Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em
SciELO
Similares em
SciELO -
 Similares em Google
Similares em Google
Compartilhar
Revista colombiana de Gastroenterología
versão impressa ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.1 Bogotá jan./mar. 2015
Comparative Study of Colonic Stenting against Laparotomy for Treating Acute Intestinal Obstruction Due to Colorectal Cancer
Rodrigo Castaño Llano MD. (1), Juliana Restrepo MD. (2), Alejandro Carvajal López MD. (3), Mario H. Ruiz MD. (3), Juan D. Puerta MD. (4), Óscar Álvarez MD. (5), Andrés Rojas MD. (6), Alejandra Álvarez (7), Luis Miguel Ruiz (7), David Restrepo (7)
(1) Gastrointestinal Surgeon and Endoscopist, Head of the Postgraduate General Surgery at the Universidad Pontificia Bolivariana, Gastro Group at the Universidad de Antioquia, Instituto de Cancerología Clínica las Américas in Medellín, Colombia.
(2) Medical Resident in General Surgery at the Universidad Pontificia Bolivariana in Medellín, Colombia.
(3) General Surgeon at the Hospital Pablo Tobón Uribe in Medellín, Colombia.
(4) Coloproctologist, General Surgeon and Professor of Surgery at the Universidad Pontificia Bolivariana and Clínica las Américas in Medellín, Colombia.
(5) Gastroenterologist, Texas Valley Coastal Bend Veterans Administration Hospital and Clinical Assistant Professor at the University of Texas Health Science Center at San Antonio, Texas.
(6) General Surgeon at the Instituto de Cancerología Clínica las Américas in Medellín, Colombia.
(7) Undergraduate Students in the Faculty of Medicine at the Universidad Pontificia Bolivariana in Medellin, Colombia.
Received: 01-07-14 Accepted: 02-02-15
Abstract
Objective: Obstructions due to colorectal cancer (CRC) are common and carry significant risks of morbidity and mortality. The use of colonic stents appears to be a good alternative and can be used with palliative therapy or as a bridge to facilitate definitive one-time-only surgery. The aim of this study is to compare the use of stents with surgery in patients with malignant colorectal obstructions in terms of morbidity, survival and the need for ostomies.
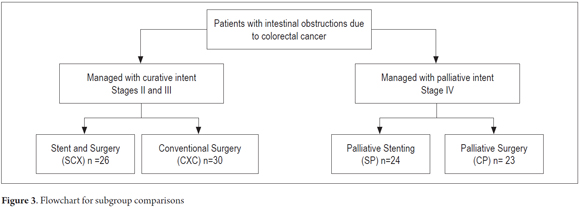
Methods: This is a descriptive study based on data collected from a cohort of 103 patients from 2004 to 2012. Data were recorded in a database designed for this purpose. Patients were retrospectively divided into four groups for purposes of analysis. One group, which was labelled SCX, had had colonic stents placed and later underwent elective surgery (n = 26). A second group labelled CXC had undergone conventional surgery (n = 30). The other two groups consisted of patients in advanced stages of the disease. One of these groups, labelled SP received palliative stents (n = 24). The final group, labelled CP, underwent palliative surgery (n = 23). Comparisons among groups were made and then descriptions of the most important findings were developed.
Results: The technical success rate was 90.0% and the clinical success rate was 82.0% in the groups of patients who received stents. There were two perforations in the SP group (8.3%) and none in the other group (SP) which received stents. Stent migration occurred in three patients (11.5%) in the SCX group and three (12.5%) in the SP group. The overall survival rate was 42.7%, but was only 4.2% in the SP group and 34.8% in the CP group. Fewer ostomies were required in the SCX group (five patients, 19.2%) than in the CXC group (eight patients, 26.7%) although the difference was not statistically significant. Similarly eight patients (26.7%) in the CXC group and 15 patients (65.2%) in the CP group required ostomies (P <0.05). Obstructions were relieved in 22 patients (84.6%) in the SCX group, but in only 18 patients (60%) in the CXC group which is a statistically significant difference (P = 0.042). The hospital average stay of 4 days (0-9 days) for the groups managed with stents (SCX and SP) was shorter than for the other two groups. Thirty patients required at least one reoperation in the CXC group, nine (39.1%) in the CP group and 8 in the group that received stents.
Conclusions: The use of colorectal stents for palliative or preoperative bridge therapy has low morbidity, and sometimes avoids temporary or permanent colostomies. Surgery and the use of ostomies can be avoided in patients with advanced disease who are undergoing palliative care, plus they have shorter hospital stays. However, prospective studies to clarify what the true role of stents in malignant colorectal obstructive disease are still needed.
Keywords
Colorectal cancer, acute colorectal obstruction, prosthetics.
INTRODUCTION
Despite the implementation of screening programs for colorectal cancer (CRC), about 20% of CRC is diagnosed as the result of obstruction of the colon (1). Independent of the state of the patient, decompression and restoration of the permeability of the colon is necessary to avoid increased risk of perforation and sepsis (2). Intestinal obstruction is one of the complications of colorectal cancer that causes the greatest amounts of morbidity and mortality (3). It is an emergency situation in as many as 8% to 29% of patients. These are usually older patients with multiple comorbidities and disturbed physiological functioning. They are usually malnourished and dehydrated and have electrolyte imbalances (4). Generally, emergency treatment involves surgery in stages including placement of colostomies which is associated with high mortality (12%) and morbidity (39%) rates (5). The current trend seeks to realize an elective procedure in which there are optimum conditions for surgery in a single step. The complication rate is lower in patients undergoing elective surgery: the average mortality rate is 3.5% (0.9% to 6%) and the morbidity rate is 23% (6). The two management options are urgent surgery and placement of a self-expanding metallic stent (SEMS) (7, 8).
For patients with resectable conditions, the first aim must be radical resection of neoplasia independent of whether it is done surgically or through placement of a stent. Nevertheless, choosing the ideal procedure is a matter of debate (9, 10). The use of stents as elective pre-cancer surgery bridge therapy for colorectal obstruction appears to be superior to emergency surgery for obstructions in cases of CRC according to various observational studies with short-term follow-up (11-15). Randomized studies show inconsistent results (16-20), and there are very few long-term studies comparing the two options (21, 22). A multicenter study in Holland was prematurely closed down after a group of patients with stage IV CRC managed with stents showed a high rate of severe complications (23). Another study had a long-term clinical failure rate of up to 50% in patients treated with colonic stents with palliative intent (24).
In 1991, Professor Song placed the first SEMs in a patient's gastrointestinal tract (25). In 1991, Dohmoto described the use of colonic stents to solve acute colorectal obstruction (26). The next year, in 1992 Spinelli followed with further description (27). Since then there have been many outcomes reported from clinical research with various kinds of self-expanding metal stents in the colon and rectum. Success rates range from 50% to 96% (16, 18, 24-35). The use of SEMS for palliation of unresectable CRC and for preoperative treatment of obstructive CRC is a therapeutic option for resolving the symptoms of colorectal obstruction which appears to be a promising alternative for urgent patient treatment. In addition to acting as a bridge for further surgery, hospitalization times and costs can be minimized without impacting patient survival.
Although there are limited studies of local experience with the use of colorectal stents have small numbers of patients and short follow-periods, studies describing the results of these interventions in our midst are needed because this appears to be a promising advance in the management of this disease (28-32). The aim of this study was to compare morbidity and survival in patients with colorectal cancer and intestinal obstructions that were managed with colonic stents with similar patients who were managed with conventional open surgery.
METHODOLOGY
This is a retrospective and descriptive study cohort which included patients diagnosed with colon and/or rectal cancer and who had intestinal obstructions. Patients were treated at various institutions in the city of Medellin, Colombia from 2004 to 2012. Patients over the age of 18 years whose diagnoses of intestinal obstruction due to colon cancer had been confirmed histopathologically were included. We excluded patients with intraoperative tumor perforation and/or radiological or clinical suspicion of peritonitis and those patients for whom there was insufficient information in the medical records.
Data was collected on a form for data extraction from medical records which was completed by the group of researchers. The information provided was directly submitted by the patient or by a previously identified close relative in cases in which the patient's condition did not allow her or him to supply complete information including cases of death. Questions were asked clearly and in terms appropriate for people outside of the medical professions. In case enough information was not provided over the phone, the information was confirmed through the clinical history available at the institution in which the patient was treated. The cutoff date for the study was July 31, 2012.
The research was approved by the ethics committee of the Faculty of Medicine of the Universidad Pontificia Bolivariana.
The following variables were analyzed: age, sex, stage of colon cancer, degree of differentiation, tumor location, filtration from intestinal anastomoses, surgical site infections, need for colostomy, complications associated with colostomies, enteric fistulas, intestinal obstructions, perforations of the colon associated with a stent, stent migration, stent obstruction, length of hospital stay, survival rates, and status at discharge.
TECHNIQUES AND PROCEDURES
The description that follows shows how to establish a diagnosis of colorectal cancer and intestinal obstruction of the colon and rectum, conventional surgery and placement of colonic stent:
Acute colon and rectal obstruction: Confirmation of suspicion and diagnosis are based on clinical and radiological data.
- Clinical: Acute abdominal pain or intermittent colic, bloating, vomiting, absence of stool or flatulence, and changes in peristalsis.
- Radiology: X-rays should only be used when there is clinical suspicion. There are two methods: a plain erect abdominal radiogram and a contrasted CT scan of the abdomen. Findings considered for a diagnosis included a minimum of three or more levels of fluid, coin stacking, inverted "J", haustra, dilated bowel loops (> 3 cm for thin loops, > 6 cm for transverse colon loops, and left colon and > 9 cm for the cecum) absence of gas in the rectal ampulla. Other specific findings from the CT scan that were considered included thickening of the walls of the colon, intestinal pneumatosis and free fluid. Patients' accounts of their medical history were used to discard abdominal adhesions resulting from prior abdominal surgery as the main cause of possible obstruction.
Cancer of the colon and rectum: A diagnosis of CRC was made by histopathological examination by a medical pathologist from a surgical specimen taken from the area between ileocecal valve and the anal-rectal dentate line. Preoperative diagnosis was also accepted for patients with known histories and diagnoses established by colonoscopy and biopsy and who had an intestinal obstruction secondary to a tumor.
Conventional open surgery (hemicolectomy, sigmoidectomy or low anterior resection of the rectum): laparotomy, layered dissection, and excision of the surgical specimen. Decision making for performing anastomosis or colostomy. The anastomosis was performed manually or with mechanical sutures. Colostomies were placed through Hartmann's procedure, loop colostomy, or double barrel colostomy. Subsequently, hemostasis was achieved and the layers of the fascia and skin were closed. A surgical specimen was sent to pathology and histopathology for analysis.
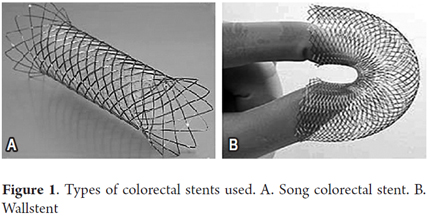
Colonic stents used: Wallstent (Boston Scientific Natick-MA, USA) and the Song colorectal stent (Tecnostent-Corpaul, Medellín-Colombia) (Figure 1).
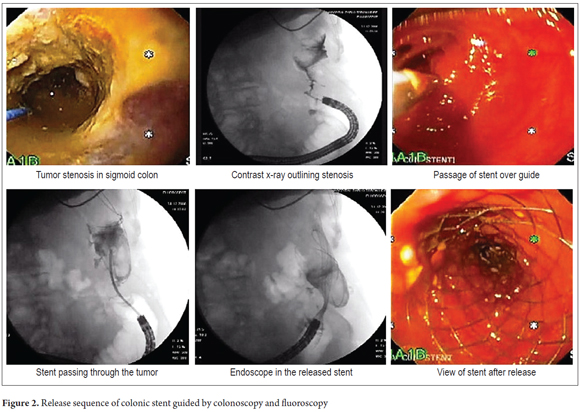
Placement Technique: For patients with upper or middle rectal lesions and for patients with more proximal left lateral lesions, the patients were supine. Generally patients were not been prepared for colonoscopy because of the obstruction, but enemas were applied prior to the procedure. The patient was sedated with midazolam plus meperidine as an analgesic. All patients were given nasal O2. No complications related to sedation occurred. The colonoscope was placed and the stenosis to be treated was located. The contrast medium was introduced under fluoroscopic control so that the morphology and length and length of the stenosis could be estimated. If data from a previous barium enema x-ray of the colon was available, this maneuver was not necessary. A rigid atraumatic 0.035 guide was then passed through the stenosis. The stenosis was not dilated, and the procedure continued with the opening of the stent according to its own system. The choice of prosthesis placed depended on the type of lesion and its length. Stent lengths were chosen to leave a margin of two to three cm on both sides of the stenosis. The remainder of the prosthesis was released slowly while constantly checking its position endoscopically and fluoroscopically (Figure 2) (29, 30).
Statistical Analysis
The following variables were descriptively analyzed: age, sex, stage of colon cancer, degree of differentiation, tumor locations, filtration from intestinal anastomosis, infection of surgical site, need for colostomy, complications associated with colostomy, enteric fistulas, intestinal obstructions, colonic perforations associated with stenting, stent migration, stent obstruction, length of hospital stay, patient survival by group, and status at discharge. Averages and standard deviations were used for quantitative variables while absolute and relative frequencies were calculated for qualitative variables. Differences in complications and clinical conditions between groups were calculated using percentages and Fisher's exact test. The Mann Whitney U test was used to estimate associations and comparisons of quantitative variables such as age and length of stay between groups. Survival rates were compared through the Kaplan Meyer test and the statistical log rank test. The Charlson comorbidity index was calculated as a predictor of mortality according as a function of the comorbidities of patients.
RESULTS
Patients were subdivided into four groups for evaluation: 26 patients who were managed with colonic stents and subsequent elective surgery (SCX), 24 patients who were managed only with palliative stenting (SP), 30 patients who underwent conventional surgery with curative intent (CXC) and 23 patients who underwent palliative surgery (CP). Characteristics listed in the method sections are analyzed below (Figure 3).
Sociodemographic, Clinical and Tumor-Associated Characteristics
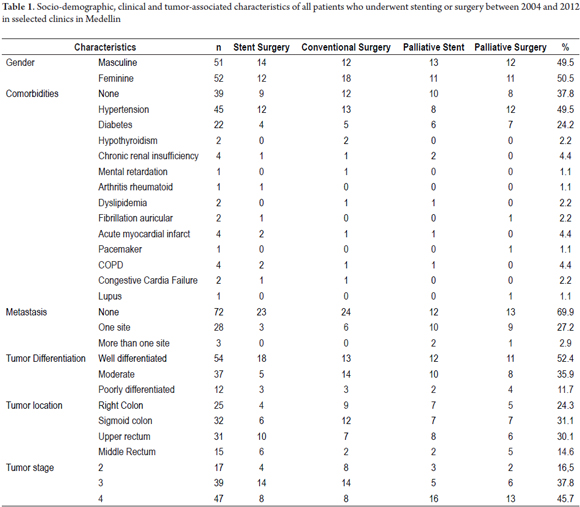
A homogeneous distribution by sex was found 49.5% men and 50.5% women. The ages of the patients ranged between 28 and 87 years, but only 2.88% were less than 35 years old. The mean age was 60 years. The most frequent comorbidities were hypertension (43.7%) and diabetes mellitus (21.3%). On or another comorbidity, including hypothyroidism, chronic renal failure, rheumatoid arthritis, mental retardation, dyslipidemia, obesity, acute myocardial infarction, chronic obstructive pulmonary disease, heart failure and Lupus occurred in 23.3% of these patients.
Depending on the stage of the disease, 72 patients (69.9%) presented either stage II or stage III and 31 patients (30.1%) were in Stage IV (advanced) with metastatic disease. Of these, 28 (27.2%) had metastases at one site (usually the liver), and three (2.9%) had metastases at more than one site. Twenty five (24.3%), tumors were in the right colon, 32 (31.1%) were in the sigmoid colon, 31 (30.1%) were in the upper rectum, and 15 (14.6%) were in the middle rectum. Sociodemographic and clinical characteristics associated with tumor are summarized according to treatment groups in Table 1.
Charlson Index
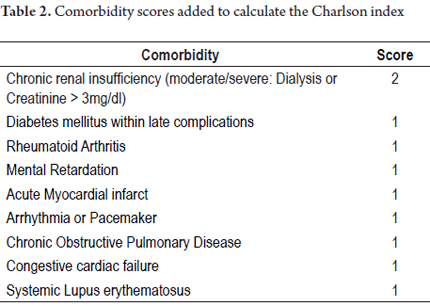
The Charlson index, which is a comorbidity index designed to predict long-term mortality (1 year) depending on associated chronic diseases, was used to analyze information included within variables (33). Points were assigned as follows: 0-1 points for when comorbidities were absent, 2 points for low levels of comorbidities, and 3 points or more for high levels of comorbidities.
The Charlson index recorded in this study was calculated and corrected for age according to each case as follows: 67.0% of patients scored 0 (12% mortality/year); 25.2% of patients scored 1 (26% mortality/year); 1.0% of patients scored 2 (26% mortality/year); and 6.8% of patients scored 3 (52% mortality/year). There were no patients with scores of five or more (85% mortality/year).
To calculate the Charlson index, comorbidity scores were added as shown in Table 2.
Early and late complications
Complications presented in the groups were described and were divided into two subgroups: early complications included those that occurred within 30 days of the procedure, and late complications included those that occurred after this time period. No complications occurred in 66.1% of the patients. Early complications occurred in 33% of patients. Surgical site infections (SSI) were recorded in 8.8% of this group and were the most common complication. Of these, 4.9% were superficial and/or deep, and 3.9% were organ space infections (abdominal collections). Other complications included urinary tract infections (4.9%), filtrations (2.9%), anastomosis (1.9%), intestinal fistulas (1.9%), hernias (1.9%), stent migration (1.9%), perforation by stent (1.9%), and intestinal obstructions due to any cause (1.9%).
Late complications occurred in 29.2% of the patients. Abdominal collections were also the most common late complication, but in smaller proportion. This occurred in 4.9% of the patients. Other late complications included hernias (4.8%, 2.9% were parastomal hernias), urinary tract infections (3.9%), intestinal obstructions from any cause (2.9%) of patients, filtration (1.9%) and intestinal anastomosis (1.9%). It is important to note that stents migrated 3.9% of the patients in each of two of the groups with this intervention.
Hospital Stays
Patients undergoing surgery without stenting had longer hospital stays than those managed with stenting. Patients in the SCX and SP groups had average stays of just four days each (0-9 days). The CXC group's average hospital stay was 10 days (6-21 days) and the CP group's average stay was 11 days (6-23 days).
Technical Success and Clinical Success
Technical success is defined as proper placement of a colonic stent without complications or setbacks during the procedure, and clinical success is defined as resolution of the patient's colonic obstruction following the procedure. Technical success was found in 90.0% of patients in whom stents were placed, and clinical success was recorded in 82.0%. Stenting failed to relieve obstructions in four patients who then underwent colostomies. The patient who suffered early stent migration required a colostomy.
Readmission and Reoperation
Sixteen patients (15.5%) were readmitted to the hospital, and one or more reoperations were required in 39 patients (37.9%). Readmission rates were similar in the four groups studied, with five patients (16.7%) in the CXC group, four patients (15.4%) in the SCX group, four (17.4%) in the CP group and three (12.5%) in the SP group. Some type of reoperation was needed for all patients in the CXC group who were readmitted. Reoperation was required in 19 patients (63.3%), two times for five patients (16.7%), three times for four patients (13.3%) and four reoperations in two patients (6.7%). In the stented groups, there was no need for reoperation of any of the patients. In the CP group, there was one reoperation each in 14 patients (60.9%) and two reoperations in six patients (26.1%).
Subgroup Comparison
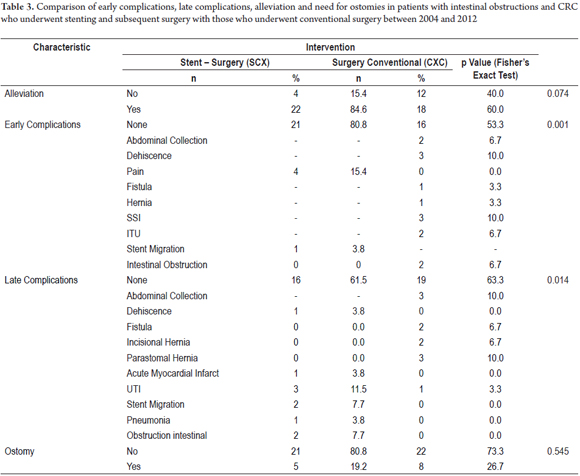
Subgroups of patients were compared to determine differences in morbidity rate findings. Initially the SCX group was compared with the CXC group (Table 3). Colostomies were needed for five (19.2%) of the 26 patients in the SCX group and in eight (26.7%) of the 30 patients in the CXC group. No early complications were found in 21 patients (80.8%) of the SCX group nor in 16 patients (53.3%) of the CXC group with a statistically significant difference (P = 0.001). One relevant finding is that alleviation of symptoms was achieved in 22 patients (84.6%) of SCX group, but, in contrast, alleviation of symptoms was achieved in only 18 patients (60%) of the CXC group, again with a statistically significant difference (P = 0.042). Also, filtration from anastomoses were found in three patients (10.0%), there were two abdominal collections (6.7%) and two intestinal obstructions (6.7%) in the CXC group, whereas there were no such complications in the SCX group. A stent migrate in one patient in this group (3.8%) (Table 3).
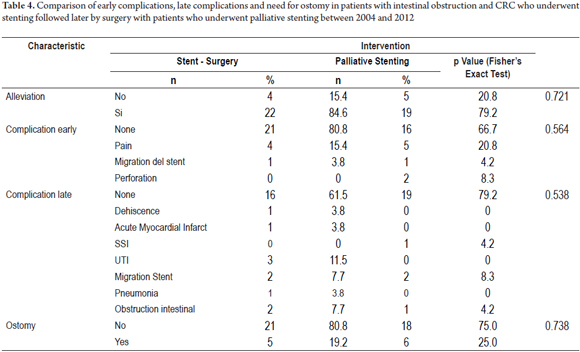
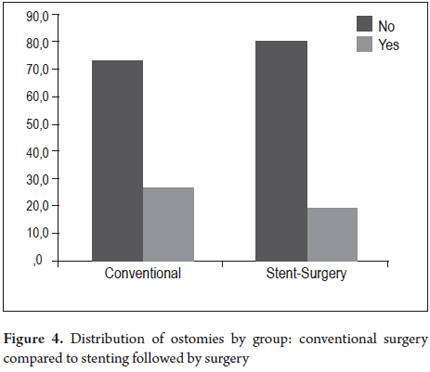
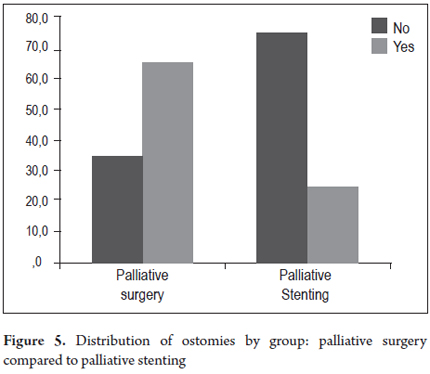
The two groups who had had colonic stents placed, the SCX group and the SP group (Table 4) were compared. In the SCX group, there were no early complications in 21 patients (80.8%) and no late complications in 16 patients (61.5%) while there were no early complications in 16 patients (66.7%) and no late complications in 19 patients (79.2%) in the SP group. Post procedure pain, which affected four patients (15.4%) in the SCX group and five patients (20.8%) in the SP group, was the most common early complication in both groups. There were two perforations (8.3%) in the SP group which were managed with colostomies, but there were no perforations in the SCX group. One patient in each group experienced the migration of a stent (4.2% of SP, and 3.8% of SCX). Both were managed with colostomies. Three other patients underwent colostomies because of unresolved symptoms of obstruction. There were two late stent migrations in each group (7.7% of SCX, 8.3% of SP). Despite the use of colonic stents, ostomies were required in five of the 26 patients (19.2%) of the SCX group and in six of the 24 patients of the SP group (25.0%). The difference was not statistically significant (Table 4, Figures 4 and 5).
Survival
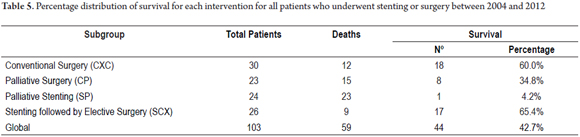
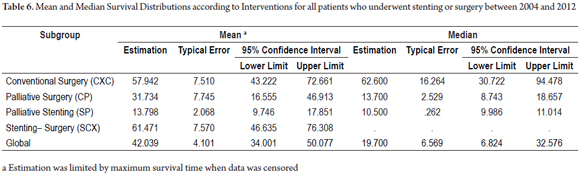
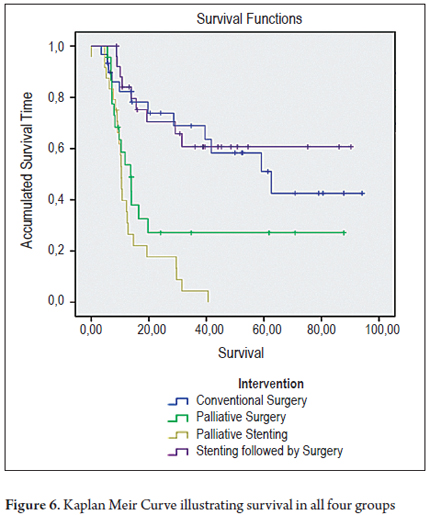
The survival distributions were found and evaluated for each group (Table 6). Groups of patients managed with curative intent had better results, and survival time was worse for patients in advanced stages. A survival rate of 60.0% was found for patients in the CXC group and a survival rate of 65.4% was found for patients in the SCX group. In contrast, palliative groups had survival rates of 34.8% in the CP group and 4.2% in the SP group. Of the period evaluated, the median survival time of patients in the CXC group was 57.9% of the period, while it was 61.4% in the SCX group, 31.7% in the CP group and 13.7% in the SP group (Tables 5 and 6, and Figure 6).
DISCUSSION
The use of colonic stents to treat intestinal obstructions in patients with CRC is a promising development for two patient scenarios: palliative management and preoperative therapy as a bridge to cancer surgery. As a bridge it has few risks, relieves the obstruction and avoids a definitive colostomy (28). In an emergency, intervention with two operations including colostomy and elective resection with primary anastomosis can still be done. The morbidity and mortality inherent in elective colorectal surgery (23% and 3.5% respectively) are much lower than those associated with emergency surgery (39% and 12% respectively) (34). In other studies, more than 50% patients treated urgently have ostomies (35). Applying a stent helps solve the acute situation, allows correction of the patient's electrolyte acidosis, and improved the patient's overall status. This allows for planning future surgery with less likelihood of morbidity, especially of early complications as this study demonstrates, and also less likelihood of mortality (36). A cost-effectiveness study has concluded that the use of a stent before surgery was more effective and less expensive than urgent surgery (37). Stenting minimizes the need for colostomies, thus improving the quality of life of these patients (38). As shown in this study, this is especially true for patients for whom stenting is used with palliative intent. Stenting also avoids multiple surgeries for patients for whom you must rebuild their intestinal transits. Stenting facilitates realization of "optimal cancer surgery" by allowing appropriate assessment of the extension and avoiding unnecessary subtotal colectomies made in an effort to avoid abandoning synchronous tumors (39, 40). Patients who receive stents have shorter hospital stays as evidenced in the two groups of patients who were stented in this study. Patients with advanced disease benefit from the use of these prostheses by avoiding a palliative intervention and a colostomy in their last days. Stenting also allows use of a colonoscopic biopsy, often not available in emergency surgery, to confirm the diagnosis.
The problems that have arisen in comparison with conventional surgery are primarily related to costs and complications. As for costs, the entire cost of the surgical procedure is saved when patients receive a definitive palliative stent. In this context, the prosthesis is certainly cheaper. This is also true for patients who have elective surgery after urgent stent placement. The objective of emergency surgery is to operate on the patient in one step to save the cost of a second operation, but this does not take into account other costs associated with possible additional complications a patient who undergoes emergency surgery is likely to develop, nor is emergency surgery generally done under the best conditions.
This study's evident limitation lies in the fact that it compared patients with colorectal cancer at all stages of disease which necessarily leads to confusion bias. Since this is a retrospective series, this bias was difficult to control. The only statistically significant differences found were in the results of the comparison between performance of ostomies, palliative stenting, and palliative surgery, the comparison of rates of relief between patients who were stented before later surgery and those who underwent conventional surgical procedures, and the shorter hospital stays of the groups managed with stenting.
Given the limitations of the number of patients in this study, reliable conclusions cannot be drawn. Nevertheless, the results of this series and other publications suggest that the use of endoluminal stents for the treatment of intestinal obstructions due to CCR is useful, safe, effective and has low morbidity rates both when used as definitive palliative treatment for patients with inoperable advanced disease and when used as a temporary treatment to resolve an obstruction with the intention of later surgery under better patient conditions.
In this study, the Charlson index which predicts mortality at one year based on associated chronic diseases showed the correlation of those diseases for the patients included in the study. It demonstrated, as expected, higher levels of mortality among patients at advanced stages of disease.
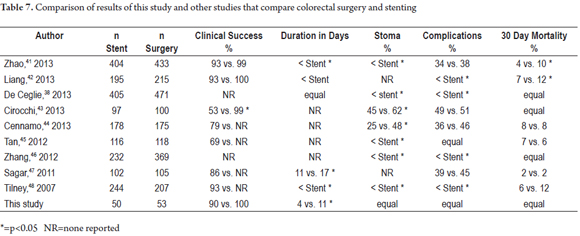
Various meta-analyses (Table 7) have tried to compare surgery and stenting for treatment of malignant colorectal obstructions, but these studies have focused on the problem differently than this study. They have either focused on the exclusive use of stenting for palliation or have evaluated stenting as a bridge therapy before definitive surgery to relieve the obstruction. Most of these studies often cite marked heterogeneity in the data measured which gives less weight to the findings of this meta-analysis. With these limitations in mind, Table 7 lists the studies published to date and compares their results with those of this series.
Meta-analyses that compare colonic stenting and surgery for treatment of colonic obstruction have had mixed results. Liu (49), Ye (50), and Sagar (51) did not find stenting to be superior to surgery in terms of complications and clinical success while Huang (52) and Tilney (48) found stents to be effective and safe as bridging therapy for treatment of colonic obstructions with lower rates of ostomies and shorter hospital stays.
Recently, Sabbagh and colleagues (53) reviewed histological findings from patients who had been stented before later surgery. They purported to show adverse effects of stenting on survival. These included significantly more frequent ulceration of the tumor, peritumoral ulceration, perineural invasion and lymph node involvement in stented patients than in patients treated only with surgery. The same group suggests worse cancer prognoses with lower survival rates in patients receiving the stent than in patients who immediately undergo surgery (54). Recent studies have even suggested increased perineural compromise in patients receiving stents (55). In contrast, another long term follow-up study shows stenting results in lower incidence of incisional hernias (6% vs. 22%, p = 0.04) less stomata (6% vs. 26%, p = 0.01) and better survival (log-rank test, 0.004) than immediate surgery (56). Meanwhile, Tung surgery found no difference in survival after 65 months of follow-up (48% vs. 52%, p = 0.63) (57). Knight's findings were similar (58). All of this suggests that more research is needed to explain what implications histological changes associated with stent insertion have for these patients.
CONCLUSIONS
Obstructions of the colon and rectum are usually secondary to malignancy. Obstructive colonic neoplasms require emergency surgery to avoid complications and eradicate the primary pathology. Obstructive colon tumors increase morbidity and mortality associated with surgical resection, especially in the context of urgency. Ideally, these procedures should be conducted in a way that results in the least possible perioperative risk for the patient.
There are literature reviews that provide evidence about the safety of segmental resection and primary anastomosis in a single stage for patients with obstructive colon cancer, particularly those limited to the left colon. Subtotal colectomy should be considered for patients who develop complications such as obstruction and perforation, in favor of removing remote synchronous lesions. Ways are being sought to change the urgent state of malignant colorectal obstruction to an elective condition. Endoluminal stents can provide a method for converting urgent surgery into elective surgery in institutions where the procedure is available. Information related to endoluminal stenting is still insufficient to suggest that preoperative placement of these prostheses improves the outcome in terms of survival when surgery is performed later. Nevertheless, stenting appears to reduce the need for ostomies, although studies with greater methodological weight and larger sample sizes are need to support this hypothesis.
Conflicts of Interest Declaration
The authors declare that they have no conflicts of interest.
This study was made with the support of the Sustainability project by the Vice-Rector for Research at the University of Antioquia.
REFERENCES
1. Barnett A, Cedar A, Siddiqui F, Herzig D, Fowlkes E, Thomas CR, Jr. Colorectal cancer emergencies. J Gastrointest Cancer. 2013;44:132-42. [ Links ]
2. Dolan EA. Malignant bowel obstruction: A review of current treatment strategies. The American Journal of Hospice & Palliative Care. 2011;28:576-82. [ Links ]
3. Tuca A, Guell E, Martinez-Losada E, Codorniu N. Malignant bowel obstruction in advanced cancer patients: Epidemiology, management, and factors influencing spontaneous resolution. Cancer Management and Res. 2012;4:159-69. [ Links ]
4. Winner M, Mooney SJ, Hershman DL, et al. Incidence and Predictors of Bowel Obstruction in Elderly Patients With Stage IV Colon Cancer: A Population-Based Cohort Study. JAMA Surgery 2013:1-7. [ Links ]
5. Schwenter F, Morel P, Gervaz P. Management of obstructive and perforated colorectal cancer. Expert Review of Anticancer Therapy. 2010;10:1613-9. [ Links ]
6. Henry JC, Pouly S, Sullivan R, et al. A scoring system for the prognosis and treatment of malignant bowel obstruction. Surgery. 2012;152:747-56; discussion 56-7. [ Links ]
7. Grundmann RT. Primary colon resection or Hartmann's procedure in malignant left-sided large bowel obstruction? The use of stents as a bridge to surgery. World J of Gastrointest Surg. 2013;5:1-4. [ Links ]
8. Soreide K. Emergency management of acute obstructed left-sided colon cancer: Loops, stents or tubes? Endoscopy. 2013;45:247-8. [ Links ]
9. Dayyeh BK, Baron TH. Editorial: Endoscopic stent placement as a bridge to surgery in malignant colorectal obstruction: A balance between study validity and real-world applicability. Am J Gastroenterol. 2011;106:2181-2. [ Links ]
10. McCullough JA, Engledow AH. Treatment options in obstructed left-sided colonic cancer. Clin Oncol (R Coll Radiol). 2010;22:764-70. [ Links ]
11. Martinez-Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega-Deballon P, Moreno-Azcoita M. Self-expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: Comparison of primary anastomosis and morbidity rates. Dis Colon Rectum. 2002;45:401-6. [ Links ]
12. Lee HJ, Hong SP, Cheon JH, et al. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: Endoscopic stenting versus surgery. Gastrointest Endosc. 2011;73:535-42. [ Links ]
13. Law WL, Choi HK, Chu KW. Comparison of stenting with emergency surgery as palliative treatment for obstructing primary left-sided colorectal cancer. Br J Surg. 2003;90:1429-33. [ Links ]
14. Ng KC, Law WL, Lee YM, Choi HK, Seto CL, Ho JW. Self-expanding metallic stent as a bridge to surgery versus emergency resection for obstructing left-sided colorectal cancer: A case-matched study. J Gastrointest Surg. 2006;10:798-803. [ Links ]
15. Pessione S, Petruzzelli L, Gentilli S, Mioli P. Treatment of neoplastic stenosis of the left colon: Presurgical expandable metal stent vs emergency surgery. Comparison of results and survival rates. Chir Ital. 2007;59:661-9. [ Links ]
16. Angenete E, Asplund D, Bergstrom M, Park PO. Stenting for colorectal cancer obstruction compared to surgery: A study of consecutive patients in a single institution. Int J Colorectal Dis. 2012;27:665-70. [ Links ]
17. van Hooft JE, Bemelman WA, Oldenburg B, et al. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: A multicentre randomised trial. Lancet Oncol. 2011;12:344-52. [ Links ]
18. Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: A multicenter randomized controlled trial. Surg Endosc. 2011;25:1814-21. [ Links ]
19. Cheung HY, Chung CC, Tsang WW, Wong JC, Yau KK, Li MK. Endolaparoscopic approach vs conventional open surgery in the treatment of obstructing left-sided colon cancer: A randomized controlled trial. Arch Surg. 2009;144:1127-32. [ Links ]
20. Ho KS, Quah HM, Lim JF, Tang CL, Eu KW. Endoscopic stenting and elective surgery versus emergency surgery for left-sided malignant colonic obstruction: A prospective randomized trial. Int J Colorectal Dis. 2012;27:355-62. [ Links ]
21. Saida Y, Sumiyama Y, Nagao J, Uramatsu M. Long-term prognosis of preoperative "bridge to surgery" expandable metallic stent insertion for obstructive colorectal cancer: Comparison with emergency operation. Dis Colon Rectum. 2003;46:S44-9. [ Links ]
22. Dastur JK, Forshaw MJ, Modarai B, Solkar MM, Raymond T, Parker MC. Comparison of short-and long-term outcomes following either insertion of self-expanding metallic stents or emergency surgery in malignant large bowel obstruction. Tech Coloproctol. 2008;12:51-5. [ Links ]
23. van Hooft JE, Fockens P, Marinelli AW, et al. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40:184-91. [ Links ]
24. Fernandez-Esparrach G, Bordas JM, Giraldez MD, et al. Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol. 2010;105:1087-93. [ Links ]
25. Song HY, Choi KC, Cho BH, Ahn DS, Kim KS. Esophagogastric neoplasms: Palliation with a modified gianturco stent. Radiology. 1991;180:349-54. [ Links ]
26. Dohmoto M. New method: Endoscopic implantation of rectal stent in palliative treatment of malignant stenosis. Endosc Dig. 1991;3:1507-12. [ Links ]
27. Spinelli P, Dal Fante M, Mancini A. Self-expanding mesh stent for endoscopic palliation of rectal obstructing tumors: A preliminary report. Surg Endosc. 1992;6:72-4. [ Links ]
28. Castaño R, Puerta JD, Restrepo JI, et al. Manejo actual de la obstrucción maligna colorrectal: grandes incisiones, pequeñas incisiones o sin incisiones. Rev Col Gastroenterol. 2007;23:57-66. [ Links ]
29. Castaño R. Técnicas en stents gastrointestinales endoscópicos: cómo, cuándo, manejo de las complicaciones, selección del stent y costos. Rev Col Gastroenterol. 2012;27:32-45. [ Links ]
30. Castaño R, Puerta JD, Alvarez O, et al. Self-Expanding Metal Stents in Malignant and Benign Colonic Obstructions. Gastrointest Endosc. 2008;67:AB 151. [ Links ]
31. Kang SG, Ruiz MH, Castaño R. Stents colónicos en la obstrucción maligna del colon. In: Castaño R, Artifon E, eds. Endoscopia Oncológica: diagnóstico y terapéutica. Medellín, Colombia: Editorial Universidad de Antioquia. 2011:155-70. [ Links ]
32. Castaño R, Artifon El. Técnicas Endoscópicas en Próteses Gastrointestinais: Como e Quando Utilizar, Manejo de Complicacoes, Selecao e Custos. In: Artifon EL, de Moura E, Sakai P, eds. Próteses Endoscópicas no Aparelho Digestorio. Sao Paulo: Editora Atheneu; San Pablo. 2012. p. 123-131. [ Links ]
33. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of chronic diseases. 1987;40:373-83. [ Links ]
34. Ghazi S, Berg E, Lindblom A, Lindforss U. Clinicopathological analysis of colorectal cancer: A comparison between emergency and elective surgical cases. World J Surg Oncol. 2013;11:133. [ Links ]
35. Benizri EI, Rahili A, Benchimol D. Emergency management of acute colonic cancer obstruction. Journal of visceral surgery. 2012;149:e366-7. [ Links ]
36. Warden C, Stupart D, Goldberg P. Stenting as first-line management for all patients with nonperforating left-sided obstructing colorectal cancer. Colorectal Dis. 2013;15:e389-95. [ Links ]
37. Targownik LE, Spiegel BM, Sack J, et al. Colonic stent vs. emergency surgery for management of acute left-sided malignant colonic obstruction: A decision analysis. Gastrointest Endosc. 2004;60:865-74. [ Links ]
38. De Ceglie A, Filiberti R, Baron TH, Ceppi M, Conio M. A meta-analysis of endoscopic stenting as bridge to surgery versus emergency surgery for left-sided colorectal cancer obstruction. Crit Rev Oncol Hemat. 2013;88:387-403. [ Links ]
39. Dulucq JL, Wintringer P, Beyssac R, Barberis C, Talbi P, Mahajna A. One-stage laparoscopic colorectal resection after placement of self-expanding metallic stents for colorectal obstruction: A prospective study. Dig Dis Sci. 2006;51:2365-71. [ Links ]
40. Ramadori G, Lindhorst A, Armbrust T. Colorectal tumors with complete obstruction--endoscopic recovery of passage replacing emergency surgery? A report of two cases. BMC Gastroenterol. 2007;7:14. [ Links ]
41. Zhao XD, Cai BB, Cao RS, Shi RH. Palliative treatment for incurable malignant colorectal obstructions: A meta-analysis. World J Gastroenterol. 2013;19:5565-74. [ Links ]
42. Liang TW, Sun Y, Wei YC, Yang DX. Palliative treatment of malignant colorectal obstruction caused by advanced malignancy: A self-expanding metallic stent or surgery? A system review and meta-analysis. Surg Today. 2014;44:22-33. [ Links ]
43. Cirocchi R, Farinella E, Trastulli S, et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: A systematic review and meta-analysis. Surg Oncol. 2013;22:14-21. [ Links ]
44. Cennamo V, Luigiano C, Coccolini F, et al. Meta-analysis of randomized trials comparing endoscopic stenting and surgical decompression for colorectal cancer obstruction. Int J Colorectal Dis. 2013;28:855-63. [ Links ]
45. Tan CJ, Dasari BV, Gardiner K. Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg. 2012;99:469-76. [ Links ]
46. Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: A meta-analysis. Surg Endosc. 2012;26:110-9. [ Links ]
47. Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev. 2011:CD007378. [ Links ]
48. Tilney HS, Lovegrove RE, Purkayastha S, et al. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc. 2007;21:225-33. [ Links ]
49. Liu Z, Kang L, Li C, Huang M, Zhang X, Wang J. Meta-analysis of complications of colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction. Surg Laparosc Endosc Percutan Tech. 2014;24:73-9. [ Links ]
50. Ye GY, Cui Z, Chen L, Zhong M. Colonic stenting vs emergent surgery for acute left-sided malignant colonic obstruction: A systematic review and meta-analysis. World J Gastroenterol. 2012;18:5608-15. [ Links ]
51. Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev. 2011:CD007378. [ Links ]
52. Huang X, Lv B, Zhang S, Meng L. Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: A meta-analysis. J Gastrointest Surg. 2014;18:584-91. [ Links ]
53. Sabbagh C, Chatelain D, Trouillet N, et al. Does use of a metallic colon stent as a bridge to surgery modify the pathology data in patients with colonic obstruction? A case-matched study. Surg Endosc. 2013;27:3622-31. [ Links ]
54. Sabbagh C, Browet F, Diouf M, et al. Is stenting as "a bridge to surgery" an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg. 2013;258:107-15. [ Links ]
55. Kim HJ, Choi GS, Park JS, Park SY, Jun SH. Higher rate of perineural invasion in stent-laparoscopic approach in comparison to emergent open resection for obstructing left-sided colon cancer. Int J Colorectal Dis. 2013;28:407-14. [ Links ]
56. Gianotti L, Tamini N, Nespoli L, et al. A prospective evaluation of short-term and long-term results from colonic stenting for palliation or as a bridge to elective operation versus immediate surgery for large-bowel obstruction. Surg Endosc. 2013;27:832-42. [ Links ]
57. Tung KL, Cheung HY, Ng LW, Chung CC, Li MK. Endo-laparoscopic approach versus conventional open surgery in the treatment of obstructing left-sided colon cancer: long-term follow-up of a randomized trial. Asian J Endosc Surg. 2013;6:78-81. [ Links ]
58. Knight AL, Trompetas V, Saunders MP, Anderson HJ. Does stenting of left-sided colorectal cancer as a "bridge to surgery" adversely affect oncological outcomes? A comparison with non-obstructing elective left-sided colonic resections. Int J Colorectal Dis. 2012;27:1509-14. [ Links ]











 texto em
texto em