Services on Demand
Journal
Article
Indicators
-
 Cited by SciELO
Cited by SciELO -
 Access statistics
Access statistics
Related links
-
 Cited by Google
Cited by Google -
 Similars in
SciELO
Similars in
SciELO -
 Similars in Google
Similars in Google
Share
Revista colombiana de Gastroenterología
Print version ISSN 0120-9957
Rev Col Gastroenterol vol.30 no.1 Bogotá Jan./Mar. 2015
Helicobacter Pylori Reinfection Rate after More Than Two Years of Follow-up in a Cohort of Successfully Treated Colombian Patients
William Otero Regino MD. (1), Alba Alicia Trespalacios Rangel Bact. MSc. PhD. (2), Marcela Mercado Reyes Bact. MSc. (3)
(1) Professor of Medicine in the Gastroenterology Unit at the National University of Colombia and Gastroenterologist at Founders Clinic in, Bogotá Colombia.
(2) Professor of Microbiology in the Faculty of Science and Director of the Medical Microbiology Program at the Pontificia Universidad Javeriana in Bogotá, Colombia.
(3) Professor of Microbiology in the Faculty of Science in Bogotá, Colombia.
Received: 19-08-14 Accepted: 02-02-15
Abstract
Helicobacter pylori (H. pylori) infects at least half the world's population although its prevalence is higher in developing countries. The reinfection rate varies from region to region and may include recrudescence of infection or true recurrence. So far there are few studies that have investigated recurrence of infections in Colombia, and none had been done in Bogota, Colombia.
Objective: The objective of this study was to determine the rate of recurrence of H. pylori in patients who had been treated effectively with three different triple therapies.
Materials and Methods: This was an observational study nested in a cohort of 180 patients in whom H. pylori had been successfully eradicated during 2008 and 2009. Eradication was verified with the labeled urea breath test. The average follow-up time was 43.7 months (range: 31-56 months). Recurrence was investigated with monoclonal stool antigen tests (ImmunoCard STAT®, HpSA (Meridian Bioscience Inc.)).
Results: A total of 86 patients were monitored during the follow-up period. Out of this group the reinfection rate was 5.8% (5/86). The annual reinfection rate was 1.59% (5/313.4 patient-years x 100). The first case of reinfection was presented at 32 months, and the other cases occurred at 37, 42, 44 and 56 month of follow-up. The reinfection rate was 1.8%/year calculated after two years of monitoring.
Conclusions: The H. pylori reinfection rate in Bogotá is low and is less than that previously reported for other regions of Colombia.
Keywords
Reinfection, Helicobacter, recrudescence.
INTRODUCTION
Helicobacter pylori (H. pylori) infects approximately 50% of the world's population (1, 2), but its prevalence varies significantly among countries, social classes, ages and races (3-5). H. pylori's prevalence is usually lower in developed countries than in developing countries where it can be as high as 80% (6). It is the major causative agent of chronic gastritis, peptic ulcers and gastric adenocarcinoma (7-10). Eradication significantly reduces recurrence of peptic ulcers (11), cures 60% to 80% of gastric MALT in early stages (Lugano I and II) (12, 13), and may decrease the risk of gastric adenocarcinoma (14, 15), and the risk of recurrence of gastric adenocarcinoma after endoscopic resection (16). Eradication is cost-effective for patients with functional dyspepsia (17) and for patients with immune thrombocytopenia for whom it results in 50% improvement of platelet counts (18).
Considering the benefits of eradication of this microbe, determining the rate of reinfection is important. Reinfection means that patients' gastritis and peptic ulcers will probably recur (19, 20). The impact is even higher in countries like Colombia with high prevalences of infection (77.2% to 83% in Colombia) (21-23) and high incidences of gastric adenocarcinoma (17.4/100,000 to 48.2/100,000 people in Colombia) (24). Reinfection is a form of recurrence that is defined as the reappearance of H. pylori after a negative test performed at least four weeks after eradication therapy has ended (20). Recurrence may be secondary to "resurgence" or to a true reinfection. Recrudescence is the situation in which the strain of H. pylori present before treatment is temporarily suppressed by antibiotic treatment and undetectable when the patient is tested four weeks after treatment, but detectable when the organism again begins to replicate (20, 25). This definition implies that the bacteria identified by molecular techniques is the same bacteria that were present before treatment.
H. pylori reinfections are found that are both molecularly different and genetically identical to that found in the original bacteria that were eradicated (20). A global rates of recurrence vary, but these bacteria are generally more prevalent in the developing world. A systematic review in 2005 by Gisbert found an annual recurrence rate of 3% (20). That study considered that recurrence was probably secondary to recrudescence and not to true reinfections because the majority decline over time. Studies have shown that reinfections are uncommon after the first year of treatment and that most recurrences are due to recrudescence, however the use of inappropriate methods to verify eradication of infection and the difficultly of following-up on eradication have made it difficult to really know what the true rate of reinfection is (25).
A recent meta-analysis has found an average annual recurrence rate of 2.7% in developed countries and an average annual recurrence rate of 13% or more in developing countries (26). Over 15 years ago recurrence rates in Colombia were found to be 18% to 20% per year (27, 28). Nevertheless, that assessment was done during the first year and a half using rapid urease testing and histology with hematoxylin and eosin without special stains which leads to doubts about the likelihood of accurate results (20). A more recent multinational study in Latin America which used the urea breath test found a recurrence rate for Colombia of 18.1% (participants were from Tuquerres in Nariño Department) and an average for Latin America of 11.5% (29). Given that studies of reinfection in Colombia have found high rates using inaccurate methods and similar rates with the urea breath test only in rural areas, we decided to perform a rigorous study to determine the rate of reinfection in Bogotá in a cohort of patients treated with triple therapy and followed for two to five years.
MATERIALS AND METHODS
This was a study of a cohort of patients who had been cured of infection at Clínica Fundadores and the Faculty of Sciences of the Pontificia Universidad Javeriana.
Population and sample
A randomized, single-blind clinical trial of treatment of H. pylori using three different triple therapies was developed in 2008 and 2009. Eighty patients were included in each group. H. pylori infections were identified by the rapid urease test and histology with hematoxylin and eosin and additional Giemsa staining or by cultures. Patients received one of three types of triple therapy for 10 days: 1. Esomeprazole + levofloxacin + amoxicillin (ELA); 2. Esomeprazole + levofloxacin + clarithromycin (ELC) y 3. esomeprazole + clarithromycin + amoxicillin (ECA).
Eight weeks after treatment ended, success of H. pylori eradication was verified by Carbon 13 urea breath test (13C PAU), as previously described (19). After determining therapeutic success, patients were invited to be part of a cohort to be monitored to assess reinfection. After completion of treatment the patients were included in the follow-up for at least 1 year. They were called by telephone one year later to invite them to take a stool antigen test in order to evaluate reinfection. The stool antigen test (HpSA test) ® (Meridian BioscienceInc) is considered to be useful when you do not have the urea breath test by the Maastricht Consensus (15). The test's sensitivity is 95.4% (95% CI: 86 to 100%) and a specificity of 100%.
Overall Objectives
The overall objective of the study was to determine the annual rate of H. pylori reinfection in individuals who had been successfully treated with three different triple therapies.
Specific Objectives
1. To determine the reinfection rate over by individual follow-ups.
2. To identify potential risk factors associated with reinfection.
Exclusion criteria
Patients were excluded if they had been diagnosed with cancer, suspected of having gastric cancer, were pregnant or lactating, had histories of previous gastric surgery, or were taking antibiotics, antisecretory or bismuth medicines within a month of stool test for antigen.
Variables
Dependent Variable: The dependent variable was the reinfection rate counted from positive stool antigen tests during the monitoring period.
Independent Variables: Independent variables included sociodemographic variables of age, sex, socioeconomic status, education level, and occupation; family background variables of family member diagnosed with H. pylori infection and family history of gastroduodenal disease; type of treatment administered; and follow-up time before reinfection.
Procedure
Patient Recruitment: Subjects who met the study's inclusion criteria were invited to participate. The study was explained to them in detail, and they were asked to sign an informed consent form. Study variables were recorded on a pre-encoded form. Once consent had been given, the participant underwent the stool antigen test in order to determine the rate of reinfection.
Test procedure for ELISA: For the fecal antigen test, the patient is told that she or he will have to take a stool sample. The patient was prohibited from consuming proton pump inhibitors or antacids for at least two weeks prior to the exam. Once collected, the sample was delivered to the laboratory for analysis and was processed on the same day of collection. The ImmunoCard STAT ® test HpSAT (Meridian Bioscience Inc was used to determine the presence of H. pylori antigens in stool samples. The test was used according to the manufacturer's recommendations in catalog 750720. The test protocol is based on a fast qualitative immunoassay for in vitro detection of Helicobacter pylori antigens in human stool. A diluted stool sample from the patient is placed in the sample port of the test device. After five minutes of incubation at room temperature, the appearance of a reddish-pink color contiguous with the letter "T" in the window indicates a positive result. Both the research protocol and the informed consent form were approved by the Ethics Committee of the institution where the study was done.
Collection, processing and analysis of results: A database was created in SPSS 20 ®. A Kaplan-Meier survival curve was calculated to analyze the rate of reinfection (the dependent variable) in people/month and in terms of frequency of reinfection during the follow-up period. Univariate analysis was used to assess potential associations between reinfection and risk factors. Relative risks were calculated with their respective 95%confidence intervals. All results were considered statistically significant when significance was <0.05.
RESULTS
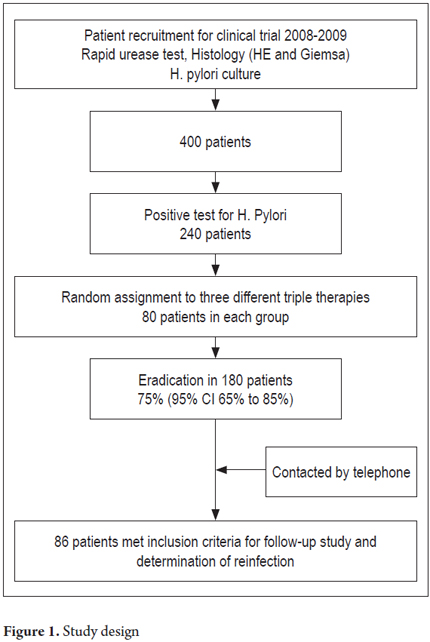
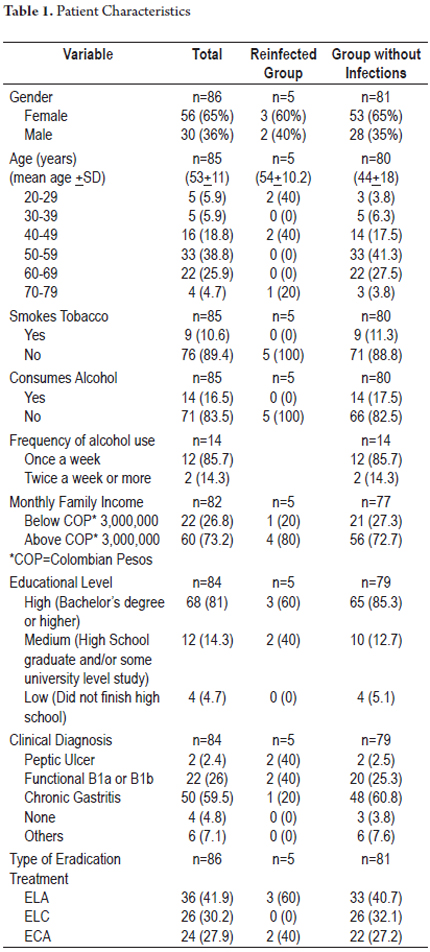
Eighty-six of 180 eligible patients were finally included in this study. Sixty patients were excluded. At the time they were called for stool antigen testing, twenty were taking antibiotics, thirty were taking proton pump inhibitors for gastro-esophageal reflux, and ten were hospitalized due to pulmonary diseases or surgery. Thirty-four patients decided not to participate. There were thirty-six patients in the group that received ELA (41.9%), twenty-six in the group that received ELC (30.2%), and twenty-four in the group that received ECA (27.9%). The mean age was 53 + 11 years (mean + SD), and the age range was 26 to 74 years. Fifty-six patients (65%) were female and thirty (36%) were men. The largest number, fifty patients (59.5%), had clinical diagnoses of chronic gastritis. This was followed by twenty-two patients (26%) with B1a or B1b functional dyspepsia and two patients (2.4%) with peptic ulcers. Figure 1 shows the design of the study. Sociodemographic and clinical information is shown in Table 1.
Monitoring and Reinfection Rate
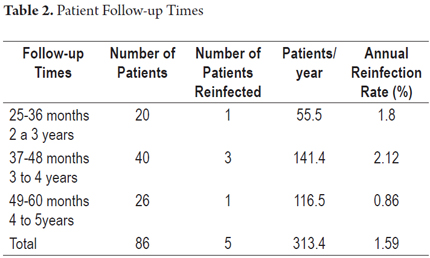
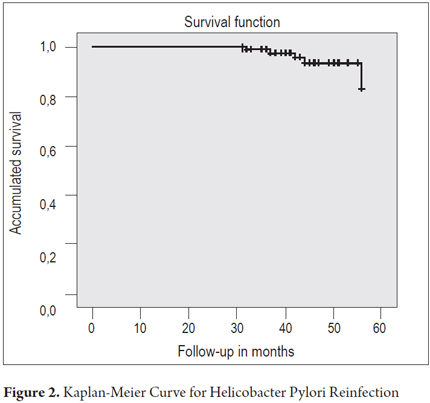
The mean duration of follow-up in the group of 86 patients was 43.7months (range, 31-56 months). The total percentage of patients with H. pylori reinfection was 5.8% (5/86). Of these cases, three out of five were in the group of patients who received ELA triple therapy, two out of five had relapses in the group who received ACE therapy, and there were no cases of reinfection in the group of patients that received ELC therapy. The annual reinfection rate was 1.59% (5/313.4 x 100 patient years). The distribution based on time tracking is shown in Table 2. The curve of reinfection occurrence shows that the first case of reinfection presented at 32 months followed by cases at 37, 42, 44 and 56 months of follow-up (Figure 2). No significant associations with reinfection risk factors were found.
DISCUSSION
In this study, the cumulative risk of recurrence of H. pylori infection from two to five years after eradication was 5.8% and the annual percentage of positive tests for H. pylori infections was 1.8% per year after two years of monitoring. There was no statistically significant differences for reinfection rates by type of therapy received, although the small number of patients who were reinfected cannot accurately determine this relationship. The late appearance of the organism favors reinfection rather than recrudescence since the latter appears during the first year or immediately after (20, 25). Even more than late appearance, the diminishing rate of recurrence during the 4th and 5th year of follow-up, during which it was 0.86%, supports the likelihood of reinfection. Although the five reinfected patients underwent endoscopies in which biopsies were taken for culturing to determine whether the molecular characterizations of the H. pylori strains recovered are different from those of the strains found before eradication therapy, this information was not available at the time this paper was written.
The low recurrence rate that we found contrasts with the rate found by the Latin American study of H. pylori recurrence of 18.2% per year recently found in Túquerres, Nariño, a rural Colombian area (29). It is also lower than the 11.5% average of Latin American countries that were part of that study. Considering that the microorganism was identified immediately after the first year in the Latin American study, the possibility exists that these higher rates reflect a mixture of recrudescence and reinfection (20, 25, 30).
Our results are also different from those found in the larger follow-up study in Latin America for a period of six years (31). In that study the reinfection rate, as determined by histology, was 5.4% per year (31).
Patients included in that study's follow-up had been part of a clinical trial conducted ten years earlier in a rural town where the initial eradication of H. pylori had been verified by urea breath test (32). That study did not specify how long after treatment eradication was verified even though this is very important for ruling out the possibility of initial false negative test results. We do not know the reason for this discrepancy in those results, although it is not excluded that patients from a small, rural population with different sanitary conditions may be exposed to H. pylori through contaminated water, improperly cooked vegetables and other environmental factors which are considered to be very important in re-exposure to H. pylori infection as has been demonstrated in underdeveloped countries with high prevalences of infection (33, 34). Other factors that may also favor reinfection in such populations include domestic transmission (true reinfection) and transmission through endoscopic and forceps biopsies that are reprocessed manually. Transmission can occur in 1% to 3% of endoscopy, even if the equipment is washed with 70% ethanol or 2% glutaraldehyde (35). Patients in the initial study underwent several endoscopies as part of a study for determining the evolution of gastric cancer precursor lesions (32).
In our study, the prevalence of pretreatment infections was 59.5% (95% CI: 54.7% to 64.4%). Patients who participated in our study were from estratos three to five in Bogotá, the capital city (Translator's note: Colombia has a system of geographical districts based on income levels. There are six estratos ranked from the lowest income level in estrato 1 to the highest income level in estrato 6). Our study was conducted 20 years after the one in Tuquerres where the infection rate in 2013 was 83% which is much higher than 59.5% found in this study (22). We believe that these factors could explain the discrepancies in the results of these two studies.
Given the design features of this study and its results, we believe that, at least in Bogotá, the current prevalence of H. pylori is lower than those previously reported for other regions (59.5% vs. 77-83%) but higher than those found in industrialized countries which are from 10% to 30% (36). We also believe that the reinfection rate is low, similar to the rate of 1.8% per year which was found in Brazil recently in a 5-year follow-up study (37). The rate here is also similar to another underdeveloped country, Morocco, which was found to have a rate of 0.8% per year (36). In other countries in the developing world reinfection rates range from 1.1% at two years in China (38) to 73% at eight months in Peru (39).
CONCLUSIONS
The results of this study demonstrate that the prevalence of infection in Bogotá as determined in a group of patients with dyspepsia or symptoms of gastroesophageal reflux disease is 59%. This is lower than rates found previously. Secondly, this study's results show that the reinfection rate is low (1.59% per year). These data justify optimizing the recommended management of this infection (15). With this new information, patients and doctors can be more relaxed about fears of future reinfection which is not "inevitable" as often thought in many places. We consider it important to continue this kind of study in other regions of Colombia, as the epidemiology of H. pylori may vary from place to place sites within the same country.
Conflicts of interest
The authors declare that they have no conflicts of interest and that the costs of this study were borne entirely by the researchers.
REFERENCES
1. Dunn BE, Cohen H., Blaser MJ. Helicobacter pylori. Clin Microbiol Rev. 1997;10:720-41. [ Links ]
2. Suerbaum S, Michetti P. Helicobacter pylori infection. N Engl J Med 2002;347:1175-86. [ Links ]
3. Grad YH, Lipsitch M., Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: Evidence for sustained race/ethnic disparities. Am J Epidemiol 2012;175:54-9. [ Links ]
4. Epplein M, Signorello LB, Zheng W, Peek RM, Michel A, Williams SM, et al. Race, African ancestry, and Helicobacter pylori infection in a low-income United States population. Cancer Epidemiol Biomarkers Prev 2011;20:826-34. [ Links ]
5. Jafar S, Jalil A, Soheila N, Sirous S. Prevalence of Helicobacter pylori infection in children, a population-based cross-sectional Study in West Iran. Iran J Pediatr 2013;23:13-8. [ Links ]
6. Sanders MK, Peura DA. Helicobacter pylori-associated diseases. Curr Gastroenterol Rep 2002;4:448-54. [ Links ]
7. NIH Consensus Conference. Helicobacter pylori in peptic ulcer disease. NIH Consensus Develpment Panel on Helicobacter pylori in Peptic Ulcer Disease. JAMA 1994;272:65-69. [ Links ]
8. McKoll KE. Clinical practice. Helicobacter pylori infection. N Engl J Med 2010;362:1597-1604. [ Links ]
9. Mbulaiteye SM, Hisada M, El-Omar EM. Helicobacter pylori associated global gastric cancer burden. Front Biosci 2009;14:1490-1504 [ Links ]
10. Piazuelo MB, Epplein M, Correa P. Gastric Cancer: An infectious disease. Infect Dis Clin N Am 2010;24:853-69. [ Links ]
11. Hopkins RJ, Girardi LS, Turney EA. Relationship between Helicobacter pylori eradication and reduced duodenal and gastric peptic ulcer recurrence: A review. Gastroenterology 1996;110:1244-52. [ Links ]
12. Chen LT, Lin JT, Tai JJ. Long-term results of anti-Helicobacter pylori Therapy in early–stage gastric high grade transformed MALT lymphoma. J Natl Cancer Inst 2005;97:1345-53. [ Links ]
13. Sthatis A, Chini C, Bertoni F. Long-term outcome following Helicobacter pylori eradication in a retrospective study of 105 patients with localized gastric marginal zone B-cell lymphoma of MALT type. Ann Oncol 2009;20:1086-93. [ Links ]
14. Wong BC, Lam SK, Wong WM. Helicobacter pylori eradication to prevent gastric cancer in a high risk region in China: A randomizes controlled trial. JAMA 2004;291:187-94. [ Links ]
15. Malfertheiner P, Megraud F, O´Morain CA. Management of Helicobacter pylori infection. The Masstricht IV/Florence Consensus Report. Gut 2012;61:646-64. [ Links ]
16. Fukase K, Kato M, Kikuchi S. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinom after endoscopic resection of early gastric cancer: An open-label randomized controlled trial. Lancet 2008;372:392-7. [ Links ]
17. Moayyedi P. Dyspepsia. Curr Opin Gastroenterol 2012;28:602-7. [ Links ]
18. Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, et al. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: A systematic review. Blood 2009;113:1231-40. [ Links ]
19. Xia HH, Talley NJ, Keane CT. Recurrence of Helicobacter pylori infection after successful eradication: Nature, possible causes and potential preventive strategies. Dig Dis Sci 1997;42:1821-24. [ Links ]
20. Gisbert J. The recurrence of Helicobacter pylori infection: Incidence and variables influencing it. A critical review. Am J Gastroenterol 2005;100:2083-99. [ Links ]
21. Campuzano-Maya G, Hoyos D, Calvo VD, Suárez OA, Lizcano D, Rojas CA. Prevalencia de la infección por Helicobacter pylori en médicos de Medellín, Colombia. Acta Gastroenterol Latinoam 2007;37:99-103. [ Links ]
22. Bravo LE, Cortés A, Carrascal E, Jaramillo R, García LS, Bravo PE, et al. Helicobacter pylori: patología y prevalencia en biopsias gástricas en Colombia. Col Med 2003;34:124-31. [ Links ]
23. Porras C, Nodora J, Sexton R, Ferrecccio C, Jiménez S, Domínguez RL, et al. Epidemiology of Helicobacter pylori infection in six Latin American countries (SWOG Trial S0701). Cancer Causes Control 2013;24:209-215. [ Links ]
24. http://globocan.iarc.fr Consultada el 7 de junio de 2013. [ Links ]
25. Zhang YY, Xia HHX, Zhuang ZH, Zhong J. Review article: "true" re-infection of Helicobacter pylori after successful eradication-worldwide annual rates, risk factors and clinical implications. Aliment Pharmacol Ther 2008;29:145-60. [ Links ]
26. Niv Y, Hazzai R. Helicobacter pylori recurrence in developed and developing countries: meta-analysis of 13C-urea breath test follow-up after eradication. Helicobacter 2008;13:56-61. [ Links ]
27. Otero W, Sierra F, Gutiérrez O. Omeprazole plus Amoxicilin in H. pylori Erradication and Duodenal Ulcer. Reinfection and Ulcer recurrence in an area of high prevalence of infection. Gastroenterology 1995;108:A185. [ Links ]
28. Sierra F, Gutiérrez O, Molano B, Otero W. H pylori: Reinfection rate after succesfull Eradication. Gastroenterology 1995;108:A21989. [ Links ]
29. Morgan DR, Torres J, Sexton R, Herrero R, Salazar-Martínez E, Greenberg ER, et al. Risk of recurrent Helicobacter pylori infection 1 year after initial eradication therapy in 7 Latin American Communities. JAMA 2013;309:578-86. [ Links ]
30. Rollan A, Giancaspero R, Fuster F, et al. The long-term reinfection rate and the course of duodenal ulcer disease after eradication of Helicobacter pylori in a developing country. Am J Gastroenterol 2000;95:50-56. [ Links ]
31. Mera R, Fotham ETH, Bravo LE, Bravo JC, Piazuelo MB, Camargo MC, et al. Long Term follow up of patients treated for Helicobacter pylori infection. Gut 2005;54:1536-40. [ Links ]
32. Correa P, Fontham ETH, Bravo JC, Bravo LE, Ruíz B, Zarama G, et al. Chemoprevention of gastric displasia: Randomized trial of antioxidant supplements and Anti-Helicobacter pylori therapy. J Natl Cancer Inst 2000;92:1881-8. [ Links ]
33. Hulten K, Han SW, Enroth H. Helicobacter pylori in the drinking water in Peru. Gastroenterology 1996; 110:1035-6. [ Links ]
34. Hopkins RJ, Vial PA, Ferreccio C. Seroprevalence of Helicobacter pylori in Chile: Vegetables may serve as one route of transmission. J Infect Dis 1993;168:222-6. [ Links ]
35. Langenberg W, Raws EAJ, Oudbier JH. Patient to patient transmission of Campylobacter pylori infection by fiberoptic gastroduodenoscopy and biopsy. J infect Dis 1990;161:507-11. [ Links ]
36. Benajah DA, Lahbabi M, Alaoui S, El-Rhazi K, El Abkari E, Nejjari C, et al. Prevalence of Helicobacter pylori and its recurrence after successful eradication in a developing nation (Morocco). Clin Res Hepatol Gasroenterol 2013 early Rel April 6. [ Links ]
37. Silva FM, Navarro-Rodríguez T, Barbuti RC, Mattar R, Hashimoto CL, Eisig JN. Helicobacter pylori reinfection in Brazilian patients with peptic ulcer disease: A 5-year follow-up. Helicobacter 2010;15:46-52. [ Links ]
38. Mitchell HM, Hu P, Chi Y. A low rate of reinfection following effective therapy against Helicobacter pylori in a developing nation (China). Gastroenterology 1998;114:256-61. [ Links ]
39. Ramírez-Ramos A, Gilman HR, Leon-Barua R. Rapid recurrence of Helicobacter pylori infection in Peruvian patients after successful eradication. Clin Infect Dis 1997;25:1027-31. [ Links ]











 text in
text in