Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.29 no.4 Bogotá Oct./Dec. 2014
Morphological Issues of Drug Induced Liver Disease
Rocío del Pilar López Panqueva MD. (1)
(1) Medical pathologist at the Hospital Universitario Fundación Santa Fe de Bogotá and Universidad de Los Andes in Bogotá, Colombia.
Received: 13-11-14 Accepted: 24-11-14
Abstract
Drug-induced liver disease is a multifaceted phenomenon which has a varied morphological spectrum that mimics other patterns of liver damage both in cases of acute drug exposure and in cases of chronic exposure to drugs. Those patients who are idiosyncratically susceptible at the therapeutic dose or to intrinsic toxicity may also be affected by other factors including genetic factors, age, sex, nutritional status, exposure to other drugs and the existence of an underlying disease. The only clinical manifestation of the disease may be the adverse effect of a drug, but it can also be accompanied by systemic manifestations and manifestations in other organs, and it can even be fatal (1).
The incidence of drug-induced liver disease is not well defined, but some studies claim that its overall annual incidence varies between 1/100,000 people and 15/100,000 people. In the United States, twenty new cases per 100,000 inhabitants occur every year. More than 900 natural and pharmaceutical drugs, herbal medicines, homeopathic products, dietary supplements and toxins have been reported to cause liver damage. This can occur whether or not they are used at normal therapeutic doses. These cases are responsible for about 15% of consultations and hospitalizations for jaundice, acute hepatitis, and chronic hepatitis in adults above the age of 50, and in up to 40% of all cases of hepatitis. Drug-induced liver disease also accounts for 11% to 50% of all cases of acute liver failure. Published data indicate that antibiotics are responsible for between 27% and 46% of cases, that drugs for diseases of the central nervous system are responsible for between 13% and 17%, anti-inflammatory and analgesic agents are responsible for between 5% and 17%, and herbal products are responsible for 9%. New biomarkers and the use of microRNA are being studied and may become promising alternatives in the near future for identifying patients susceptible to drug-induced hepatotoxicity.
There are so many types of liver damage attributed to these agents that only give some examples can be provided in this article. These examples have been chosen on the basis ofn the patterns of liver damage with emphasis on the importance of proper and thorough clinical correlation (2, 3).
Keywords
Liver biopsy, toxicity, drugs, toxins, patterns, necroinflammatory, cholestasis, steatosis, steatohepatitis, green tea, acetaminophen, steroids, amiodarone, methimazole, nitrofurantoin, chlorpromazine, oral contraceptives, methotrexate.
OVERVIEW
The liver becomes a target that is susceptible to damage because it is the first organ to come into contact with ingested agents, the most important site for concentrations of xenobiotics and the place where most biotransformations occur. Susceptibility to toxicity is modified by numerous factors. It is more common in adults than in children, and women are more likely to develop chronic toxicity. Some medicines cause damage depending on the dosage and exposure time, in other cases damage is due to metabolic idiosyncrasy or drug interactions. Often, discontinuation of medication allows the damage to heal, although it may take several weeks or months. Damage very rarely persists after suspension (4).
The diagnosis of liver damage induced by any drug, toxin, dietary supplement, vitamin, herbal or homeopathic product is often a challenge for both the clinician and the pathologist. There are no specific clinical or laboratory findings for these diagnoses. The Council for International Organizations of Medical Sciences (CIOMS) classifies these lesions as hepatocellular, cholestatic or as mixed pattern. They can mimic any acute, chronic or primary liver disease and can present any of the well-known morphological patterns including necroinflammation, steatosis, steatohepatitis, cholestasis, vascular, tumors and cirrhosis. These patterns reflect pathophysiological mechanisms that induce liver damage including hypersensitivity, autoimmune, disorders, and metabolic disorders and they may suggest the etiology of the lesions (5).
The diagnosis in these cases is made through excluding possibilities. Liver biopsies, good clinical judgment and knowledge about agents which can potentially damage the liver can all contribute to diagnosis. Although liver biopsy is still considered the gold standard for the diagnosis of drug-induced hepatotoxicity, this procedure is not used in routine medical practice although it is usually required as a diagnostic test when the clinical picture is unclear or when laboratory tests disagree with each other. A biopsy may be done to determine the pattern of liver damage, and a biopsy may be important for excluding certain toxic agents, clarifying the mechanisms of damage, or assessing the severity or chronicity of the damage. A biopsy may also be useful when there is no clinical suspicion of a particular diagnosis and differential is difficult (6, 7).
In this article we will study the most frequent forms of presentation according to their morphological patterns and attempt to demonstrate a practical approach to the diagnosis of drug or toxin-induced liver disease. The various tables list just some of the agents usually involved in each of the patterns described.
ACUTE DAMAGE
Acute damage occurs in 90% of cases when there has been less than three months. There are several spectra of liver damage possible in these cases (4-6, 8, 9).
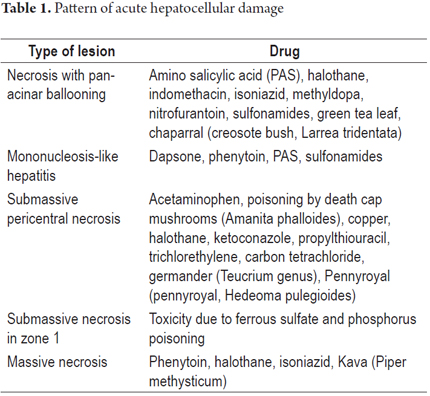
1. Hepatocellular pattern. This pattern is based on cytotoxic or cytolytic damage characterized by damage to hepatocytes that results in hepatocellular necrosis which can be massive or restricted to certain zones. Damage can occur with or without steatosis. Clinical symptoms resemble acute viral hepatitis with hepatocellular jaundice, elevated aminotransferases (8-100 times normal), normal or only slightly elevated alkaline phosphatase. In this group of patients there is tendency to fulminant hepatic failure. The differential diagnosis includes viral diseases, microorganisms, autoimmune hepatitis and Wilson's disease (Table 1).
2. Pattern of acute steatosis resembling NASH. This can be either macrovesicular steatosis or microvesicular steatosis. Patients have mild jaundice and their aminotransferase levels are lower than those seen when there is hepatocellular necrosis. In some cases such as those accompanied by liver failure, there is lactic acidosis with no or minimal disruption of aminotransferases. These cases can have severe systemic involvement and poor prognoses. Damage to the liver is caused by disturbance of the fat metabolism that inhibits mitochondrial function. Depending on the agent, symptoms can begin anywhere between seven and twenty-eight days after initial exposure. We should include alcoholic steatohepatitis, obesity, diabetes mellitus, Wilson's disease and viral hepatitis C in the differential diagnosis. If the vesicles affected by steatosis are small, remember to exclude rare entities such as Reye syndrome and acute fatty liver of pregnancy (Table 2).
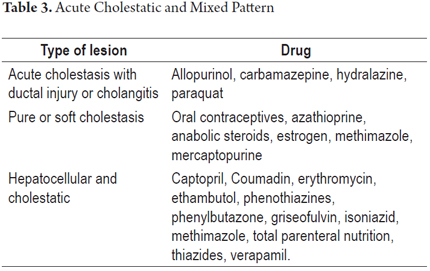
3. Cholestatic pattern. This pattern clinically resembles an obstruction of the bile duct, although septic processes, heart failure, and viral hepatitis must be ruled out before making this diagnosis. It manifests with obstructive jaundice with itching and may be accompanied by abdominal pain. Biochemical parameters that indicate cholestasis include elevated levels of alkaline phosphatase and gamma glutamyl transferase. There may be hyperbilirubinemia, and aminotransferase levels rarely exceed 5 times their normal value.
4. Mixed pattern of cholestatic and hepatocellular damage. This pattern combines clinical and pathological manifestations of the first and third patterns. It is one of the most frequent patterns, and its diagnosis is fairly clear since similar patterns rarely occur in acute liver disease due to other causes. The clinical manifestations mimic acute hepatitis accompanied by itching and fatigue. Levels of serum alkaline phosphatase (ALP), ALT and AST are all elevated. The ALT/FA ratio ranges between two and five defining the mixed pattern (Table 3).
Histopathological findings
Pattern of Acute Hepatocellular Damage (4, 6, 7)
- This pattern consists of hepatocellular compromise with necrosis and ballooning degeneration compromising entire acini with minimal or no cholestasis. It clinically resembles the viral etiology of acute hepatitis with diffuse parenchymal damage and lobular inflammation involving lymphocytes, macrophages and plasma cells. Usually, this pattern is rich in eosinophils whose location varies between acinar location and portal. Minimal portal inflammation and apoptosis are observed in some cases. In some cases, lobular disarray with fragmentation of the reticular pattern, rosette formation of hepatocytes, accompanied by regenerative changes, binucleation, mitosis and thick trabeculae can be seen.
- One variant is associated with sinusoidal lymphocytosis and marked activation of Kupffer cells. This variant resembles hepatitis due to mononucleosis (pseudomononucleosis or mononucleosis-like syndrome) with focal necrosis and activity.
- Another variant is submassive necrosis in the pericentral zone (zone 3) which is accompanied by some degree of inflammation and the collapse of the reticular pattern, rapid reproduction of Kupffer cells which are filled with liposfuscin, and mild cholestasis.
- Another variant is submassive necrosis in zone 1 (periportal) with coagulation necrosis. There is and minimal inflammatory response, or it is accompanied by accumulation of fat in large vacuoles.
- When there is massive necrosis, it creates a pattern of damage like that seen in acute fulminant viral hepatitis
Pattern of Acute Steatosis (10, 11)
- Steatosis of small or micro vacuoles resembling acute fatty liver of pregnancy or Reye's disease with small droplets of fat occupying the cytoplasm of the hepatocytes which have hyperchromatic nuclei.
- Steatosis of macro vacuoles in which a large fat vacuole replaces the cytoplasm and displaces the nucleus to the periphery.
- Steatohepatitis with steatosis, usually a medium sized or large vacuole. Hepatocellular necrosis or ballooning and inflammation, with or without Mallory-Denk bodies.
Acute cholestatic pattern (4, 7, 9, 12)
- Bile deposits are observed in hepatocytes and/or canaliculi together with biliary stasis, portal inflammation, cholangiolar proliferation and/or damage to the bile ducts.
- Pure or soft cholestasis is characterized by marked presence of jaundice, dark urine and itching in patients who otherwise feel well. There are minimal or no alterations of alkaline phosphatase and aminotransferase. When these levels are elevated, they are always below two times normal.
Mixed Necroinflammatory and Cholestatic Pattern (4, 7, 9)
A liver biopsy will show marked necrosis of hepatocytes, apoptotic cells (confluent necrosis), inflammation accompanied by biliary stasis, cholestasis and proliferation of hepatocanalicular cholestasis.
CHRONIC DRUG INDUCED LIVER DISEASE
Progression to chronic damage occurs when the patient has been exposed to the toxic agent for more than 6 months with persistent occurrence of biochemical damage. This has been reported in 11% to 13% of cases. Many of the drugs and toxic agents discussed above as causing acute liver damage can also cause chronic damage. Both clinical and histopathological changes resemble chronic hepatitis, sometimes there are even significant levels of fibrosis and cirrhosis (4-8).
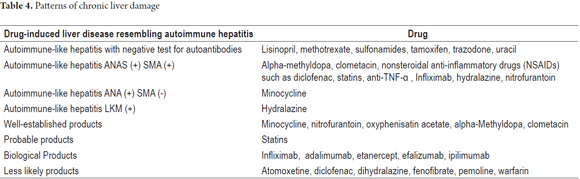
1. The clinical and pathological findings in cases of the chronic necroinflammatory pattern are indistinguishable from those of chronic hepatitis, especially autoimmune hepatitis. A blood test for viral hepatitis must be used to discard this possibility. There are reports of at least twenty-four products related to this pathology. Some of them are listed in Table 4. This pattern occurs more frequently in women. It presents signs of hypersensitivity with rash, arthralgia, and eosinophilia. There may be autoantibodies (ANA, ASMA, hypergammaglobulinemia). Differential diagnosis between chronic hepatitis and drug-induced autoimmune hepatitis is very difficult. The severity varies, withdrawal of the drug should lead to full resolution, and response to corticosteroid treatment supports the diagnosis (Table 4) (13-17).
2. Clinically and histopathologically, chronic cholestatic lesions are very similar to primary biliary cirrhosis, primary sclerosing cholangitis and ductopenia. Most cases of drug-induced cholestasis are followed by rapid improvement of symptoms and complete recovery after removal of the drug. In some cases, biochemical anomalies persist for several month. Anti-mitochondrial antibodies (AMA) are an exception because they are not detectable (Table 5).
Histopathological findings
- The chronic necroinflammatory pattern is seen in drug-induced autoimmune hepatitis with or without autoimmune markers. This pattern resembles classical autoimmune hepatitis with marked portal and lobular inflammation of predominantly plasma cells with some lymphocytes and eosinophils. There is interface hepatitis and hepatocellular necrosis. In drug induced autoimmune hepatitis without autoimmune markers, the histological features are indistinguishable from chronic viral or autoimmune hepatitis including progression to fibrosis and cirrhosis (13-17).
- Pattern resembling primary biliary cirrhosis. Morphological findings are characterized by evidence of chronic cholestasis, pseudoxanthomatous changes of hepatocytes, accumulation of copper and its binding protein in periportal hepatocytes, mild to moderate portal swelling in patch with variable ductopenia. The term "vanishing ducts" is used to describe cases with marked ductopenia.
- Pattern resembling primary sclerosing cholangitis with ductal sclerosis of the extrahepatic bile duct or intrahepatic ducts.
Other less common but equally important patterns to note include the following:
VASCULAR DAMAGE
Vascular damage can be subdivided into several morphological types (4, 18-19):
- Sinusoidal dilation is associated with prolonged use of oral contraceptives. It occurs most frequently in Zone 1. It can also occur as a result of azathioprine and heroin.
- Peliosis hepatis is characterized by large spaces filled with red blood cells without endothelial lining. It has been observed as the result of anabolic steroids, azathioprine, oral contraceptives, danazol, hypervitaminosis A and tamoxifen.
- Phlebosclerosis is related to alcohol and heroin
- Ischemic hepatitis is related to amiodarone administered intravenously
- Veno-occlusive disease is related to ingestion of teas containing the pyrrolizidine alkaloids. It can also be related to alcohol, excessive vitamin A, azathioprine and dacarbazine. It can also occur in patients on immunosuppressive therapy with cyclophosphamide and subsequent to colon carcinoma therapies with oxipltinum and can be a complication of chemo therapy and radiation therapy following bone marrow transplantation.
- Budd-Chiari syndrome has been reported after use of oral contraceptives and in children receiving total parenteral nutrition
- Damage to hepatic arteries and arterioles results from hypersensitivity to some drugs which produces angiitis leading to thickening of the arterial walls, endothelial swelling, myocyte necrosis and eosinophil inflammation. Occasionally giant cell arteritis occurs in the portals arteries. Sulfonylureas, penicillin, phenytoin and allopurinol are included in this group.
- Thrombosis and stroke can be related to oral contraceptives.
- Hepatoportal sclerosis can be due to exposure to arsenic, azathioprine or to antiretrovirals such as didanosine.
GRANULOMATOUS REACTIONS
Various entities including infectious processes can cause granulomas. Among these are sarcoidosis, chronic cholestatic or systemic diseases, lymphomas and drugs. Noncaseating epithelioid granulomas are observed in portal, periportal or acini areas in up to 15% of liver biopsies. Some are accompanied by a small amount of cholestasis or focal necrosis. Examples of drugs that may be involved are antimicrobials such as amoxicillin, cephalexin, dicloxacillin, interferon, isoniazid, nitrofurantoin, quinine, penicillin, and sulfonamides. Others that may be involved are anticonvulsants such as carbamazepine, chlorpromazine, diazepam, and phenytoin. Still others include allopurinol, amiodarone, aspirin, anti TB vaccine, dapsone, and methyldopa. Prolonged use of mineral oils can cause constipation lipogranulomas. Talc, especially among intravenous uses of psychoactive substances, and materials used for sutures in intra-abdominal procedures can lead to granulomas on the liver's surface (4, 7).
LIVER FIBROSIS AND CIRRHOSIS
Although most people recover from the vast majority of adverse reactions to drugs without ever developing clinically significant fibrosis, sometimes periportal, pericentral or acinar fibrosis does develop.
Fibrosis sometimes occurs in Zone 1, especially among those whose drug reaction has caused chronic necroinflammatory disease such as autoimmune-like hepatitis and chronic cholestatic.
Fibrosis occurs in zone 3 at the pericellular or sub-sinusoidal level in alcoholic liver disease and hypervitaminosis A.
Hepar lobatum in which significant fibrosis forms large lobes has been described after chemotherapy in patients with metastatic carcinoma of the mammary gland or rectum (21, 22).
Cirrhosis has most especially been observed in patients who have had submassive hepatocellular necrosis which evolves into fibrosis, nodular regeneration and subsequent cirrhosis.
Cirrhosis established as a complication of prolonged therapy with methotrexate or amiodarone has also be seen. Cirrhosis following prolonged use of isoniazid, iproniazid, and valproic acid has also been observed (4).
NEOPLASIAS AND PSEUDOTUMORS
In the past, thorotrast, whose use has now been discontinued, and therapy with arsenic were well known for their relations with hepatic angiosarcoma, hepatocellular carcinoma and cholangiocarcinoma. Exposure to vinyl chloride has long been associated with angiosarcoma and epithelioid hemangioendothelioma (23, 24).
The association of oral contraceptives and liver adenomas and focal nodular hyperplasia is also well established. There have also been some cases of hepatocellular carcinoma. Other agents such as anabolic steroids, danazol and carbamazepine have also been associated with hepatocellular adenoma (25), and azathioprine has been associated with the development of nodular regenerative hyperplasia (26).
Some examples of the patterns described above are shown in the following histories and photographs.
Acute hepatitis due to green tea consumption (Camellia sinensis) (Figure 1)
The photo shows a 47 year old woman who had had jaundice accompanied by nausea and abdominal pain for two months. She had been drinking green tea for four months refers for weight loss. She had no other relevant history. Her aminotransferase level was ten times its normal value and total bilirubin elevated at the expense of direct bilirubin. Her alkaline phosphatase level was normal. Blood tests were negative for viral infections and autoantibodies.
A liver biopsy revealed cellular edema, predominantly macrovacuolar with marked fatty changes and portal inflammation, a lobular appearance, interface hepatitis and occasional apoptotic hepatocytes without fibrosis.
There are numerous descriptions of hepatotoxic herbal products, dietary supplements and teas, as well as hepatotoxic extracts and components of these products. Nevertheless, the incidence of hepatotoxicity is unknown with reports ranging from 2% to 50%.
Hepatotoxicity resulting from drinking green tea characteristically presents as acute liver damage. Sometimes it has resulted in severe liver failure leading to death although most cases reported have been considered "probable" according to their CIOMS scores. There are over 100 products containing green tea extract, and it has been shown that doses lower than 1.6 grams are well tolerated. The mechanism of action and adverse effects have not been well elucidated, and there may be periods of latency periods ranging from ten days to seven months after the initial onset. The most common pattern is hepatocellular damage, although there are reports of the cholestatic and mixed pattern (27).
Submassive Hepatitis due to acetaminophen overdose (Figure 2)
The photo shows a 25 year old woman who suffers from obesity. She had taken thirty 500 mg acetaminophen tablets accompanied by a large amount of alcohol in a suicide attempt. The day after, she came to the emergency room after suffering numerous episodes of nausea and vomiting. Upon admission she had jaundice, tachycardia, hypotension, and asterixis and was confused, but she had no fever. Her total bilirubin was 15.5 mg/dl, her direct bilirubin was 14.2 mg/dl, and her aminotransferase levels were marked elevated (ALT 15.570 U/L and AST 18.733 U/L). Here alkaline phosphatase level was 129 U/L and her PT lasted three times longer than normal. Her acetaminophen level was 141mcg/ml. A liver transplantation was performed. The explant's architecture was complete disrupted by trabecular disarray due to submassive hepatocellular necrosis with collapse of the reticular pattern, the presence of ballooned cells and cholangiolar proliferation with minimal inflammation.
The toxic damage from acetaminophen is directly hepatocellular. It is involved in about 40% of all cases of acute liver failure, usually resulting from an overdose (more than 7.5 grams/day in adults). Symptoms begin within 24 to 96 hours after ingestion and can quickly lead to liver failure, often accompanied by renal failure, and can result in death. It can occur unintentionally especially in malnourished alcoholics and chronic liver disease patients. At toxic doses there is severe depletion of glutathione saturating all conjugation mechanisms which frees the original components which are then metabolized into intermediate substances which are very harmful for liver cells. The toxic level of acetaminophen can be lowered by the consumption of alcohol and drugs that induce the cytochrome P-450 system as well as by being overweight (4).
Steatohepatitis due to steroids (Figure 3)
The photo shows 30 year old woman who had been diagnosed with systemic lupus erythematosus at the age of 20. She had received betamethasone for 5 years with very good clinical response from the underlying disease. The dose had been decreased from six mg/day to one mg/day. Although her aminotransferase levels had been normal at the beginning of her last year of treatment, follow-up examinations showed that her aminotransferase levels had progressively risen to three to four times the normal value. There were no other associated symptoms, and all other liver function tests and blood tests for viral infections were normal. The patient was found to have an enlarged liver, so it was decided to perform a liver biopsy. The biopsy showed marked macrovesicular pan-lobular steatosis, minimal portal inflammation and mild portal and sub-sinusoidal fibrous expansion.
One of the adverse effects of prolonged glucocorticoid use is liver toxicity which can trigger or worsen steatosis. Steatohepatitis and chronic hepatitis occur less frequently. Symptomatic and progressive liver damage is equally unusual. These adverse effects may be due to the same effect of glucocorticoids on insulin resistance or in the alteration of the fatty acid metabolism. They may also be the result of the patient being overweight which commonly occurs among patients receiving long term corticosteroid therapy. While steatosis is rapidly reversible, steatohepatitis may take a long time to reverse after treatment is stopped (28).
Steatohepatitis with marked fibrosis due to amiodarone (Figure 4)
The photo shows an 80 year old woman who was referred for examination because of weight loss, fatigue, weakness and jaundice associated with diffuse abdominal pain. She had a history of hypertension and supraventricular arrhythmia and had been receiving 50 mg/day of metoprolol, 50 mg/day of losartan 50, and 200 mg/day of amiodarone for the previous ten years. She had hepatomegaly and mild ascites. Her laboratory studies showed total bilirubin of 9.6 mg/dl, indirect bilirubin of 3.48 mg/dl, direct bilirubin of 6.12 mg/dl, AST at 51 IU/L, ALT at 42 IU/L, an alkaline phosphatase level of 282 IU/L, albumin at 2.03 g/dL, total protein at 4.89 g/dL, globulin at 2.86 g/dL, and a prothrombin time of 13.7/10.7.
A liver biopsy showed marked hepatocellular ballooning with frequent presence of Mallory bodies in zone 1, mild inflammation, hepatocellular cholestasis, macrovacuolar fatty alterations (Figure 4A) associated with sub-sinusoidal fibrosis and bridging (Figure 4B).
Amiodarone elevates aminotransferase levels in up to 30% of patients and causes steatohepatitis in one or two percent of patients treated at high doses for a long time. The total cumulative dose can persist in tissue even after treatment has been stopped. The morphological picture varies from hepatocellular damage to steatohepatitis, cholestasis, ischemic hepatitis, and cirrhosis.
There may be mitochondrial abnormalities with lysosomes charged with phospholipids (phospholipidosis). In these cases, light microscopy will show the cytoplasm to be clear and foamy or will show granular hepatocytes and Kupffer cells which are lysosomal lamellar inclusions in the ultrastructure. This has also been associated with rare cases of Reye's syndrome. The mechanism of action appears to be direct damage of the lipid membrane with lysosomal and mitochondrial changes which explain the presence of steatosis and Mallory body formation with subsequent fibrosis (4, 8).
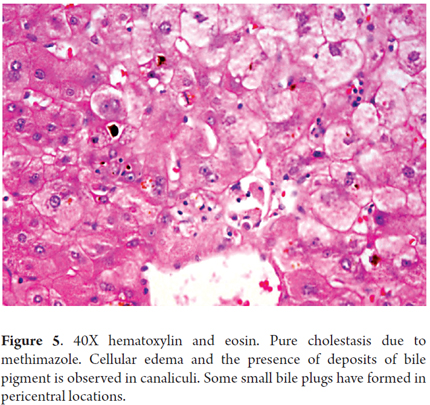
Pure Cholestasis due to Methimazole (Figure 5)
The photo shows a 33 year old woman with Graves' disease who had been treated with twice daily 20 mg doses of methimazole. Three weeks after treatment started she developed jaundice, acholia, dark urine, fatigue, weakness and intense itching. Widespread skin and mucosal jaundice was found. There were no other relevant findings. Total bilirubin: 17.89 mg/dL, direct bilirubin: 12.95 mg/dL, indirect bilirubin: 4.94 mg/dL, Alkaline phosphatase (ALP): 342 IU/L, Gamma-glutamyl transpeptidase (GGT): 23 IU/L, AST: 82 IU/L, ALT: 93 IU/L, PT: 12/10 seconds, INR: 1.0, TPT: 25/23 seconds, albumin 3.4 g/dL. Blood tests for viral infections, antinuclear antibodies (ANA), antimitochondrial antibodies (AMA), and smooth muscle antibodies (SMA) were all negative. A liver biopsy showed only marked hepatocanalicular cholestasis.
Methimazole hepatotoxicity is usually transient with asymptomatic elevation of aminotransferases. Cholestatic hepatitis, isolated cholestasis and ductopenia are all rare. The mechanism of action is unknown, but it is believed that there is an immune reaction to one of methimazole's metabolic products. When cholestasis occurs, it may be due to enzymatic alteration due to a genetic cause, to glutathione depletion or to increased drug concentration in the liver which produces a toxic effect within the hepatocytes, or to direct action of one of its metabolites (12).
Autoimmune-like hepatitis due to nitrofurantoin (Figure 6)
The photo shows a 60 year old woman who had had several episodes of urinary tract infections for which she had been treated with antibiotics. A month consulting a physician because of malaise and nausea, she had again presented urinary symptoms and had self-medicated with 50 mg/day of Nitrofurantoin, a medication she had received in the past. She told the physician that she had been suffering from fatigue and tiredness for six months. Her aminotransferases levels were three to four times normal, but alkaline phosphatase and bilirubin levels were normal. She tested positive 1:80 for smooth muscle antibodies (SMA), but ANA and AMA tests were negative as were tests for viral infections. Given the persistent elevation of transaminases, a liver biopsy was taken and analyzed. It found portal inflammation with damage to the limiting plate. The infiltrate was lymphoplasmacytic with lobular hepatitis and the presence of some apoptotic hepatocytes and fibrous portal expansion.
Differentiation of clinical and morphological findings between autoimmune hepatitis and autoimmune-like drug-induced hepatitis can be very difficult or impossible. Clinical judgment is key for proper diagnosis. Antibiotics are responsible for a significant percentage of cases, and for nine percent of those that specifically resemble autoimmune hepatitis. Nitrofurantoin and minocycline are responsible for 90% of these cases. They occur predominantly in women without other risk factors that would suggest classical autoimmune hepatitis. Genetic factors, polymorphisms, and the presence of leukocyte antigen modulators of the immune response and the formation of neoantigens, CD8 T cell activation and deficiency in immune regulation have been identified as potential triggers for hepatocyte damage. The suspension of the drug is essential for spontaneous improvement, but if there are severe clinical and pathological manifestations, steroid use is indicated (14, 15).
Chronic cholestatic pattern with primary biliary cirrhosis due to Chlorpromazine (Figure 7)
The photo shows a 55 year old man who had been receiving chlorpromazine for depression for two months. He had started with 50mg/day and had increased the dosage to 75 mg/day. Upon consultation he was suffering from fatigue, jaundice and itching, and his urine was dark. His bilirubin level was elevated to 7 mg/dl, his alkaline phosphatase level was 220 IU/L, he had slightly elevated aminotransferases, and he had peripheral eosinophilia. It was decided to suspend chlorpromazine and start administration of antihistamines and cholestyramine. Nevertheless, symptoms persisted and his itching became worse. Six months later, liver function tests observed showed his levels were double what they had been initially. A liver biopsy found portal infiltrates, inflammation and degenerative ductal changes (Figure 7A). The patient's fatigue continued, jaundice and itching worsened, and the patient began to lose weight and to present steatorrhea. Two years later, another biopsy showed evidence of chronic cholestasis with mild portal inflammation, periseptal xanthomatous changes, ductopenia affecting 50% of the ducts, and portal and septal fibrosis (Figure 7B).
Abnormalities in liver function tests have been reported in up to 40% of patients on long-term treatment with chlorpromazine. This is usually self-limiting and can even be reversed without stopping the medication. Acute and chronic cholestasis are common in these cases. Manifestations such as fever, rashes and eosinophilia occur in some cases. The mechanism of action is not known although a mechanism of hypersensitivity has been established on the basis of clinical manifestations and recurrence after renewed administration of medication (4).
Budd-Chiari syndrome due to oral contraceptives (Figure 8)
The photo shows a 30 year old woman with pain and bloating. She had no relevant medical history and did not drink alcohol. She had been taking oral contraceptives for 7 years. Upon physical examination, ascites and leg edema were found. A colonoscopy showed internal hemorrhoids and ultrasound found a slightly enlarged liver with slight enlargement of the caudate but no chronic stigmata or masses. An MRI demonstrated thrombosis of the hepatic vein. Blood tests for viral infections and autoimmunity studies were negative, and she had no deficiency of protein C, protein S, or clotting factors V and II. There were no procoagulant factors present and her liver function tests showed no abnormalities. A liver biopsy showed serious sinusoidal dilation with mild congestion without evidence of inflammation or necrosis.
Budd-Chiari syndrome and hepatic adenomas can occur in women after prolonged use of oral contraceptives. Symptoms resolve spontaneously and/or tumor size markedly decreases after suspension of oral contraceptives. It has been suggested that a combination of environmental exposure (ACO) and genetic predisposition could be responsible for Budd-Chiari syndrome in these cases but no causes have been demonstrated for adenomas (4, 28).
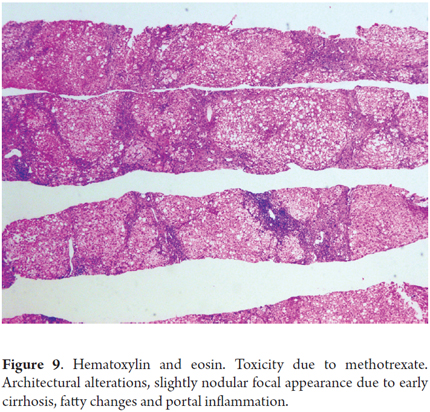
Fibrosis due to methotrexate (Figure 9)
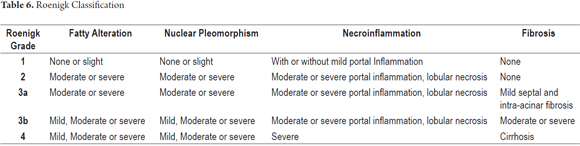
The photo shows a 55 year old man who had been diagnosed 15 years earlier with active rheumatoid arthritis. He had initially received treatment with prednisone and penicillamine but had responded poorly. Treatment with 7.5 mg/week of methotrexate and 500 mg/day of aspirin was initiated. At the beginning of this treatment his liver function tests were normal. He did not drink alcohol, and his viral serology was negative. The dosage was gradually increased up to 15 mg/week. Two years later, tests showed a slight increase of aminotransferase levels (1 time the normal value). A liver biopsy showed Roenigk 1 liver damage with mild steatosis and portal inflammation without fibrosis or anisonucleosis. Treatment continued with significant improvement of the patient's joint symptoms while aminotransferase levels never rose to values greater than two times normal.
After seven years of therapy, the cumulative dose was 4.5 grams. The patient presented weight gain, bloating, and ascites. Liver tests showed slightly elevated aminotransferases (two times normal), normal alkaline phosphatase, prothrombin time increased to 18 seconds (normal value 12 seconds), and an albumin level of 2 .5 g/dl. A new liver biopsy showed mild steatosis and mild inflammation with marked fibrosis and areas of early cirrhosis (stage 6.5). No cirrhosis was established. Liver damage was rated as Roenigk 3b. Methotrexate was suspended and patient's ascites decreased progressively and liver function tests, albumin and prothrombin time returned to normal.
Long term use of methotrexate, even at low doses, to treat psoriasis, rheumatoid arthritis or chronic inflammatory bowel disease carries the risk of liver toxicity. The risk increases very significantly if there is exposure to alcohol, if there are other bases for liver disease, or if the patient is obese or has diabetes. These patients may already have elevated liver enzymes that does not in itself represent a toxic effect secondary to methotrexate. A biopsy may contribute to patient management and the medical decision to continue or discontinue the medication.
The morphological spectrum in these cases varies, so morphological analysis must consider steatosis, anisonucleosis, hepatocellular necrosis and fibrosis. The classification described by Roenigk is the most widely accepted and frequently used grading system for liver damage (Table 6) (29, 30).
CONCLUSION
Liver damage induced by drugs, toxins, herbal products or homeopathic treatments can mimic any pattern of primary liver disease. This greatly complicates correct identification of the pathology. A liver biopsy is sometimes considered to be a valuable tool for evaluation of these patients. The report should be accompanied by a detailed description of the pattern which will be very useful for assessing possible differential diagnoses, determining the severity of the damage, and guiding medical treatment. This must always be linked to judicious clinical analysis of the patient's complete medical history and a search of the literature to establish a correct diagnosis since many of these substances share morphological patterns.
REFERENCES
1. Hou FQ, Zeng Z, Wang GQ. Hospital admissions for drug-induced liver injury: clinical features, therapy, and outcomes. Cell Biochem Biophys 2012; 64: 77-83. [ Links ]
2. Leise MD, Poterucha JJ, Talwalkar JA. Drug induced liver injury. Mayo Clin Proc 2014; 89(1): 95-106. [ Links ]
3. Rangnekar AS, Fontana RJ. An update on drug induced liver injury. Minerva Gastroenterol Dietol 2011; 57(2): 213-29. [ Links ]
4. Ramachandran R, Kakar S. Histological patterns in drug-induced liver disease. J Clin Pathol 2009; 62: 481-92. [ Links ]
5. Davern TJ. Drug-induced liver disease. Clin Liver Dis 2012; 16: 231-45. [ Links ]
6. Kleiner DE. The pathology of drug-induced liver injury. Semin Liver Dis 2009; 29: 364-72. [ Links ]
7. Xuchen Zhang, Jie Ouyang, Swan N. Thung. Histopathologic Manifestations of Drug-induced Hepatotoxicity. Clin Liver Dis 2013; 17: 547-564. [ Links ]
8. Bjornsson E, Kalaitzakis E, Av Klinteberg V, et al. Long-term follow-up of patients with mild to moderate drug-induced liver injury. Aliment Pharmacol Ther 2007; 26: 79-85. [ Links ]
9. Andrade RJ, Lucena MI, Kaplowitz N, et al. Outcome of acute idiosyncratic drug-induced liver injury: long-term follow-up in a hepatotoxicity registry. Hepatology 2006; 44: 1581-8. [ Links ]
10. Oshea, Darasathy, and Mc Cullough Alcoholic Liver Disease. Hepatology 2010; 51(1). [ Links ]
11. Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis 2012; 32: 3-13. [ Links ]
12. Rocío del Pilar López-P, y col. Ictericia colestásica inducida por metimazol en una paciente con hipertiroidismo. Acta Gastroenterol Latinoam 2014; 44: 52-58. [ Links ]
13. Abraham C, Hart J, Locke SM and Baker AL. A case of indometacin-induced acute hepatitis developing into chronic autoimmune hepatitis. Gastroenterology & Hepatology 2008; 5(3): 172 -176. [ Links ]
14. Czaja AJ. Drug-induced autoimmune-like hepatitis. Dig Dis Sci 2011; 56: 958-76. [ Links ]
15. Suzuki A, Brunt EM, Kleiner DE, et al. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology 2011; 54: 931-9. [ Links ]
16. Lewis JH. Diagnosis: liver biopsy differentiates DILI from autoimmune hepatitis. Nat Rev Gastroenterol Hepatol 2011; 8: 540-2. [ Links ]
17. Andrew S. deLemos, David M. Foureau, Carl Jacobs, Will Ahrens, Mark W. Russo, Herbert L. Bonkovsky. Drug-Induced Liver injury with Autoimmune Features. Semin Liver Dis 2014; 34(02): 194-20. [ Links ]
18. Gluck N, Fried M and Porat R. Acute Amiodarone Liver Toxicity Likely Due to Ischemic Hepatitis. IMAJ 2011; 748-752. [ Links ]
19. Mallet VO, Bralet MP, Pol S. Response to Schiano, et al. Hepatoportal sclerosis as a cause of noncirrhotic portal hypertension in patients with HIV. Am J Gastroenterol 2008; 103: 808-9. [ Links ]
20. Khan AZ, Morris-Stiff G, Makuuchi M. Patterns of chemotherapy-induced hepatic injury and their implications for patients undergoing liver resection for colorectal liver metastases. J Hepatobiliary Pancreat Surg 2009; 16: 137-44. [ Links ]
21. Teke Z, Nessar G, Kiremitci S, Aksoy E, Elbir O. Hepar Lobatum Carcinomatosum Associated with Metastatic Rectal Carcinoma: An Unusual Cause of Liver Dysmorphy. Med Princ Pract 2011; 20(1): 93-6. [ Links ]
22. Gravel DH, Bégin LR, Brisson ML, Lamoureux E. Metastatic carcinoma resulting in hepar lobatum. Am J Clin Pathol 1996; 105(5): 621-7. [ Links ]
23. Infante PF, Petty SE, Groth DH, Markowitz G, Rosner D. Vinyl chloride propellant in hair spray and angiosarcoma of the liver among hairdressers and barbers: case reports. Int J Occup Environ Health 2009; 15(1): 36-42. [ Links ]
24. Hozo I Miric D, Bojic L, Giunio L, Lusic I, Culic V, Simunic M. Liver angiosarcoma and hemangiopericytoma after occupational exposure to vinyl chloride monomer. Environ Health Perspect 2000; 108(8): 793-5. [ Links ]
25. Dhingra S, Fiel MI. Update on the new classification of hepatic adenomas: clinical, molecular, and pathologic characteristics. Arch Pathol Lab Med 2014; 138(8): 1090-7. [ Links ]
26. Daniel F, Cadranel JF, Seksik P, Cazier A, Duong Van Huyen JP, Ziol M, Coutarel P, Loison P, Jian R, Marteau P. Azathioprine induced nodular regenerative hyperplasia in IBD patients. Gastroenterol Clin Biol 2005; 29(5): 600-3. [ Links ]
27. Bunchorntavakul C, Reddy KR. Herbal and dietary supplement hepatotoxicity. Aliment Pharmacol Ther 2013; 37: 3-17. [ Links ]
28. Chitturi S, Farrell GC. Corticosteroids. Adverse effects of hormones and hormone antagonists on the liver. En Kaplowitz N, DeLeve LD, eds. Drug-induced liver disease. 3rd ed. Amsterdam: Elsevier; 2013. p. 613-4. [ Links ]
29. GP Aithal, et al. Monitoring methotrexate-induced hepatic fibrosis in patients with psoriasis: are serial liver biopsies justified? Aliment Pharmacol Ther 2004; 19: 391-399. [ Links ]
30. MAM Berends, et al. Reliability of the Roenigk Classification of Liver Damage after Methotrexate Treatment for Psoriasis. Arch Dermatol 2007; 143(12): 1515-1519. [ Links ]











 text in
text in