleen in 26 patients (16%) and telangiectasias in 27 patients (16.6%). The diagnosis of cirrhosis was established in 52 patients (31.9%, n = 163). Thirty-five of them were classified on the CHILD scale: 21 patients were classified as Child A (60%), 13 patients were classified as Child B (37.1%) and one patient was classified as Child 1 (2.9%) as shown in Figure 2. Liver biopsies were taken from 71 patients (43.5%). Ten of them (14.1%) were classified as stage F4 cirrhosis on the Metavir scale. Other distributions are shown in Figure 3.
Hepatitis C Virus Genotypes
The viral genotypes of hepatitis C were analyzed for 56 patients: forty-four patients had genotype 1B (78.5%) and eight patients had genotype 1A (14.2%). Genotypes 2B, 1A-1B, IV and 1 (without subtyping) accounted for 1.7% of the patients each. The average viral load was 803,683 IU/ml with a median of 247,719 IU/ml.
Treatment
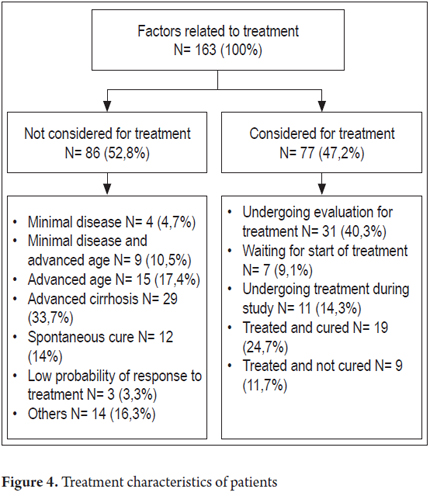
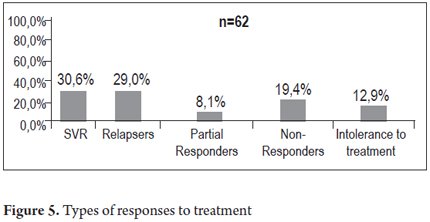
The first analysis of 84 patients who needed treatment or had already been treated at least once divided the patients according to the number of treatments they had received as follows: 23 patients were "naive" (27.3%) patients who had not received any treatment; 43 patients (51.2%) had had one prior treatment, 13 patients (15.5%) had had 2 treatments, 4 patients (4.8%) had had three treatments, and one patient (1.2%) had had four or more treatments. Sixty of the patients who had received treatment in the past or recently had treatment response data. Of these, 19 patients (30.6%) achieved sustained viral response (SVR), 18 patients (29.0%) relapsed after treatment, 5 patients (8.1%) were partial responders, 12 patients (19.4%) had had no response and 8 patients (12.9%) had discontinued treatment because of intolerance. A second analysis of all 77 patients who were treated during the study either because they were naive patients, relapsers, or partial responders is shown in Figure 4. Fourteen of these patients received triple therapy including boceprevir and/or telaprevir. Of the 163 patients in the study, 86 patients (52.8%) were not considered to be suitable for HCV treatment for the following reasons: minimal disease in four patients (4.7%), minimal disease combined with advanced age in nine patients (10.5%), advanced age in 15 patients (17.4%), advanced cirrhosis in 29 patients (33.7%), 12 patients (14%) whose disease was cured spontaneously, three patients (3.3%) who were unlikely to respond, and 14 other patients (16.3%). Treatment was considered to be appropriate for 77 patients. Of these, thirty-one patients (40.3%) were undergoing evaluation, seven patients (9.1%) were waiting to start treatment, eleven patients (14.3%) were undergoing treatment, nineteen (24.7%) had finished treatment and were cured, and nine (11.7%) had been treated but were not cured (Figures 4 and 5).
Clinical Staging of Hepatitis C
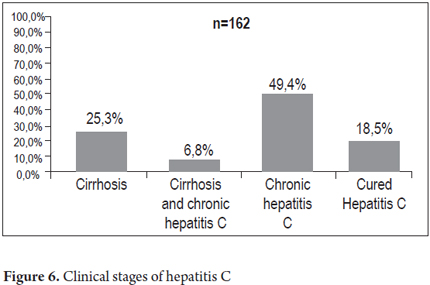
The clinical, laboratory, pathology and treatment response information studies of 162 patients were analyzed to establish the clinical stage of disease. The analysis resulted in the following distribution: 41 patients (25.3%) had cirrhosis, 11 patients (6.8%) had hepatocellular carcinoma and cirrhosis, 80 patients (49.4%) had chronic hepatitis C, and 30 patients (18.5%) were cured either spontaneously or as the result of treatment. Concomitant fatty liver disease diagnosed by biopsy and ultrasound occurred in 54 patients (34.8%), 39 patients (72.2%) had NAFLD (Non-Alcoholic Fatty Liver Disease) and 15 patients (27.8%) had NASH (Non-Alcoholic Steatohepatitis) (Figure 6).
DISCUSSION
The highest percentage of patients (62%) were women, and the average age of all patients was 58.2 years. The reason for this is that the most frequently identified risk factor for acquisition of hepatitis C in our study was a history of transfusion before 1992 (101 patients, 62%). Most of these patients were women who had received transfusions due to pregnancy-related surgery 20 or 30 years ago. The bimodal distribution associated with intravenous drug use among people aged 30-49 and described in other series years was not seen in our study as only 2% of the patients in our series suffered from drug addiction (41). For 40 patients (24.5%), the form of acquisition could not be determined. This percentage is around 40% in studies like that of McCarthy from other countries (46). We believe that these data are due to higher rates of post-transfusion transmission in our country. Five of the patients (3.1%) were health care workers which is worrisome and raises the issue of strengthening nosocomial biosecurity measures (8-10). As in the previous study of our environment (14-16), our study found that genotype 1 is the predominant genotype of hepatitis C virus here: it was found in 96.4% of the patients who had an average viral load of 803,683 IU/ml. The viral load is important since it is known that fibrosis increases with higher viral loads (values greater than 800,000 IU/ml) as demonstrated in the study of Hadziyanis (36). Also, genotype 1 implies greater difficulty in treatment with lower resulting rates of SVR (15, 47, 49).
Liver biopsy data were obtained for 71 patients the majority of whom were among the group of patients considered for treatment according to the recommendations of international standards (38, 42, 43, 44, 49).
Treatment was considered appropriate for 47% of the patients in our series and was begun for 46 patients (28.2%). SVR was achieved in 19 patients (67% of those treated, 24% of those considered for treatment, 11% of all patients). The classic studies of treated patients by Mans, Fried and McHutchinson show SVR rates of 42%, 46% and 40% respectively (22, 23, 53). In a study by Butt of 134,934 veterans infected with HCV, only 11% of the patients began treatment, and of these only 22% completed 48 weeks (50). Although the data found in our study are not comparable with these international studies, they give us an initial indication of how HCV patients here respond to treatment and provide us with an incentive to expand the number of patients in the series.
In the group of patients (53%) for whom no treatment was considered, it is noteworthy that the fifteen elderly patients (17.4%) together with the 29 patients with advanced cirrhosis (33.7%) accounted for more than 50% of the total group. These patients would have benefited from earlier detection of their HCV infections when it might have been possible to treat them. In our series, the group of patients who were cured spontaneously accounted for 14% of the patients whereas the international literature reports rates of spontaneous cures of 25% to 35% (53-56). The only association that can be established is the presence of genotype one in over 90% of our patients.
It should be emphasized that 80 patients (49%), almost half of all the patients, were diagnosed with chronic hepatitis C which is a condition which by definition should be considered for treatment (26-28). Liver cirrhosis was diagnosed in 52 patients (31.9%) (Including 11 with cirrhosis and HCC). These patients may, at some point in the natural history of the disease, become candidates for liver transplantation. We know that today in the USA hepatitis C is the main indication for transplantation. In our country this could become a problem of gigantic proportions considering that the likely prevalence of the disease in our midst is around 1%, then we face approximately 500,000 cases of hepatitis C which have not yet been diagnosed (10).
This study describes clinical and serological characteristics and responses to treatment of patients diagnosed with chronic hepatitis C. They were primarily treated with dual therapy, but some patients were with incomplete data were also treated with triple therapy. The need to extend this series in order to consolidate data about risk factors and treatment responses in our country is clear. Consequently, we issue an invitation for proposals for population studies of prevalence so that we can understand the true importance of HCV in Colombia.
REFERENCES
1. Armstrong GL, Wasley A, Simard EP, McQuillan GM, Kuhnert WL, Alter MJ. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med 2006; 144: 705-14. [ Links ]
2. Averhoff FM, Glass N, Holtzman D, Global burden of hepatitis C: considerations for healthcare providers in the United States, Clin Infect Di 2012; 55(Suppl. 1): S10-5. [ Links ]
3. Louie K, St Laurent S, Forssen U, Mundy L, Pimenta J. The high comorbidity burden of the hepatitis C virus infected population in the United States. BMC Infectious Diseases 2012; 1286. [ Links ]
4. Burguete-García A, Conde-González C, Jiménez-Méndez R, Juárez-Díaz Y, Meda-Monzón E, Madrid-Marina V, et al. Hepatitis C seroprevalence and correlation between viral load and viral genotype among primary care clients in Mexico. Salud Pública de México 2011; 53S7-S12. [ Links ]
5. Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W; Practice Guidelines Committee of the American Association for the Study of Liver Diseases; Practice Parameters Committee of the American College of Gastroenterology. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology 2007; 46: 922-38. [ Links ]
6. Lavanchi D. Evolving Epidemiology of Hepatitis C Virus. Clin Microbiol Infect 2011; 17: 107-115. [ Links ]
7. Aman W, Mousa S, Shiha G, Mousa S. Current status and future directions in the management of chronic hepatitis C. Virology Journal 2012; 957. [ Links ]
8. Louie KS, Laurent S, Forssen UM, Mundy LM, Pimenta JM. The high comorbidity burden of the hepatitis C virus infected population in the United States, BMC Infectious Diseases 2012; 12: 86. [ Links ]
9. Sistema Nacional de Vigilancia en Salud Pública - SIVIGILA. Informe comportamiento de la hepatitis b y c II trimestre 2011 SIVIGILA II trimestre 2011. [ Links ]
10. Guías de diagnóstico y tratamiento de hepatitis C. Rev Col Gastroenterol 2012; 27 (Supl. 4). [ Links ]
11. Horner SM, Gale M Jr. Intracellular innate immune cascades and interferon defenses that control hepatitis C virus. J Interferon Cytokine Res 2009; 29: 489-98. [ Links ]
12. Thio CL, Thomas DL. Interleukin-28b: a key piece of the hepatitis C virus recovery puzzle. Gastroenterology 2010; 138: 1240-3. [ Links ]
13. Beltrán M, Navas MC, De la Hoz F, Mercedes Muñoz M, Jaramillo S, et al. Hepatitis C virus seroprevalence in multi-transfused patients in Colombia. J Clin Virol 2005; 34(Suppl. 2): S33-38. [ Links ]
14. Arias Y R, Echeverry S J, Castro M A, Rios M F, Martinez O, frecuencia de genotipos y subtipos de virus de la hepatitis c en pacientes colombianos con infección crónica. Rev Medica Sanitas 2010; 13 (3): 10-19. [ Links ]
15. Farfan YA, Garzón MA, Rey MH. Prevalencia de hepatitis C por reacción de cadena de la polimerasa (PCR), en donantes de Banco de sangre. Revista Colombiana de Gastroenterología 2007; 22(4): 308-312. [ Links ]
16. Botero R, Idrovo V, et al. Genotipos de VHC. Revista Colombiana de Gastroenterología 1998; XII: 25-27. [ Links ]
17. Leonard B. Seeff, Natural History of Chronic Hepatitis C. Hepatology 2002; 36(5). [ Links ]
18. Oh R, Hustead T. Causes and evaluation of mildly elevated liver transaminase levels. American Family Physician 2011; 84(9): 1003-1008. [ Links ]
19. Seeff LB. Why is there such difficulty in defining the natural history of hepatitis? Transfusion 2000; 40: 1161-1164. [ Links ]
20. Paul H. Hayashi, Adrian M. Di Bisceglie, The progression of hepatitis B– and C–infections to chronic liver disease and hepatocellular carcinoma: epidemiology and pathogenesis, Med Clin N Am 2005; 89 371-389. [ Links ]
21. Hugo R. Rosen, Chronic Hepatitis C Infection, N Engl J Med 2011; 364: 2429-38. [ Links ]
22. Manns MP, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001; 358: 958-965. [ Links ]
23. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002; 347: 975-82. [ Links ]
24. Simmonds P, Bukh J, Combet C, Deleage G, Enomoto N, Feinstone S, et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 2005; 42: 962-973. [ Links ]
25. Vanegas N, Román J, Febles M, Sánchez C, Suárez J, López M, et al. Tratamiento de la infección crónica por el virus de la hepatitis C. Factores predictores de respuesta. Revista Española De Quimioterapia 2011; 24(4): 198-203. [ Links ]
26. Poordad F, McCone J Jr, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al; SPRINT-2 Investigators. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011; 364: 1195-206. [ Links ]
27. Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al; ADVANCE Study Team. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011; 364: 2405-16. [ Links ]
28. Zeuzem Stefan, Andreone Pietro, Pol Stanislas, Lawitz Eric, Diago Moises, Roberts Stuart, et al, Telaprevir for Retreatment of HCV Infection, N Engl J Med 2011; 364: 2417-28. [ Links ]
29. Bacon R. Bruce, Gordon C Stuart, Lawitz Eric, Marcellin Patrick, Vierling M John, Zeuzem Stefan, Boceprevir for Previously Treated Chronic HCV Genotype 1 Infection, N Engl J Med 2011; 364: 1207-17. [ Links ]
30. Shan Liu, Lauren E. Cipriano, Mark Holodniy, Douglas K. Owens, Jeremy D. Goldhaber-Fiebert, New Protease Inhibitors for the Treatment of Chronic Hepatitis C, A Cost-Effectiveness Analysis, Annals of Internal Medicine 2012; 156: 4. [ Links ]
31. Bezemer Geert et al. Long-term effects of treatment and response in patients with chronic hepatitis C on quality of life. An international, multicenter, randomized, controlled study, BMC Gastroenterology 2012; 12: 11. [ Links ]
32. Haider S, Ahmad J. Update of old and emerging therapies in chronic hepatitis C. JPMA. The Journal of The Pakistan Medical Association 2011; 61(12): 1226-1230. [ Links ]
33. Cisneros-Garza L. Nuevos avances en el manejo de la hepatitis C. Salud Pública de México 2011; 53S52-S60. [ Links ]
34. T. Jake Liang, Marc G. Ghany, Current and Future Therapies for Hepatitis C Virus Infection, N Engl J Med 2013; 368: 1907-17. [ Links ]
35. Chao D1, Botwin GJ, Morgan TR. Update on Recently Approved Treatments for Hepatitis C. Curr Treat Options Gastroenterol 2014. [Epub ahead of print] [ Links ].
36. Hadziyannis SJ, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med 2004; 140: 346-355. [ Links ]
37. Brunt EM. Grading and staging the histopathological lesions of chronic hepatitis: the Knodell histology activity index and beyond. Hepatology 2000; 31: 241-246. [ Links ]
38. Wong JB, Koff RS. Watchful waiting with periodic liver biopsy versus immediate empirical therapy for histologically mild chronic hepatitis C. A cost-effectiveness analysis. Ann Intern Med 2000; 133: 665-675. [ Links ]
39. Lee J, Payette M, Osiecki J. Viral hepatitis: targeted tests and therapies contribute to improved outcomes. MLO: Medical Laboratory Observer 2012; 44(3): 18-20. [ Links ]
40. Mach T, Cieśla A, Warunek W, Janas-Skulina U, Cibor D, Ciećko-Michalska I, et al. Efficacy of pegylated interferon alfa-2a or alfa-2b in combination with ribavirin in the treatment of chronic hepatitis caused by hepatitis C virus genotype 1b. Polskie Archiwum Medycyny Wewnętrznej 2011; 121(12): 434-439. [ Links ]
41. Ghany, et al. Diagnosis, Management, and Treatment of Hepatitis C: An Update. Hepatology 2009; 49(4). [ Links ]
42. Kleiner DE. The liver biopsy in chronic hepatitis C: a view from the other side of the microscope. Semin Liver Dis 2005; 25: 52-64. [ Links ]
43. Gebo A Kelly, et al. Role of Liver Biopsy in Management of Chronic Hepatitis C: A Systematic Review. Hepatology 2002; 36(5): 1. [ Links ]
44. Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22: 696-699. [ Links ]
45. Abraira García, Luisa, García Sierra, Alberto, Guillán Pavón, Begoña. Otero Antón, Esteban, Suárez López, Francisco. Guía de práctica clínica Hepatitis C. Santiago de Compostela 2009. [ Links ]
46. McCarthy M, Wilkinson ML. Hepatology. BMJ (Clinical Research Ed.) 2000; 318: 1256-9. [ Links ]
47. Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology 2011; 54: 1433-I444. [ Links ]
48. Foster GR, Fried MW, Hadziyannis SJ, Messinger D, Freivogel K,Welland O Prediction of sustained virological response in chronic hepatitis C patients treated with peginterferon alfa -2a and ribavirin. Scan J Gastroenterol 2007; 42(7): 247-255. [ Links ]
49. Valva P, Casciato P, Diaz Carrasco JM, Gadano A, Galdame O, et al. The Role of Serum Biomarkers in Predicting Fibrosis Progression in Pediatric and Adult Hepatitis C Virus Chronic Infection, 2011 PLoS ONE 6(8): e23218. [ Links ]
50. Falck-Ytter Y, Kale H, Mullen KD, et al. Surprisingly small effect of antiviral treatment in patients with hepatitis C. Ann Intern Med 2002; 136: 288. [ Links ]
51. Butt AA, McGinnis KA, Skanderson M, Justice AC. Hepatitis C treatment completion rates in routine clinical care. Liver Int 2010; 30: 240. [ Links ]
52. Kanwal F, Hoang T, Spiegel BM, et al. Predictors of treatment in patients with chronic hepatitis C infection - role of patient versus nonpatient factors. Hepatology 2007; 46: 1741. [ Links ]
53. McHutchison G John, Lawitz J Eric, Shiffman Mitchell, Muir J Andrew, Galler W Greg, McCone Jonathan, Peginterferon Alfa-2b or Alfa-2a with Ribavirin for Treatment of Hepatitis C Infection, N Engl J Med 2009; 361: 580-93. [ Links ]
54. Barrett S, Goh J, Coughlan B, et al. The natural course of hepatitis C virus infection after 22 years in a unique homogenous cohort: spontaneous viral clearance and chronic HCV infection. Gut 2001; 49: 423. [ Links ]
55. Datz C, Cramp M, Haas T, et al. The natural course of hepatitis C virus infection 18 years after an epidemic outbreak of non-A, non-B hepatitis in a plasmapheresis centre. Gut 1999; 44: 563. [ Links ]
56. Seeff LB, Miller RN, Rabkin CS, et al. 45-year follow-up of hepatitis C virus infection in healthy young adults. Ann Intern Med 2000; 132: 10. [ Links ]











 text in
text in