Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.1 Bogotá Jan./Mar. 2015
Helicobacter Pylori Eradication: Survey conducted by the Colombian Association of Gastroenterology
Martín Gómez MD. (1), Oscar Ruíz MD. (2), David Páramo Hernández MD. (3), Rosario Albis MD. (4), Luis Carlos Sabbagh MD. (5)
(1) Internist and Gastroenterologist at the Hospital El Tunal and Associate Professor of Gastroenterology at the National University of Colombia in Bogotá, Colombia.
(2) Internist and Gastroenterologist at the Hospital de Occidente de Kennedy in Bogotá, Colombia.
(3) Gastroenterologist and Clinical Epidemiologist at Hospital Santa Clara and Universidad El Bosque in Bogotá, Colombia.
(4) General Surgeon and Gastrointestinal Surgeon at the Instituto Nacional de Cancerología (INC) and theGastroenterology at Clinica Colsanitas in Bogotá, Colombia.
(5) Internist and Gastroenterologist, Chief 0f the Gastroenterology Unit at Clinical Colsanitas and President of the Colombian Association of Gastroenterology. Bogotá, Colombia.
Received: 30-07-14 Accepted: 02-02-15
Abstract
The aim of this study is to present the results of an online survey about strategies and schemes used by members of the Colombian Association of Gastroenterology (ACG - Asociación Colombiana de Gastroenterología) to diagnose and eradicate Helicobacter pylori infections in light of the Maastricht IV/ Florence Consensus Report. Information was prospectively collected between December 2013 and May 2014 through a virtual questionnaire on the official website of the Colombian Association of Gastroenterology (ACG).
The survey was answered by 114 physicians, of whom 60 (52%) were internists and gastroenterologists located in major cities. The results show that 61% (n = 71) of respondents adhere to the recommendations of Maastricht for the first line eradication scheme and 66% (n = 76) adhere to the consensus' recommendations for the second line scheme. Another aspect of the survey that should be highlighted is that, in contrast to the recommendations of Maastricht that monoclonal fecal antigens or the urea breath test be used for assessment of eradication, the diagnostic test favored by 63% (n = 72) of respondents was upper endoscopy plus a biopsy with Giemsa staining. This is probably because of availability and costs in our country.
The results of the survey show treatment schemes for eradication of Helicobacter preferred by the professionals who responded, and allow us to evaluate the acceptability of, and adherence to, the recommendations on treatment regimens of the consensus. This includes contrasts such as following up eradication treatment by endoscopy rather than the methods recommended by the consensus.
Keywords
Survey, helicobacter pylori, Maastricht IV Consensus.
INTRODUCTION
Helicobacter pylori (H. pylori) is the organism responsible for the most common chronic bacterial infections in the world. Genetic studies suggest that humans have been infected with H. pylori for about 58,000 years (1). The bacteria were first observed over 100 years ago (2), but their real implication and their relationship with many pathologies were not fully established until 1982 when Marshall and Warren identified and subsequently cultivated this micro-organism. They initially called it Campylobacter pyloridis and later reclassified it as Helicobacter pylori (H. pylori) (3-5).
Infection with this organism always causes chronic gastritis, but only 20% of those infected develop any clinical disease (6).
Chronic infections affect almost half the world's population, and in developing countries the prevalence is as high as 90%. In developed countries except for Japan, its prevalence is less than 40% (7, 9). In our population rates, blood and laboratory tests of adults indicate a prevalence of 70% to 78% (8-12).
Based on current evidence, it is known that this organism is causally associated with chronic gastritis and gastro-duodenal ulcers. People who are infected with the bacteria have a 1% to 10% chance of developing these conditions. H. pylori is also associated with pathologies like noncardia gastric adenocarcinoma with a risk for infected patients of 0.1% to 3% and functional dyspepsia with a risk for infected patients of 1% (6, 13-18). A causal relationship between the bacteria and mucosa-associated lymphoid tissue (MALT) has also been proposed with a reported risk of development of less than 0.01% in infected patients. The main risk factors that have been reported for acquiring infections are poor living conditions including large numbers of siblings, poor access to education, overcrowding, and poor or little access to potable water (19-22). High rates of infection may also be association with genetic factors related to ethnicity since infections are more common in African Americans and indigenous people (23-24).
In Colombia, gastric cancer is the second most common type of cancer among men and the fourth among women, but it is the leading cause of death from cancer with an incidence of 20.7 per 100,000 inhabitants between 1995 and 1999. In other words, this is a high risk population (25). A randomized test conducted in Shandong, China has shown that eradication of H pylori using amoxicillin and omeprazole decreased the incidence of gastric cancer by 39% over a period of 15 years (26).
That study confirmed the findings of other studies (27-32) and demonstrates that eradication reduces recurrence (33). Based on the available evidence, the consensus of the Conference on Gastric Cancer recommended detection and treatment of H. pylori in asymptomatic persons in high-risk populations (Populations in which the incidence of gastric cancer is higher than 20/100,000) in order to prevent gastric cancer (34). This suggests that our country could benefit from this recommendation.
Considering the above, Helicobacter pylori eradication is not only useful for the treatment of gastric and duodenal ulcers, but also for the treatment and prevention of diseases associated with H. pylori such as gastric cancer and for diminishing the spread of this infection (9, 26, 35). Nevertheless, the success of these treatments has already been compromised by the increased antimicrobial resistance exhibited by H pylori (36, 37).
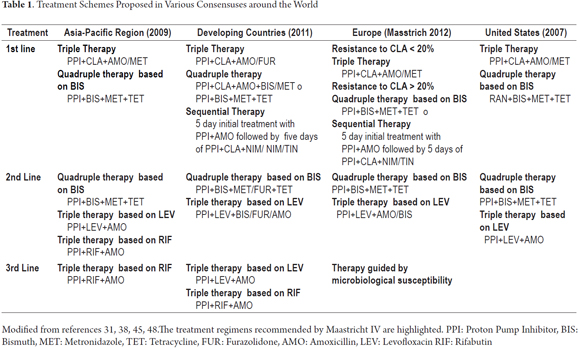
Given the critical importance of this infection and the need for effective treatments to eradicate it, international consensus meetings have been held, the most influential of which has been the Maastricht consensus. It was last revised in 2012 in the Maastricht IV/ Florence Consensus Report (38). It is crucial that medical personnel in our country learn the various diagnosis and eradication strategies (39).
The purpose of this study was to use a survey to determine which strategies and schemes are currently used by our colleagues and partners for the diagnosis and eradication of Helicobacter Pylori, and then to compare them with the proposal of the Maastricht IV/ Florence Consensus Report. Scientific surveys help us determine variations in the practice of gastroenterology colleagues and provide a framework to identify areas that need improvement through future research and conferences.
METHODS
This is an observational cross-sectional study in which information was taken prospectively between December 2013 and May 2014 with online survey on the official website of the Colombian Association of Gastroenterology (www.gastrocol.com/) which is still available at goo.gl/CNsQak. The questionnaire was answered anonymously and voluntarily by general practitioners, internists, gastroenterological internists, surgeons, gastroenterologists, gastrointestinal surgeons, and colorectal surgeons from various regions of our country where the Internet is available. (Table 1)
STATISTICAL ANALYSIS
Records of survey responses were stored in data tables at Google drive and were subsequently downloaded to Excel tables for statistical analysis. Variables were coded as qualitative or quantitative and then qualitative variables were categorized as nominal or ordinal and quantitative variables were categorized discrete or continuous. Descriptive statistics were analyzed using absolute and relative frequencies. Measures of central tendency such as mean or median were calculated for numerical quantitative variables. The results tables present the relative frequencies.
RESULTS
During the response period, a total of 114 medical professionals filled out the questionnaire: ten general practitioners (8.8%), one internist (0.9%), sixty gastroenterological internists (52.6%), two general surgeons (1.8%), fourteen gastrointestinal surgeons (12.3%), twenty-two surgical gastroenterologists (19.2%), two surgical coloproctologists (1.8%), and three physicians from other specialties (2.6%). Respondents were located in 14 Colombian cities as follows: fifty-nine in Bogotá (52%), eight in Medellín (7%), seven in Bucaramanga (6%), six in Cali (5%), five in Barranquilla (4%), and five in Manizales (4.5%).
Answers to specific questions:
1. Do you agree with the strategy of treatment for H. pylori whenever a patient's blood test is positive?
Yes: 27 (24%), No: 87 (76%)
2. Do you agree with the use of eradication therapy when the patient has:
a. Functional dyspepsia? Yes: 66 (58%), No: 48 (42%)
b. GERD? Yes: 19 (17%), No: 95 (83%)
c. Received non-selective NSAIDs including low-dose acetylsalicylic acid for more than four weeks? Yes: 66 (58%), No: 48 (42%)
d. Received PPIs for more than 8 weeks? Yes: 55 (46%), No: 59 (52%)
e. A diagnosis of gastric intestinal gastric metaplasia? Yes: 97 (85%), No: 17 (15%)
f. Low-grade MALT (First-line treatment for patients with histological diagnosis)? Yes: 105 (92%), No: 9 (8%)
g. A confirmed diagnosis of iron deficiency anemia? Yes: 75 (66%), No: 39 (34%)
h. A confirmed diagnosis of idiopathic thrombocytopenic purpura? Yes: 78 (68%), No: 36 (32%)
i. A confirmed diagnosis of anemia vitamin B12 deficiency? Yes: 66 (58%), No: 48 (42%)
j. A confirmed diagnosis of asthma? Yes: 22 (19%), No: 92 (81%)
k. A confirmed diagnosis of atopy? Yes: 27 (24%), No: 87 (76%)
l. A confirmed diagnosis of morbid obesity? Yes: 45 (39%), No: 69, (61%)
m. A bleeding peptic ulcer? Yes: 83 (73%), No: 31 (27%)
3. Do you think the isolation of fecal monoclonal antigens for detection of H pylori performs similarly to the urea breath test?
Yes: 81 (71%), No: 33 (29%)
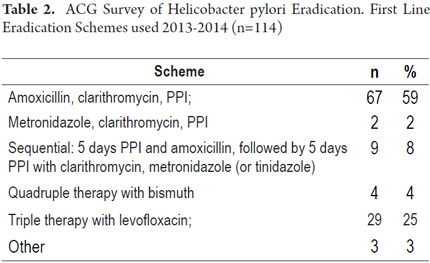
4. What is first line the combination of drugs you currently use for eradication of H pylori? (See Table 2)
A comparison of responses to the two first line treatment schemes for eradication of Helicobacter pylori recommended by the Maastricht Consensus showed that 67 (59%) adhere to the scheme of amoxicillin, clarithromycin and PPIs, and two (2 %) adhere to the scheme of metronidazole, clarithromycin, and PPIs. In total, 71 respondents (61%) adhered to one or the other scheme.
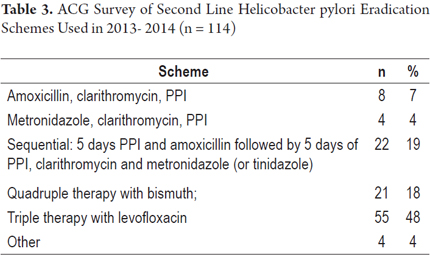
5. What combination of drugs do you use as the second line treatment for eradication of H pylori? (See Table 3)
A comparison of responses with the two Maastricht Consensus recommendations for two second line treatment schemes for eradication of Helicobacter pylori shows that 21 (18%) adhere to quadruple therapy with bismuth and 55 (48%) adhere to triple therapy with levofloxacin. In total, 76 respondents (66%) adhered to one or the other scheme.
6. How do you use PPIs in the eradication scheme?
- Single daily dose: 17 (15%)
- Twice daily dose: 96 (84%)
- Other. 1 (1%)
7. How long is do you prescribe for H pylori eradication therapy?
- Seven days: 8 (7%)
- 10 - 14 days: 106 (93%)
8. What indications do you use to decide to culture for H. pylori?
- Before starting first-line eradication treatment: 8 (7%)
- Following therapeutic failure of first-line treatment: 8 (7%)
- Following therapeutic failure of second-line treatment: 83 (73%)
- Other: 15 (13%)
9. For which patients do you confirm eradication of H. pylori?
(Number and percentage of "yes" responses) Patients with:
- Gastric ulcers: 77 (68%)
- Duodenal ulcers: 66 (58%)
- Gastric Lymphoma: 92 (80%)
- Early gastric cancer: 81 (71%)
- Dyspepsia: 13 (11%)
- GERD: 7 (6%)
- Barrett's Esophagus: 8 (7%)
- Other: 9 (8%)
10. How long do you monitor patient after eradication therapy?
- 2 weeks: 1 (1%)
- 3 weeks: 2 (2%)
- 4 weeks: 22 (19%)
- 6 weeks: 15 (13%)
- 8 weeks: 65 (57%)
- Other: 9 (8%)
11. What diagnostic test do you use to monitor eradication?
- Upper endoscopy plus biopsy (Giemsa stain): 72 (63%)
- Fecal Monoclonal Antigen: 13 (11%)
- Urea breath test: 22 (19%)
- IgG blood test: 3 (3%)
- Other: 4 (4%)
12. Have treatment failures occurred indicating H. pylori resistant to treatment? Number of treatment failures:
- None: 12 (11%)
- One: 31 (27%)
- Two: 42 (37%)
- Three: 9 (8%)
- More than three: 20 (18%)
DISCUSSION
The study was conducted through an online survey at the website of the Colombian Association of Gastroenterology (Asociación Colombiana de Gastroenterología). One hundred and fourteen physicians answered the survey. Of the respondents 60 (52%) were internists and gastroenterologists located in major cities. If we consider that our association is composed of 310 members, the response rate was 36% which is very significant when similar studies using this data collection tool are considered.
The results for adherence to Maastricht Consensus recommendations for first and second Helicobacter pylori eradication schemes of 61% (n = 71) of respondents and 66% (n = 76) respectively are worth noting. Nevertheless, adherence to Maastricht does not necessarily represent a guarantee of the efficacy of eradication efforts. Even though the survey explores aspects of efficacy of the schemes used and bacterial resistance to those schemes, questions about these two issues remain. As a basic rule, regimens that have a local eradication rates of 90% or more, preferably 95% or more, should be prescribed. If there are no schemes available with an eradication rate over 90%, the most effective regimen available locally should be used (40, 41).
Considering that the principal basis for most of these schemes is clarithromycin and/or metronidazole, resistance to these antibiotics must be highlighted especially since they are widely used as empirical therapy for infections other than H pylori infections. They are included in first-line therapies in international consensuses even though eradication rates have not been improved and despite the necessity of increased doses and longer treatment times (26, 35, 42, 43). Recently a systematic review showed that the prevalence of primary resistance to antimicrobials in Latin America among adults was 12% for clarithromycin (n = 35 studies), 53% for metronidazole (n = 34), 4% for amoxicillin (n = 28) 6% for tetracycline (n = 20), 3% for furazolidone (n = 6), 15% for fluoroquinolones (n = 5), and 8% for dual resistance to clarithromycin and metronidazole (n = 10). Prevalences of resistance vary significantly by country which leads top declining efficacy of first line helicobacter pylori eradication schemes (44, 45). The search for new therapeutic schemes that are more effective at the local level is a research challenge for the entire region.
Finally, another aspect of the survey that should be highlighted in contrast to the recommendations of Maastricht are the diagnostic tests favored here in Colombia to assess eradication. While the Maastricht consensus recommends fecal monoclonal antigen testing and the urea breath test, survey respondents prefer upper endoscopy with Giemsa staining of biopsies (63%, n = 72). This is probably because of availability and cost in our country.
A similar study by one of the authors found that, currently, the Colombian medical community has major difficulties with eradicating Helicobacter pylori (46). Five years ago that study surveyed a broader population including general practitioners, internists and gastroenterologists. The percentage of general practitioners responding was 68% compared to 31% in our survey. The earlier study found a wide range of treatment regimens were used (41 in total) and drew attention to the facts that more than half of those physicians used metronidazole within their schemes, treatment usually lasted less than 7 days, and very clear indications of eradication such as peptic ulcers were followed up by only 30% of the respondents. This could suggest an improvement in the education level of physicians or better updating about management of Helicobacter pylori infections (45).
There are no other studies of our population, but globally there have been similar studies in other countries. In Germany family physicians and gastroenterologists were evaluated. That study found that gastroenterologists treated H. pylori infections better than family physicians did because gastroenterologists identified the causal relationship between infections and associated diseases while family physicians were less discriminating (47). A study in Israel that also evaluated primary care physicians found that they made no clear diagnoses and treatment had no direct relationship with certain diseases (48). A recent study in Iran has evaluated a population of general practitioners and internists. Consistent with the findings in our country, the internists obtained better results (49).
It should be noted that our survey was conducted among members of the Colombian Association of Gastroenterology who are specialists whose knowledgeable about infectious diseases, antibiotics and suitable treatment schemes has increased thanks to the ease of access to information, including the Maastricht IV consensus, and greater awareness of the importance of knowledge about this topic among our members.
As intended, this study has allowed us to learn about trends in management of Helicobacter pylori eradication among Colombian medical professionals. It allows us to establish the fact that there is majority acceptance of, and adherence to, the recommendations for treatment regimens of the Maastricht IV consensus although it stands in contrast to it in areas such as monitoring of eradication by endoscopy.
REFERENCES
1. Goodwin CS, Worsley BW. Microbiology of Helicobacter pylori. Gastroenterol Clin North Am 1993;22:5-19. [ Links ]
2. Marshall BJ. History of the discovery of C. pylori. In: Campylobacter Pylori in Gastritis and Peptic Ulcer Disease, Blaser MJ (Ed), Igaku-Shoin, New York 1989. p7. [ Links ]
3. Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311. [ Links ]
4. Oleastro M, Ménard A. The role of Helicobacter pylori Outer membrane proteins in adherence and pathogenesis. biology. 2013;2:1110-34. [ Links ]
5. Costa F, D'Elios M. Management of Helicobacter pylori infection. Anti Infect Ther 2010;8:887-92. [ Links ]
6. Otero W, Trespalacios A, Otero E. Helicobacter pylori: Current treatment. An important challenge for gastroenterology. Rev Col Gastroenterol 2009;24:280-92. [ Links ]
7. Trespalacios A, Otero W, Mercado M. Helicobacter pylori resistance to metronidazole, clarithromycin and amoxicillin in Colombian patients. Rev Col Gastroenterol 2010;25:31-8. [ Links ]
8. McColl KE. Helicobacter pylori infection. N Engl J Med 2010;362:1597-604. [ Links ]
9. Watari J, Chen N, Amenta PS, Fukui H, Oshima T, Tomita T, et al. Helicobacter pylori associated chronic gastritis, clinical syndromes, precancerous lesions, and pathogenesis of gastric cancer development. World J Gastroenterol 2014;20:5461-73. [ Links ]
10. Garza-González E, Pérez-Pérez GI, Maldonado-Garza HJ, Bosques-Padilla FJ. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol 2014;20:1438-49. [ Links ]
11. Gutiérrez O, Aponte D, Páramo D, Sabbag LC, Angel LA, Cardona H, et al. Seroprevalencia y factores de riesgo asociados con la infección por Helicobacter pylori en niños. Rev Col Gastroenterol 2001;16:19-22. [ Links ]
12. Bravo LE, Cortés A, Carrascal E, Jaramillo R, García LS, Bravo PE, et al. Helicobacter pylori: patología y prevalencia en biopsias gástricas en Colombia. Col Med 2003;34:124-31. [ Links ]
13. Banic M, Franceschi F, Babić Z, Gasbarrini A. Extragastric manifestations of Helicobacter pylori infection. Helicobacter. 2012;17(Suppl 1):49-55. [ Links ]
14. Chen BF, Xu X, Deng Y, Ma SC, Tang LQ, Zhang SB, et al. Relationship between Helicobacter pylori infection and serum interleukin-18 in patients with carotid atherosclerosis. Helicobacter. 2013;18:124-8. [ Links ]
15. Bang CS, Baik GH. Attempts to enhance the eradication rate of Helicobacter pylori infection. World J Gastroenterol 2014;20:5252-62. [ Links ]
16. Malfertheiner P, Venerito M,. Selgrad M. Helicobacter pylori infection: selected aspects in clinical Management. Curr Opin Gastroenterol 2013;29:669-75. [ Links ]
17. O'Connor A, Molina-Infante J, Gisbert JP, O'Morain C. Treatment of Helicobacter pylori infection 2013. Helicobacter 2013;18(Suppl 1):58-65. [ Links ]
18. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010;59:1143-53. [ Links ]
19. Goh KL, Chan WK, Shiota S, Yamaoka Y. Epidemiology of Helicobacter pylori infection and public health implications. Helicobacter 2011;16(Suppl 1):1-9. [ Links ]
20. Dattoli VC, Veiga RV, da Cunha SS, Pontes-de-Carvalho LC, Barreto ML, Alcântara-Neves NM. Seroprevalence and potential risk factors for Helicobacter pylori infection in Brazilian children. Helicobacter. 2010;15:273-8. [ Links ]
21. Fialho AM, Braga AB, Braga Neto MB, Carneiro JG, Rocha AM, Rodrigues MN, et al. Younger siblings play a major role in Helicobacter pylori transmission among children from a low-income community in the Northeast of Brazil. Helicobacter 2010;15:491-6. [ Links ]
22. Trebel K, Rolle-Kampczyk U, Richter M, Kindler A, Richter T, Schlink U. A rigorous small area modelling-study for the Helicobacter pylori epidemiology. Sci Total Environ 2010;408:3931-42. [ Links ]
23. Epplein M, Signorello LB, Zheng W, Peek RM Jr, Michel A, Williams SM, et al. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer Epidemiol Biomarkers Prev 2011;20:826-34. [ Links ]
24. Arnold M, Moore SP, Hassler S, Ellison-Loschmann L, Forman D, Bray F. The burden of stomach cancer in indigenous populations: A systematic review and global assessment Gut. 2014;63:64-71. [ Links ]
25. Gómez M, Otero W, Caminos JE. Gastric cancer in young patients in Colombia. Rev Col Gastroenterol 2012;27:166-72. [ Links ]
26. Asaka M, Kato M, Takahashi S, Fukuda Y, Sugiyama T, Ota H, et al. Guidelines for the management of Helicobacter pylori infection in Japan: 2009 revised edition. Helicobacter 2010;15:1-20. [ Links ]
27. Ma JL, Zhang L, Brown LM, Li JY, Shen L, Pan KF, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst 2012;104:488-92. [ Links ]
28. Wong BC, Lam SK, Wong WM, Chen JS, Zheng TT, Feng RE, et al. China Gastric Cancer Study Group. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: A randomized controlled trial. JAMA 2004;291:187-94. [ Links ]
29. Fuccio L, Zagari RM, Eusebi LH, Laterza L, Cennamo V, Ceroni L, et al. Meta-analysis: Can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann Intern Med 2009;151:121-28. [ Links ]
30. Osborn JF, Cattaruzza MS, Ferri AM, De Angelis F, Renzi D, Marani A, et al. How long will it take to reduce gastric cancer incidence by eradicating Helicobacter pylori infection? Cancer Prev Res (Phila) 2013;6:695-700. [ Links ]
31. Shiota S, Yamaoka Y. Management of Helicobacter pylori. Med Rep 2010;15:2. pii:20. [ Links ]
32. Bae SE, Jung HY, Kang J, Park YS, Baek S, Jung JH, et al. Effect of Helicobacter pylori eradication on metachronous recurrence after endoscopic resection of gastric neoplasm. Am J Gastroenterol 2014;109:60-7. [ Links ]
33. Bang CS, Baik GH. Attempts to enhance the eradication rate of Helicobacter pylori infection. World J Gastroenterol 2014;20:5252-62. [ Links ]
34. Lansdorp-Vogelaar L, Sharp L. Cost-effectiveness of screening and treating Helicobacter pylori for gastric cancer prevention. Best Pract Res Clin Gastroenterol 2013;27:933-47. [ Links ]
35. Yang JC, Lu CW, Lin CJ. Treatment of Helicobacter pylori infection: Currentstatus and future concepts. World J Gastroenterol 2014;14;20:5283-93. [ Links ]
36. Megraud F, Coenen S, Versporten A, Kist M, Lopez-Brea M, Hirschl AM, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013;62:34-42. [ Links ]
37. Wu TS, Hu HM, Kuo FC, Kuo CH. Eradication of Helicobacter pylori infection. Kaohsiung J Med Scienc 2014;30:167e1-72. [ Links ]
38. Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection the Maastricht IV/Florence Consensus Report. Gut 2012;61:646-64. [ Links ]
39. Otero Regino W. La importancia de cultivar Helicobacter pylori. Rev Col Gastroenterol 2013;28:87-92. [ Links ]
40. Rimbara E, Fischbach LA, Graham DY. Optimal therapy for Helicobacter pylori infections. Nat Rev Gastroenterol Hepatol 2011;8:79-88. [ Links ]
41. Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut 2010;59:1143-53. [ Links ]
42. Hunt RH, Xiao SD, Megraud F, Leon-Barua R, Bazzoli F, van der Merwe S, et al. Helicobacter pylori in developing countries. World Gastroenterology Organisation Global Guideline. J Gastrointestin Liver Dis 2011;20:299-304. [ Links ]
43. Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 2007;102:1808-25. [ Links ]
44. Camargo MC, García A, Riquelme A, Otero W, Camargo CA, Hernández-García T, et al. The problem of Helicobacter pylori resistance to antibiotics: A systematic review in Latin America. Am J Gastroenterol 2014;109:485-95. [ Links ]
45. Chuah SK, Tsay FW, Hsu PI, Wu DC. A new look at anti-Helicobacter pylori therapy. World J Gastroenterol. 2011;17:3971e5. [ Links ]
46. Gómez M, Otero W, Gutiérrez O. Tratamiento de la infección gástrica con Helicobacter pylori: encuesta a un grupo de médicos generales y especialistas en Colombia/Treatmet of gastric infection with H. pylori. Acta Med Col 2011;26: 273-9. [ Links ]
47. Breuer T, Sudhop T, Goodman KJ. How do practicing clinicians manage Helicobacter pylori-related gastrointestinal diseases in Germany? Helicobacter. 1998;1:1-8. [ Links ]
48. Shirin H, Birkenfeld S, Shevah O, Levine A, Epstein J, Boaz M, et al. Application of Maastricht 2-2000 Guidelines for the Management of Helicobacter pylori Among Specialists and Primary Care Physicians in Israel: Are We Missing the Malignant Potential of Helicobacter pylori? J Clinic Gastroenterol 2004;4:322-5. [ Links ]
49. Ghanaei FM, Joukar F, Soati M, Gharib S, et al. Knowledge and Practice of General Practitioners and Internists about Helicobacter pylori infection in Guilan, Iran. Middle East J Diges Diseases 2011;3:119-25. [ Links ]











 text in
text in