Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.4 Bogotá Oct./Dec. 2014
Case Report of Fibrolamellar Hepatocarcinoma, a Rare Tumor of Young Adults
Luis Guillermo Toro Rendón MD. (1), Vanessa García MD. (2), Juan Camilo Peréz Cadavid MD. (3), Sergio Iván Hoyos Duque MD. (4), Jaime Fernando Chávez Trujillo MD. (4), Juan Ignacio Marín Zuluaga MD. (5), Óscar Mauricio Santos MD. (5), Octavio German Muñoz MD. (5), Juan Carlos Restrepo MD. (6)
(1) Internist and Hepatology Fellow at the Universidad de Antioquia in Medellín, Colombia luguto@une.net.co.
(2) Radiologist at the Hospital Pablo Tobón Uribe and Professor at the Universidad de Antioquia in Medellín, Colombia.
(3) Pathologist at the Hospital Pablo Tobón Uribe and Dinámica IPS in Medellín, Colombia.
(4) Liver and Biliary Tract Surgeon at the Hospital Pablo Tobón Uribe and Professor at the Universidad de Antioquia in Medellín, Colombia.
(5) Hepatology and Liver Transplant Unit at the Hospital Pablo Tobón Uribe in Medellín, Colombia.
(6) Hepatology and Liver Transplant Unit at the Hospital Pablo Tobón Uribe, Professor and Member of the Gastrohepatology Group at the Universidad de Antioquia in Medellín, Colombia.
Received: 09-01-14 Accepted: 05-11-14
Abstract
Fibrolamellar hepatocellular carcinoma (FL-HCC) is a rare variant of hepatocellular carcinoma (HCC) which occurs most often in young adults without regard to sex. It develops in its characteristic way on a previously healthy liver. The classical presentation is a palpable mass in the right upper quadrant, pain, and weight loss.
In the absence of a prior history of liver disease, it is usual to find a large advanced neoplasia which has characteristic findings in a CT scan or MRI. A high percentage of cases can be diagnosed solely with images.
The best treatment for this type of tumor is surgical resection which provides a 5-year survival rate of 58% to 82% but with very high rates of relapse. In published studies rates of relapse vary between 33% and 100%.
We report the case of a young patient with no history of liver disease in which fibrolamellar hepatocellular carcinoma was diagnosed using contrast organ-specific MRI. The tumor was treated with radical surgical resection.
Keywords
Fibrolamellar hepatocellular carcinoma, hepatocellular carcinoma, hepatectomy, hepatic neoplasia.
CASE DESCRIPTION
The patient was a 26 year old man who had developed malaise and fever six months earlier following heavy consumption of liquor. He went to a Level 2 medical clinical where laboratory tests showed transaminases four times above normal levels. Abdominal ultrasound of the liver showed a lesion that measured 9.8cm x 9.3 cm x 6.3 cm that was suggestive of focal nodular hyperplasia (FNH). The patient was referred to the hepatology service where an abdominal MRI with contrast was consistent with the possibility of FNH but did not rule out the possibility of HCC. Faced with this doubtful diagnosis, and considering the potential consequences of a liver biopsy (spread of HCC in the puncture tract, bleeding, and poor performance diagnosing FNH) it was decided to perform another MRI using gadoxetic acid, an organ-specific contrast medium which had become available. The second MRI showed a heterogeneous, encapsulated and well circumscribed lesion with lobed edges which compromised the lateral segment of the liver. A central lesion and septa radiated toward the periphery. It was hypodense in all sequences, and focal areas of necrosis and diffusion appeared to be restricted in all images. In the hepatobiliary phase, endoscopy was limited to capsule only, so no biopsy sample could be taken to aid in the diagnosis of FHCC.
The lesion was staged and the possibility that it had metastasized was discarded. The patient was scheduled for left hepatectomy with curative intent. During surgery, a giant liver tumor was found. It compromised segments II, III, IVA, IVB and partially compromised segments V and VIII. There was no evidence of liver or peritoneal metastasis. An extended hepatectomy with hilar node lymph dissection was performed, but we were unable to preserve the middle hepatic vein in the transection plane.
Histopathology reported FHCC. Its largest dimension was 15 cm, it was graded as 3-4 on the Edmondson-Steiner grading scale¾, but there was no metastasis to the lymph nodes.
INTRODUCTION
FHCC is a rare variant of HCC that was initially described by Edmondson in 1956 (1). In 1980 several reports established it as a separate entity (2). FHCC accounts for less than 1% of all primary tumors of the liver. A retrospective study by Hashem et al. analyzed cancer survey registries from the Surveillance, Epidemiology and End Results (SEER) program of the National Cancer Institute of the United States for the years between 1986 and 2000. They found that FHCC accounted for 0.85% of all primary liver cancers (3). Various other reports place this percentage between 0.6% and 8.6% of all cases of HCC (4). FHCC affects men and women equally (3, 5), but occurs most frequently among young adults under the age of 40 who have had no history of liver disease. The average age of these patients is 25 years old. Population studies have reported that only 6% of patients with FHCC are over 50 years, while over 85% are 35 years old or younger. This is very different from the epidemiology of HCC in which tumor incidence increases with age, peaks at age 70, is four to eight times more common in men than women, and in which 90% of cases are associated with a known risk factor. The most important of these are cirrhosis of any etiology and Hepatitis B infection (6, 7).
The usual presentation of FHCC is a single tumor mass surrounded by a fibrous capsule which may or may not have metastasized to nearby lymph nodes. FHCC is not associated with viral hepatitis or cirrhosis (4).
The etiology of FHCC is unknown, and no histological precursor related to its development has been identified (8). Cieply et al. have reported upregulation of β-catenin phosphorylation of tyrosine residues in these patients. The signaling pathways of β-catenin play a multitude of roles in liver biology including regulation of the proliferation of hepatoblasts and differentiation and control of some of their metabolic processes. It has been shown that β-catenin can be activated via tyrosine phosphorylation. The study by Cieply et al. suggests that aberrant activation of this pathway may cause dysregulation of various genes which play important roles in tumor pathogenesis (9, 10).
CLINICAL PRESENTATION
The classic triad of this entity is a palpable mass in the right upper quadrant, pain and weight loss. Nevertheless, there are usually other nonspecific symptoms such as abdominal fullness, anorexia, nausea and fever. Jaundice occurs in 40% of cases, and there have been rare reports of Budd Chiari syndrome, hemoperitoneum, biliary obstruction, gynecomastia (due to the production of aromatase by neoplastic cells and consequent conversion of androgens to estrogens), bone metastasis, acute liver failure and paraneoplastic syndromes such as hypoglycemia, hyperthyroidism and deep vein thrombosis (5, 11, 12).
A study published by Stevens in 1995 reported that 70% of patients had nodal spread at the moment of diagnosis (13), and that the most frequent site of metastasis was the hepatic hilum (14).
DIAGNOSIS
FHCC can be diagnosed by CAT scan or MRI. In cases in which the images do not provide a clear diagnosis, laparoscopic or fine needle aspiration biopsies can be useful (15).
Differential diagnosis should consider FNH, HCC, hepatic adenoma, hemangioma, adenosquamous carcinoma, bladder carcinoma extending to the liver, and fibrosis associated with metastatic tumors (breast, carcinoid, cholangiocarcinoma and thyroid) (5).
LABORATORY TESTS
Unlike HCC which usually has high levels of alpha-fetoprotein is usually high, only 10% to 15% of the patients who have FHCC have abnormal levels of alpha-fetoprotein, and they usually do not exceed 200 ng/ml. High levels of neurotensin and des-gamma carboxyprothrombin have been reported, as well as high levels of Vitamin B-12 binding capacity which can be used to monitor the progression of patients (15).
IMAGING FEATURES
FHCC can be detected in an X-ray where it will appear as a large liver mass or as a calcification in the right hypochondrium. It can appear to be nodular or starry and small relative to the size of the tumor (16).
An ultrasound image will show a single, well-defined mass which may be lobed. It will present mixed echogenicity in 60% of cases in which isoechoic and hyperechoic components predominate. The central lesion appears as a hyperechoic area in 33% to 60% of cases (17).
Frequently CAT scans without contrast medium show FHCC as hypodense compared to liver tissue. In these images any calcification present at the central lesion will be much more evident than in other types of images.
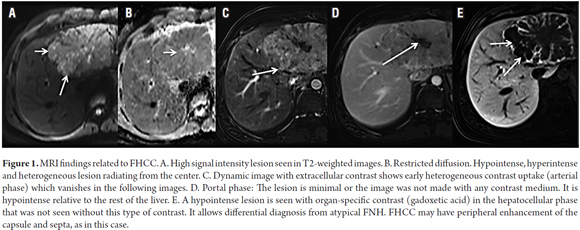
Magnetic resonance imaging will show a large well defined focal lesion which will have low signal intensity on T1 weighted images and high signal intensity T2 weighted images. With contrast, it will present early and heterogeneous image capture which disappears in later images (18). When there is a doubtful diagnosis, MRI can be performed with organ specific contrast which will produce intense enhancement of liver parenchyma in the cellular stage in which non-hepatocyte lesions do not capture the contrast. This can provide better characterization of the lesion. An important feature found in 70% of cases is the presence of a central lesion composed of fibrous tissue (19). This type of lesion may be starry or amorphous with hypointense tumor tissue surrounding images without contrast and those in the arterial phase (see Figure 1). This helps to differentiate the lesion from an FNH. In delayed images the central lesion will have minimal enhancement in 50% of cases (20).
PATHOLOGY
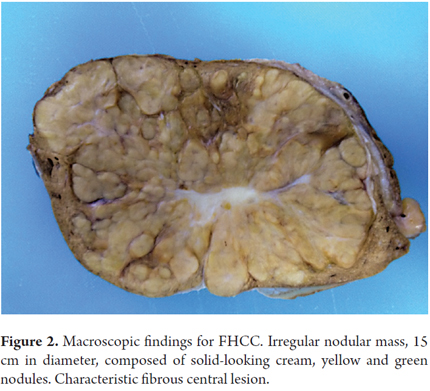
Macroscopically, this type of tumor is a single mass in 56% of cases of which 66% are well defined unencapsulated tumors in the left lobe. The cut has solid consistency. The tumor has irregular edges and the color can be gray, brown or even green when there is a large amount of bile production. The tumor has many fibrous septa that subdivide the hepatic tissue into lobes and which may connect to a central lesion (see Figure 2). This is similar to FNH and may cause confusion. Calcifications can be found in the central lesion. The non-neoplastic parenchyma of the liver is the usual appearance without evidence of fibrosis or cirrhosis, although coexistence of cirrhosis has been reported in fewer than 4% of the cases.
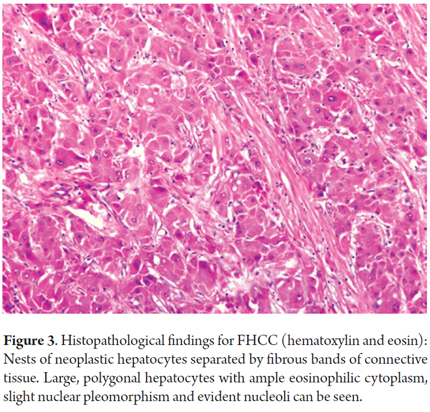
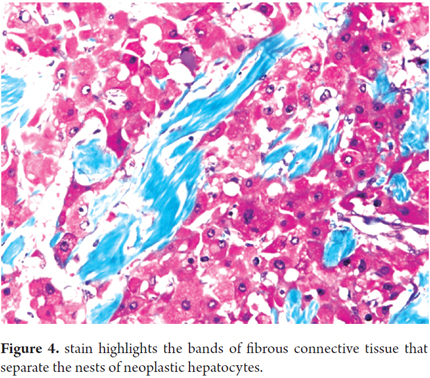
Histology will show large polygonal tumor cells with deeply eosinophilic cytoplasm, macronucleoli and abundant fibrous stroma around the tumor cells which is the reason they have been called fibrolamellar (4, 21). In 75% of cases, these fibrotic bands come together to form a central lesion which is associated with radiating bands which can also be found in the metastatic lesions (see Figures 3 and 4) (6).
The eosinophilic appearance of tumor cells is secondary to mitochondrial proliferation (22), and hyaline bodies and cytoplasmic inclusions called "pale bodies" can be found 50% of the time. The composition of pale bodies is not well defined, but they are immunoreactive for fibrinogen. It is very rare to find microvesicular steatosis, and mitotic figures are less common than in HCC (16).
Small calcifications are very common and are usually are deposited in the central lesion or in fibrotic bands (16).
Vascular invasion is present in 40% of these tumors (12). 50% are partially encapsulated, and only a few are completely encapsulated.
Copper deposits can be found because of cholestasis which is common in these tumors. Structural similarity with neuroendocrine tumors has also been reported (4).
Coexistence of HCC and FHCC in the same patient is rare, but when they are found together they are effectively separated. Stirpa et al. reported that 7% of the cases of FHCC (3/41) that they studied had areas with HCC features (13).
TREATMENT
Characteristically these tumors are only diagnosed when they have reached an advanced stage and have become malignant. Data on treatment and prognosis are scarce due to the low incidence of this tumor. The information that is available comes from case series and small cohorts.
The first series of cases that were published in the 1980s described FHCC as a neoplasia with a slow growth rate and a more favorable prognosis than HCC (2). However, recent studies show that the prognosis for FHCC is actually not as favorable as it was believed (23).
A systematic review of the literature published by Mavros et al. in 2012 found that 93% of patients survived at least one year after a hepatectomy, 80% survived for at least three years, and 70% survived for at least 5 years was 93%, 80% and 70%. The average survival time was 222 months. Among patients who had liver transplants the one year survival rate was 86%, the three year survival rates was 42%, and the five year survival rate was 34%. The average survival time was 32 months. Patients who were not candidates for either procedure had a one year survival rate of 81%, and a three year survival rate of 12%. None survived for five years. The average survival time was 20 months (24).
Based on the available data, the best treatment is surgical resection whenever it is possible. Resection provides a 5-year survival rate of 58% to 82% (25). This group of patients may have less advanced cases of the disease than those who undergo transplantation and those who do not undergo any surgical procedure which could be a factor in the high survival rates.
Recurrences occur frequently after curative resection. In the series studied relapse rates ranged from 36% to 100% with average time before relapse ranging from 10 to 33 months (5, 15, 25, 26).
Factors that have been associated with poor survival are being female, advanced state of the disease, lymph node tumor involvement, macrovascular invasion and unresectable disease (4).
In cases of advanced disease, there are no established chemotherapy schemes because of the lack of specific clinical trials. To date, chemotherapy as neoadjuvant, adjuvant or palliative treatment has not been shown to be useful, so their use is not standard. There are reports of the use of gemcitabine and oxaliplatin (27), cisplatin, vincristine and 5-fluorouracil (7), cisplatin and doxorubicin (7), and 5-fluorouracil and interferon alfa-2b that have varying response rates (28). There is no information on the use of sorafenib in this group of patients since most clinical studies of patients with HCC exclude patients who also have FHCC (29).
As a conclusion, it should be said that, in contrast to patients with HCC, many patients with FHCC benefit from surgical resection. This is influenced by age, absence of cirrhosis and portal hypertension. The most important prognostic factor is the possibility of surgical resection which provides an average 5-year survival rate of 70% even though the high rates of relapse necessitate new strategies and therapies to prevent recurrence of the disease.
REFERENCES
1. Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA journal of diseases of children 1956; 91(2): 168-86. [ Links ]
2. Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer 1980; 46(2): 372-9. [ Links ]
3. El-Serag HB, Davila JA. Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology 2004; 39(3): 798-803. [ Links ]
4. Ang CS, Kelley RK, Choti MA, Cosgrove DP, Chou JF, Klimstra D, et al. Clinicopathologic characteristics and survival outcomes of patients with fibrolamellar carcinoma: data from the fibrolamellar carcinoma consortium. Gastrointestinal cancer research: GCR 2013; 6(1): 3-9. [ Links ]
5. Liu S, Chan KW, Wang B, Qiao L. Fibrolamellar hepatocellular carcinoma. The American journal of gastroenterology 2009; 104(10): 2617-24; quiz 25. [ Links ]
6. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Journal of hepatology 2012; 56(4): 908-43. [ Links ]
7. Katzenstein HM, Krailo MD, Malogolowkin MH, Ortega JA, Qu W, Douglass EC, et al. Fibrolamellar hepatocellular carcinoma in children and adolescents. Cancer 2003; 97(8): 2006-12. [ Links ]
8. Klein WM, Molmenti EP, Colombani PM, Grover DS, Schwarz KB, Boitnott J, et al. Primary liver carcinoma arising in people younger than 30 years. American journal of clinical pathology 2005; 124(4): 512-8. [ Links ]
9. Cieply B, Zeng G, Proverbs-Singh T, Geller DA, Monga SP. Unique phenotype of hepatocellular cancers with exon-3 mutations in beta-catenin gene. Hepatology 2009; 49(3): 821-31. [ Links ]
10. White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology 2012; 142(2): 219-32. [ Links ]
11. Rageshree Ramachandran SK. Fibrolamellar hepatocellular carcinoma. Diagnostic Histopathology 2010; 16(8): 388-96. [ Links ]
12. Asrani SK, LaRusso NF. Fibrolamellar hepatocellular carcinoma presenting with Budd-Chiari syndrome, right atrial thrombus, and pulmonary emboli. Hepatology 2012; 55(3): 977-8. [ Links ]
13. Stevens WR, Johnson CD, Stephens DH, Nagorney DM. Fibrolamellar hepatocellular carcinoma: stage at presentation and results of aggressive surgical management. AJR American journal of roentgenology 1995; 164(5): 1153-8. [ Links ]
14. Chun YS, Zimmitti G. Fibrolamellar variant of hepatocellular carcinoma. Recent results in cancer research Fortschritte der Krebsforschung. Progres dans les recherches sur le cancer 2013; 190: 101-10. [ Links ]
15. Stipa F, Yoon SS, Liau KH, Fong Y, Jarnagin WR, DAngelica M, et al. Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer 2006; 106(6): 1331-8. [ Links ]
16. Friedman AC, Lichtenstein JE, Goodman Z, Fishman EK, Siegelman SS, Dachman AH. Fibrolamellar hepatocellular carcinoma. Radiology 1985; 157(3): 583-7. [ Links ]
17. McLarney JK, Rucker PT, Bender GN, Goodman ZD, Kashitani N, Ros PR. Fibrolamellar carcinoma of the liver: radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc 1999; 19(2): 453-71. [ Links ]
18. Elsayes KM, Narra VR, Yin Y, Mukundan G, Lammle M, Brown JJ. Focal hepatic lesions: diagnostic value of enhancement pattern approach with contrast-enhanced 3D gradient-echo MR imaging. Radiographics: a review publication of the Radiological Society of North America, Inc 2005; 25(5): 1299-320. [ Links ]
19. Ichikawa T, Federle MP, Grazioli L, Madariaga J, Nalesnik M, Marsh W. Fibrolamellar hepatocellular carcinoma: imaging and pathologic findings in 31 recent cases. Radiology 1999; 213(2): 352-61. [ Links ]
20. Elsayes KM, Peterson CM, Menias CO. The central scar: pathophysiology and imaging features. Current problems in diagnostic radiology 2007; 36(6): 247-57. [ Links ]
21. Kakar S, Burgart LJ, Batts KP, Garcia J, Jain D, Ferrell LD. Clinicopathologic features and survival in fibrolamellar carcinoma: comparison with conventional hepatocellular carcinoma with and without cirrhosis. Modern pathology: an official journal of the United States and Canadian Academy of Pathology, Inc 2005; 18(11): 1417-23. [ Links ]
22. Torbenson M. Review of the clinicopathologic features of fibrolamellar carcinoma. Advances in anatomic pathology 2007; 14(3): 217-23. [ Links ]
23. Weeda VB, Murawski M, McCabe AJ, Maibach R, Brugieres L, Roebuck D, et al. Fibrolamellar variant of hepatocellular carcinoma does not have a better survival than conventional hepatocellular carcinoma--results and treatment recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) experience. Eur J Cancer 2013; 49(12): 2698-704. [ Links ]
24. Mavros MN, Mayo SC, Hyder O, Pawlik TM. A systematic review: treatment and prognosis of patients with fibrolamellar hepatocellular carcinoma. Journal of the American College of Surgeons 2012; 215(6): 820-30. [ Links ]
25. Maniaci V, Davidson BR, Rolles K, Dhillon AP, Hackshaw A, Begent RH, et al. Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology 2009; 35(6): 617-21. [ Links ]
26. Ichikawa T, Federle MP, Grazioli L, Marsh W. Fibrolamellar hepatocellular carcinoma: pre- and posttherapy evaluation with CT and MR imaging. Radiology 2000; 217(1): 145-51. [ Links ]
27. Gras P, Truant S, Boige V, Ladrat L, Rougier P, Pruvot FR, et al. Prolonged Complete Response after GEMOX Chemotherapy in a Patient with Advanced Fibrolamellar Hepatocellular Carcinoma. Case reports in oncology 2012; 5(1): 169-72. [ Links ]
28. Patt YZ, Hassan MM, Lozano RD, Brown TD, Vauthey JN, Curley SA, et al. Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon Alfa-2b for treatment of hepatocellular carcinoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2003; 21(3): 421-7. [ Links ]
29. Malouf GG, Brugieres L, Le Deley MC, Faivre S, Fabre M, Paradis V, et al. Pure and mixed fibrolamellar hepatocellular carcinomas differ in natural history and prognosis after complete surgical resection. Cancer 2012; 118(20): 4981-90. [ Links ]











 text in
text in